Abstract
Background:
Guillain–Barre syndrome (GBS) is an autoimmune acute inflammatory demyelinating polyneuropathy affecting the peripheral nervous system treated with high-dose immunoglobulin, physical therapy, or plasmapheresis. Immunoglobulins are expensive and even plasmapheresis might not be affordable to patients visiting government set-ups.
Aims:
This study was undertaken to emphasize the efficacy of plasmapheresis in treatment of adult GBS patients and to narrate methods of reducing the economic burden in the treatment of these patients using modified plasmapheresis.
Methods:
A study was conducted on 12 adult GBS patients at Sir Takhtasinhji General Hospital, Bhavnagar from July 2012 to July 2014. Patients were assessed on a 6-point disability scale. They were treated with plasmapheresis over 10 days with REF627 kit from Haemonetics Corporation Limited on MCS+ machine. Improvement was noted by the change in the disability scale score and expenses of various modes of treatment were also considered.
Results:
Seventy-five percent showed improvement at the end of the treatment. The cost of modified plasmapheresis was Rs. 8000/cycle, i.e., Rs. 40,000/patient.
Conclusion:
Plasmapheresis along with proper supportive measures is a more cost-effective efficacious mode of therapy in adult patients of GBS. Further, modified plasmapheresis using REF627 kit and 6% hexastarch as replacement fluid on MCS+ apheresis machine reduces the cost of therapy for poor patients visiting government set-ups.
Key words: Economical, Guillain–Barre syndrome, plasmapheresis
Introduction
Guillain–Barre syndrome (GBS) is an acute inflammatory demyelinating polyneuropathy (AIDP), an autoimmune disease affecting the peripheral nervous system that is usually triggered by an acute infectious process. There is no cure for the disorder, but several treatments can ease symptoms and reduce the duration of the illness and assist in the patient's recovery.
Treatments may include:[1]
High-dose immunoglobulin therapy
Physical therapy
Plasmapheresis.
Immunoglobulins cost around Rs. 3000–3500/ml and given in a dose of 2 ml/kg. Therefore, the cost of such therapy in adults is more than 2 lakhs usually. Plasmapheresis on the other hand using MCS+ and hemonetics kit costs Rs. 1.4 lakhs/patient. Patients visiting government set-ups like the principal investigator's set-up cannot afford such treatment.
This study was undertaken to emphasize the efficacy of plasmapheresis in treatment of adult GBS patients and to narrate methods of reducing the economic burden in the treatment of these patients using modified plasmapheresis which reduced the cost to Rs. 40,000/patient.
Methods
A study was conducted on 12 adult patients of GBS admitted to Sir Takhtasinhji General Hospital, Bhavnagar during 2 years tenure from July 2012 to July 2014. They were treated with plasmapheresis over 10 days. Improvement was assessed by noting the change in the disability scale score after completion of the plasmapheresis cycles. The expense of various modes of treatment of GBS was also considered.
Please note that the population under study is small as incidence of cases of GBS is less in our set-up. Furthermore, immunoglobulin therapy was not given due to the availability of plasmapheresis.
Selection and description of participants
Inclusion criteria
Adults with confirmed diagnosis of GBS with weight more than 40 kg, normal serum sodium and potassium levels, serum protein >2 g% and patients without any cardiac compensation.
All these patients were assessed as per the standard six point disability scale for GBS[2] as mentioned in Table 1.
Table 1.
GBS disability scale

Technical information
They were treated with plasmapheresis with REF627 kit from Haemonetics Corporation Limited on MCS+ apheresis machine.
Anticoagulant acid citrate dextrose (ACD) was used and ACD: Whole blood ratio adjusted between 1:9 and 1:16 depending on the platelet count.
Maximum plasma volume to be removed per cycle was calculated using the following formula: Plasma volume = Blood volume × (1 − hematocrit in decimals).
Replacement fluids used: 6% Hexastarch (HES), 0.9% normal saline, and fresh frozen plasma (FFP).
Ethics
The procedures followed were in accordance with ethical standards and written consent was taken in each patient.
Results
All the 12 patients of GBS were graded on the base of a disability scale as mentioned in Table 1 before plasmapheresis and after completion of all cycles of plasmapheresis. Seven patients underwent five cycles each, three patients underwent six cycles each, one patient underwent seven cycles, and one patient (who died due to severe respiratory distress) underwent only one cycle of plasmapheresis. Changes in the disability score were recorded as mentioned in Table 2.
Table 2.
Impact of plasmapheresis
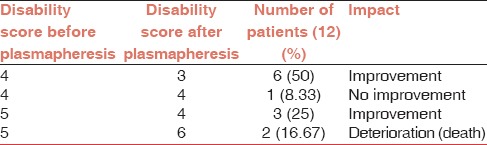
Table 2 shows improvement in 75% cases. No complications were noted except for vasovagal syncope in one cycle in one patient (8.33%). Please note that the deaths in two cases were not owing to the procedure.
Statistics
All the 12 patients presented within 2 weeks of the onset of disease.
The improvement was noted in 75% cases.
To assess improvement disability score was compared before and after plasmapheresis as elaborated under Table 2 in results. As sample size is small (<30) and it is a quantitative analysis, paired t-test was applied to assess improvement and thereby efficacy of plasmapheresis. P value was found to be 0.0271, i.e., statistically significant (0.05 is significant).
No complications were noted except for vasovagal syncope in one cycle in one patient (8.33%).
Discussion
Acute Inflammatory Demyelinating Polyneuropathy (AIDP; Guillain-Barre Syndrome [GBS]) is an acute progressive paralyzing illness affecting both motor and sensory peripheral nerves. Typically the disease begins with symmetrical muscle weakness and paresthesias that spread proximally. Weakness progresses over a period of 12 h to 28 days before the nadir is reached and may involve respiratory and oropharyngeal muscles in more severe cases. Thus, mechanical ventilation is required for approximately 25% of patients. Autonomic dysfunction can cause variability in blood pressure and heart rate. Spontaneous recovery may occur, however, up to 75% of patients develop long-term neurologic deficits. Mortality is estimated at 5%. The Miller–Fisher variant is characterized by ophthalmoplegia, ataxia, and areflexia. An autoimmune pathogenesis is strongly suggested due to the presence of antibodies against four gangliosides GM1, GD1a, GT1a, and GQ1b which differ by the number and position of sialic acids (M, D, T, and Q represent mono-, di-, tri-, and quadric sialosyl groups) in the majority of patients as well as in animal models of the disease. Observations of preceding infectious illness, such as campylobacter suggest cross-reactive antibodies may be a component in disease pathogenesis. There are several scales to evaluate severity and prognosis of the disease (e.g., GBS disability score, Medical Research Council sum score, erasmus GBS respiratory insufficiency score, and erasmus GBS outcome score).[2]
The goal of the treatment plan in GBS is to lessen the severity of the illness and to assist in the patient's recovery. High-quality intensive care remains the most important aspect of the management of severe cases of GBS. Treatments may include high-dose immunoglobulin therapy, physical therapy, plasmapheresis.[1]
The mechanism by which intravenous immunoglobulins (IVIgs) works in GBS is unclear. IVIg has minimal side effects including headache, local skin reaction at infusion site and flu-like symptoms, Aseptic meningitis, thromboembolic events such as pulmonary embolism due to increasing viscosity of blood, are seen rarely.[3] IVIgs are given in a dose of 2 g/kg body weight.
Plasma exchange removes antibodies from the bloodstream. It involves connecting the patient's blood circulation to a machine which exchanges the plasma for a substitute solution, usually albumin.[4] In GBS, 5–6 therapeutic plasma exchanges (TPEs) over 14 days are recommended with 5% albumin replacement.[2]
In this study, plasmapheresis performed for 12 patients of GBS using REF627 kit and 6% hexastarch, 0.9% normal saline, FFP, and with proper supportive measures produced a significant improvement in 75% cases. This emphasizes the fact that plasmapheresis is efficacious in the treatment of patients of GBS. Various other studies comparing the use of plasmapheresis and IVIg in GBS have found them to be equally efficacious. This fact is further supported by many other studies and trials.[5,6,7,8,9]
Further in support to our study, the study by Gajjar et al. also showed the cost comparison of TPE and IVIg in treatment of patients of GBS and concluded that TPE was more cost-effective than IVIg as the treatment modality in GBS taking into account the shortening of time interval in Intensive Care Unit and hospital.[4]
The cost per cycle of plasmapheresis in this set-up was Rs. 8000/cycle, i.e., on an average Rs. 40,000/patient as shown in Table 3. This cost included the cost of the kit REF627, replacement fluids (6% hexastarch and 0.9% normal saline) intravenous cannula and needles and was decided by the hospital authorities. Hospital stay and FFPs were provided free of cost.
Table 3.
Expense of plasmapheresis in our setup and estimated expenses for other modes of treatment in the same patients

Table 3 shows that use of REF627 kit on MCS+ and use of 6% HES in place of 5% human albumin can drastically reduce the economic burden of plasmapheresis. The selection of the type and amount of replacement fluids is an important consideration in the prescription of plasmapheresis. In most plasmapheresis procedures, replacement by colloidal agents is essential to maintain hemodynamic stability. In practice, this is limited to albumin, generally in the form of 5% solution, or FFP. FFP has the advantage of being similar to the filtrate from the patient but is associated with side effects such as allergic reactions, hypocalcemic reactions from the citrate in the plasma and a small but measurable incidence of transmission of hepatitis B (0.0005%/unit), hepatitis C (0.03%/unit), and HIV (0.0004%/unit). Finally, since plasmapheresis also depletes coagulation factors, replacement by albumin and crystalloids alone may deplete these factors and place the patient at increased risk of bleeding. It is recommended that if prothrombin time and partial thromboplastin time measured before the procedures are 1.5 times greater than control samples, at least, 2–3 units of FFP should be infused as part of the replacement solution. Because of the above concerns with the use of FFP, it is recommended that the initial replacement solution should be albumin. Albumin, at a concentration of 5 g/dL, can be replaced in equivolumes to the filtrate. It has an excellent overall safety record. A pitfall in the routine use of albumin is its cost and the lack of clotting factors. In recent years, decreased availability, rising costs, recognition of drug interactions with albumin (i.e., angiotensin-converting enzyme inhibitors) and a fear of disease transmission have led several groups to reconsider the use of colloid starches as partial or full replacement for plasma exchange. A 3% solution of hetastarch (HES) has been utilized for the first liter of albumin replacement. This technique provides 25–50% of the total fluid replacement per procedure and offers a decreased risk of allergic and anaphylactoid reactions compared to albumin. Furthermore, there are potential annual savings in the cost. Six percent HES and 10% pentastarch, both are cleared by urinary excretion. Pentastarch is eliminated twice as fast in a 24 h period, which makes it preferable. However, pentastarch is only licensed to use during leukapheresis and as a volume expander.[10] Hydroxyethyl starches are biochemically similar to glycogen, which likely explains the lack of immunogenicity and lack of adverse reactions. Substantial cost savings are associated with the substitution of starch for albumin. HES is well-tolerated and cost-effective as full or partial volume replacement with plasma exchange.[11] Colloid starch should be avoided in patients with renal failure, congestive heart failure, pulmonary edema, hyperviscosity, corn or starch allergy, coagulopathies, and liver failure.[10] No side effect due to use of FFP and 6% HES was observed in this study.
Conclusion
Plasmapheresis along with proper supportive measures is a more cost-effective efficacious mode of therapy in adult patients of GBS. It is a better option, especially in a government set-up, where most of the patients are not able to afford an expensive modality of treatment such as IVIg and immunosuppressive drugs. Further, this method can be made less expensive and economic burden reduced by REF627 kit and 6% hexastarch as replacement fluid on MCS+ apheresis machine. The financial advantage to the patient is of returning to work earlier and lesser hospital payments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Patients and their relatives, Faculty and Staff, Departments of Medicine, Anaesthesia and PSM.
References
- 1.Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS) Pharmacol Rep. 2010;62:220–32. doi: 10.1016/s1734-1140(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The sixth special issue. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 3.Koc AF, Kilic NB, Yerdelen D, Bozemir H. Guillain-Barre syndrome; etiology, clinical findings and therapeutic plasma exchange. J Neurol Sci. 2005;22:267–73. [Google Scholar]
- 4.Gajjar MD, Shah SD, Shah MC, Bhatnagar NM, Soni S, Patel T. Efficacy and cost effectiveness of therapeutic plasma exchange in patient of Guillain-Barre syndrome – A prospective study. Southeast Asian J Case Rep Rev. 2013;2:218–28. [Google Scholar]
- 5.Winer JB. Treatment of Guillian-Barre syndrome. Q J Med. 2002;95:717–21. doi: 10.1093/qjmed/95.11.717. [DOI] [PubMed] [Google Scholar]
- 6.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet. 1997;349:225–30. [PubMed] [Google Scholar]
- 7.Hughes RA. Plasma exchange versus intravenous immunoglobulin for Guillain-Barré syndrome. Ther Apher. 1997;1:129–30. doi: 10.1111/j.1744-9987.1997.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 8.Ravasio A, Pasquinelli M, Currò Dossi B, Neri W, Guidi C, Gessaroli M, et al. High dose intravenous immune globulins and plasma exchange in Guillain-Barré syndrome. Ital J Neurol Sci. 1995;16:487–92. [PubMed] [Google Scholar]
- 9.Guillian-Barre syndrome treatment approach – Epocrates online. BMJ Publishing Group Ltd. 2014. Available at https://online.epocrates.com/u/2941176/Guillain-Barre+syndrome .
- 10.Ismail N, Neyra R, Hakim R. Plasmapheresis. In: Mallache HH, editor. Ch. II. Dialysis, Clinical Nephrology, Dialysis and Transplantation. 1999. pp. 1–38. [Google Scholar]
- 11.Brecher ME, Owen HG, Bandarenko N. Alternatives to albumin: Starch replacement for plasma exchange. J Clin Apher. 1997;12:146–53. doi: 10.1002/(sici)1098-1101(1997)12:3<146::aid-jca8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


