Abstract
Introduction
The aim of this study was to investigate and analyze the incidence of early-onset colorectal cancer in Arizona, using the Arizona Cancer Registry.
Methods
We performed a retrospective analysis of patients with colorectal cancer reported in the Arizona Cancer Registry from 1995-2010. Outcome measure: incidence of CRC in patients younger than 50 years.
Results
39,623 cases of colorectal cancer were reported to the Arizona Cancer Registry during a period of 15 years. Overall, there was a 17% decrease in the incidence of CRC. However, there was a 23% increase in incidence among patients in the age group 10-50. During the same time period, 15% and 41% increase in the incidence of colon and rectal cancer was observed, respectively. The most significant increase (102%) in overall CRC incidence was seen in the age group 10-29. The highest increase (110%) in incidence of colon cancer was observed in the same age group, while the most significant increase in incidence rates (225%) of rectal cancer was seen in the age group 30-34.
Conclusion
Although there is an overall decrease in incidence of colorectal cancer in Arizona, alarming increase in incidence of early-onset CRC was observed; mirroring the national trends.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer related deaths in the United States (1, 2). The American Cancer Society estimates that in 2015 about 132,700 people will be diagnosed with colorectal cancer and about 49,700 patients will die from this disease (3). Although the overall CRC incidence and mortality have been declining in both men and women since about 1990 (4), most likely due to progressive improvements in population screening, several recent studies have shown alarming increase in the incidence of CRC in patients younger than 50 (5, 6). CRC incidence among patients younger than 50 years ranges from 0.85 per 100,000 for the age group 20-24 to 28.8 per 100,000 for the age group 45-49 (6). Recent studies that used the Surveillance, Epidemiology, and End Results (SEER) database have shown that more than one-tenth of the newly diagnosed CRC cases (11% of colon cancers and 18% of rectal cancers) have a young onset and that the associated mortality rates for those with young-onset CRC have been increasing by approximately 2% annually since 2009 (6-9). By contrast, the age-adjusted mortality rates have shown decreasing trends in older patients by 2% to 3% annually between 1992 and 2009 (2, 4).
Arizona is the sixth largest state in the US. The burden of colorectal cancer in Arizona has not been well defined in the literature, as Arizona does not participate in the SEER database. According to a recent statistical analysis, nearly 2,600 of new CRC cases will be diagnosed and approximately 900 people will die in Arizona this year from colorectal cancer; and almost 50% of these colorectal cancers will be diagnosed after the cancer has spread beyond the colon (10). Therefore, the aim of our study was to evaluate the trends of colorectal cancer in Arizona from 1995 to 2010, with particular focus on early-onset disease using the Arizona Cancer Registry.
METHODS
We performed a retrospective analysis of patients with colorectal cancer reported to the Arizona Cancer Registry from 1995-2010. Arizona Cancer Registry is a part of the Centers for Disease Control and Prevention's National Program of Cancer Registries. The registry is a member of the North American Association of Central Cancer Registries, which sets standards for data quality. All hospitals, clinics, and physicians in Arizona report cancer cases, clinical characteristics, and selected demographic information for cases to the Arizona Cancer Registry.
We obtained the following data points from the registry: age at diagnosis, gender, race, location of tumor, and stage of disease. The age of diagnosis was categorized as 0-29 years of age, and then arranged in increments of 10 years to 85+ years old for incidence of colon, rectal, and overall CRC. We compared these variables for patient's older (≥50yrs) and younger (<50yrs) to assess: 1. Incidence in the two age subsets, and 2. Stages of presentation of colon cancer for years 2005-2010.
Colorectal cancer was defined using the following ICD O3 codes: 8000 8010 8012 8020 8041 8070 8140 8070 8140 8143 8210 8211 8221 8240 8241 8243 8244 8245 8246 8261 8262 8263 8310 8470 8480 8481 8490 8560 8890. We defined location of colorectal Cancer as colon, recto sigmoid region, and rectum. We defined stages of the Colon Cancer as following: Stage I: Cancer present in the mucosa; Stage II: localized, invades through submucosa; stage III: lymph node involvement, and stage IV: distant spread with metastasis.
Statistical analysis was performed using SAS 9.4.We performed linear regression analyses testing the correlation between year of diagnosis and incidence of disease overall and for several subgroups. We used a chi square test to compare the distribution of stages of colorectal cancers for under and above 50. Standardized residuals were computed to assess the deviation from the null hypothesis of no difference between younger and older patients. A p-value ≤ 0.05 was considered statistically significant.
RESULTS
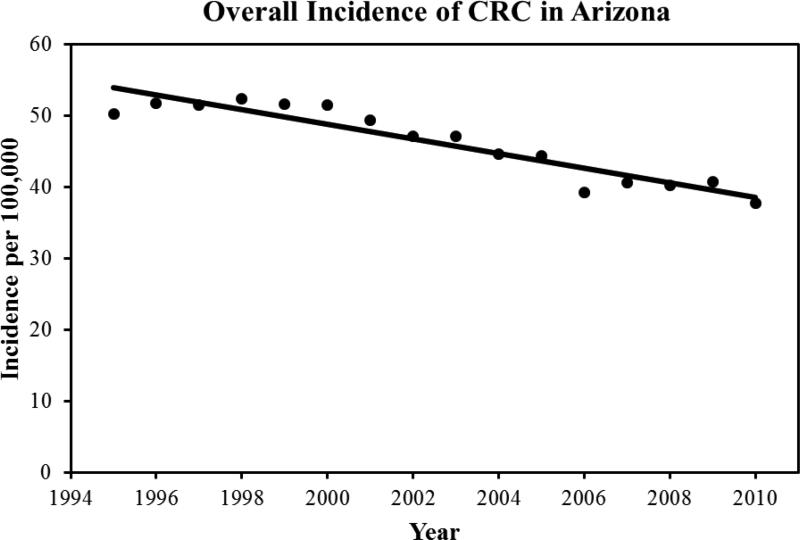
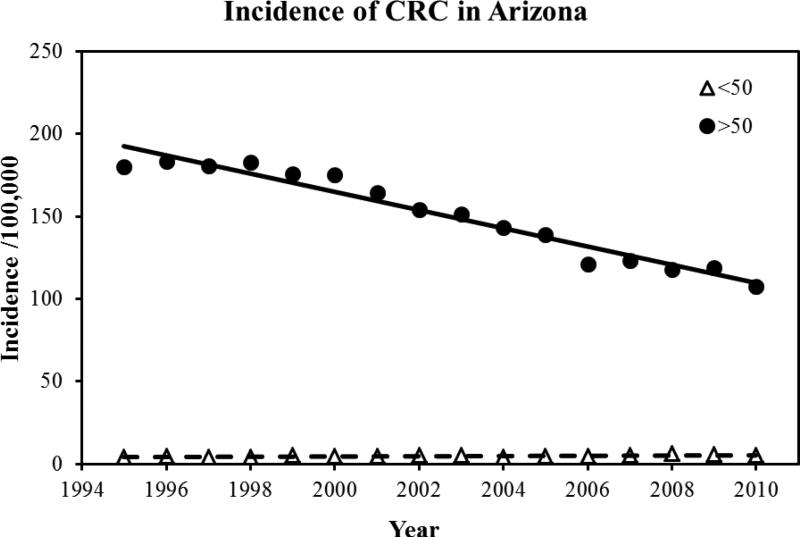
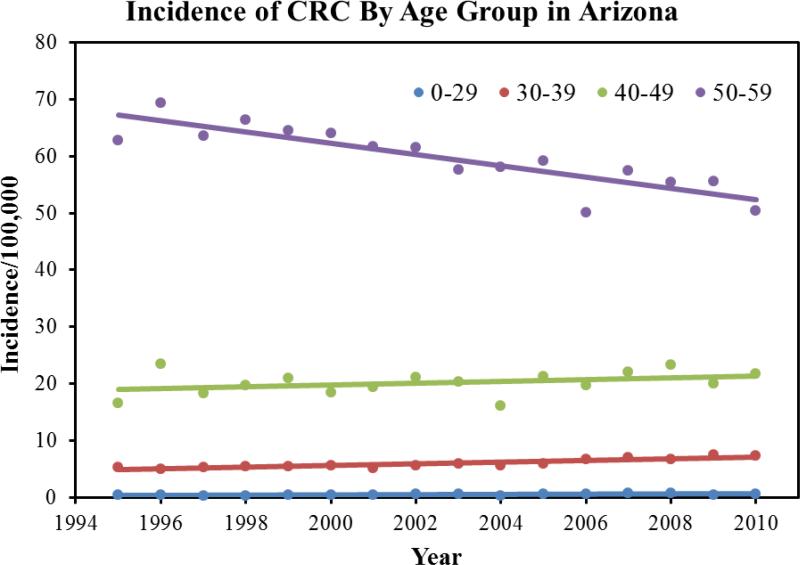
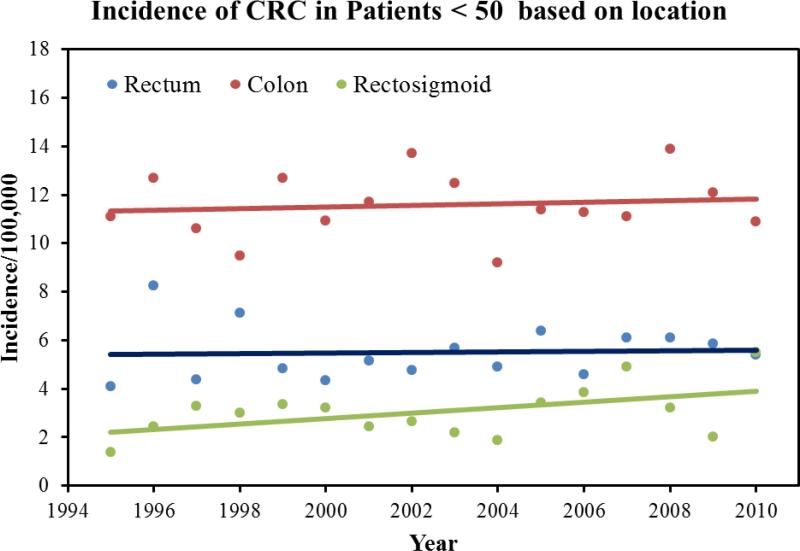
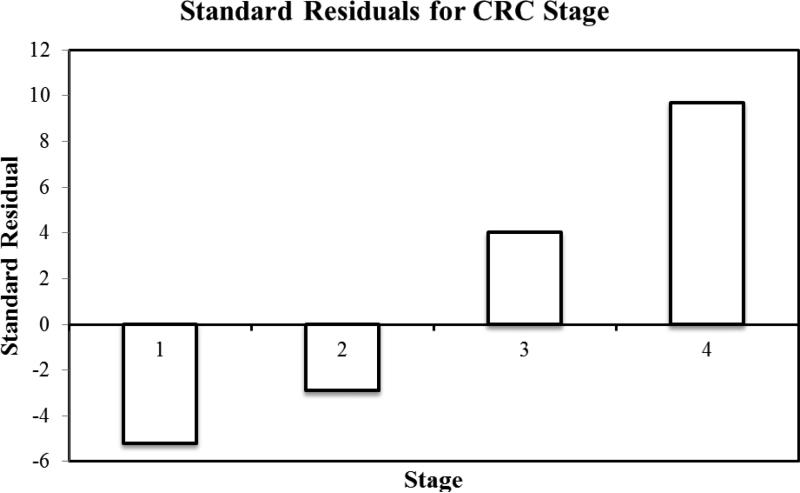
39,623 cases of colorectal cancer were reported to the Arizona Cancer Registry from 1995-2010. There were 53.1% males and the mean age at diagnosis was 69.5±23.3 years. The mean age of diagnosis in females was 70.1±24.3 years. 92% of the patient with a diagnosis of CRC was above the age of 50. In terms of ethnicity, 85% of the patients were White followed by the following ethnicities: Black: 2%; Hispanic: 8%, American Indians: 1.6%, and Asian and Pacific islanders: 1.1%. During the study period, we found a 3.1% decrease in the average age of diagnosis, 70.8 years to 68.1 years. Overall, there was a 17% decrease in the incidence of CRC. The mean incidence was 54 per 100,000 in the year 1994, which declined to 38 per 100,000 in the year 2010. Figure 1 highlights the total CRC incidence for all ages. Once we dichotomized the population into two age groups (<50 and <50), we found that there was a 23% increase in incidence in patients younger than 50. The incidence of colorectal cancer in patients younger than 50 increased from 11 per 100,000 in 1994 to 21.9 per 100,000 in 2010 (p-value: 001). Figure 2 highlights the total CRC incidence for the two age groups. Further analysis of the data in terms of 10 years increments revealed that there is a statistically significant rise in incidence of colorectal cancer in patients aged 45 compared to patients aged 35 (p-value<0.001). Table 1 highlights the incidence in 10 year increments. Similarly, we found a 58% increase in the incidence of colorectal cancer in patients younger than 29 years, 26% increase in CRC incidence in patients aged 30-39, and an 8% increase in CRC incidence in patients aged 40-49. A 225% increase in incidence of rectal cancer was seen in a group of patients 30-34. For colon cancer, a 110% increase in incidence was seen in patient group 10-29. Figure 3 highlights the incidence in four age groups. Analysis of the data in terms of location of CRC in patients younger than 50 revealed that there has been a 76% increase in the incidence of cancer in the recto-sigmoid region, 3% increase in the rectal region, and 5% increase in the colon region. Figure 4 highlights the incidence based on location of the tumor. In patients under the age of 50, 39% of patients presented with stage IV disease, followed by stage I disease in 27% of the patients. CRC Stage II and III occurred in 21% and 13% of the patients, respectively. Age based (<50 years vs. >50 years) comparison of the grades of CRC in presentation, we observed that the younger age group was more likely to present with stage IV disease compared to its older counterparts (23.5% vs. 16.5%; p<0.001). Figure 5 highlights the results using standard residuals.
Figure 1.
Overall incidence of colorectal cancer in Arizona State between 1995 and 2010.
Figure 2.
Incidence of colorectal cancer in Arizona among two age groups, <50 and >50.
Table 1.
Incidence of Colorectal Cancer in age groups
| 15 | 35 | 45 | 55 | 65 | 75 | 85 | |
|---|---|---|---|---|---|---|---|
| 1995 | 0.21 | 3.2 | 11.1 | 40.7 | 107.4 | 192.9 | 337.4 |
| 2000 | 0.353 | 2.75 | 10.92 | 36.9 | 105.8 | 203.7 | 324.8 |
| 2005 | 0.158 | 3.43 | 11.4 | 34.6 | 87.8 | 168.3 | 269.1 |
| 2010 | 0.37 | 4.44 | 10.9 | 30 | 64.5 | 127.4 | 207.5 |
Incidence is reported per population of 100,000
Figure 3.
Incidence of colorectal cancer in various age groups.
Figure 4.
Incidence of colorectal cancer in a group of patients younger than 50 based on anatomic location of tumor.
Figure 5.
Standard residuals for colorectal cancer stages.
DISCUSSION
Our study highlights increasing incidence of colorectal cancer in younger patients in the state of Arizona. Although the overall rate of CRC in Arizona decreased by 17% during the 15 year period; we found an alarming increase in the incidence of colorectal cancer in patients younger than 50 years.
In this study, we showed that the incidence of colorectal cancer in patients younger than 50 increased from 11 per 100,000 in 1994 to 21.9 per 100,000 in 2010. This rise appears to be in concordance with other studies using other national databases. Using the SEER database, Davis et al. showed that the most significant increase in incidence of CRC was in the age group 40-44, with an increase from 10.7 per 100,000 in 1988 to 17.9 per 100,000 in 2006 (5). Similarly, a study using the National Cancer Database reported that early-onset CRC incidence rates increased from year 1998 to 2007 (annual percent change (APC): 2.1%; 95% CI: 1.1% - 3.1%), whereas late-onset incidence decreased (APC: −2.5%; 95% CI: 3.0% - 2.0%) (8, 11). We have also observed that early-onset CRC tumors are more likely to be diagnosed at more advanced stages what is in agreement with one large-scale study reporting that patients with early-onset CRC are significantly more likely to present with stage III/IV disease compared to CRC patients with late-onset disease. For colon cancer, 63.4% of early-onset patients and 49.0% of late-onset patients were diagnosed with more advanced (stage III/IV) disease (p<0.01). 57.3% of early-onset and 46.2% of late-onset rectal tumors were diagnosed at later stages (p<0.01) (11, 12). We believe that later stage at the time of diagnosis is possibly related to the lower screening rates and/or failure to recognize and evaluate colonic symptoms in younger individuals, which is concerning because it likely leads to worse outcomes in this patient population. Similarly, a SEER database study confirmed that young (age group 20-40) patients with colon cancer have a poorer overall survival compared with their 60-80 year old counterparts (11, 12). The underlying etiology of increase in CRC among younger patients remains an area of active research. The increase in awareness likely contributes to the increased incidence, as patients with asymptomatic rectal bleeding are more likely to receive a colonoscopy today than they were in the past; however, this is unlikely to be the only explanation contributing to the large increase in incidence that was seen in our study. We believe that early-onset CRC is multifactorial, as genetic mutations along with changes in lifestyle such as more sedentary lifestyle, increased obesity, and increased rates of diabetes are likely contributory (13-15). Our study revealed a 76% increase in incidence of recto-sigmoid tumors, and because most of the early-onset CRC occur in the rectum, recto-sigmoid colon, and distal colon, sigmoidoscopy may be particularly useful in establishing or ruling out early-onset CRC. However, based on multiple studies, 30% of lesions in patients younger than 50 years of age can occur proximal to the splenic flexure and would be missed by sigmoidoscopy (7). Based on the increasing trends of early-onset disease, late stage presentation, and high incidence of CRC tumors in patients age 45, we recommend a reconsideration of current CRC screening colonoscopy guidelines; from age 50 to age 45. Cost-effectiveness data is limited, but Bleyer et al. have reported colonoscopy to be cost-effective in evaluating individuals between 25 and 45 years of age with rectal bleeding and no other symptoms (8).
Results of this study should be interpreted in the context of its limitations. This study could not establish the mechanisms underlying observed socio-demographic disparities, nor did it explore the molecular basis of early-onset CRC (familial adenomatous polyposis was excluded). In addition, prognosis of early-onset CRC as well as the relationship based on racial differences is not discussed in this manuscript, although several previous studies have highlighted a significance of racial disparities in CRC incidence. Not with standing, this data call for increased investigation, awareness, and action against early-onset CRC. At a minimum, symptomatic young patients should undergo timely sigmoidoscopy, if not a full colonoscopy. Identifying high risk cohorts for targeted screening should be a priority.
CONCLUSION
Overall, there is a decreasing incidence of colorectal cancer in Arizona. However, an alarming increase in incidence of early-onset CRC was observed mirroring the national trend. In addition, a shift in mean age of diagnosis from 70.8 to 68.1 was seen. Rigorous screening and early detection have led to the overall decrease in the incidence of CRC, however many studies, including this one, have shown an alarming increase in the incidence of early-onset CRC. These trends are likely multifactorial and further studies are necessary to better elucidate the underlying social, environmental, and molecular mechanisms.
Footnotes
Author Contribution
Conception and design: HA, VNN
Analysis and interpretation: ACG, JJ, VNN
Data collection: HA, RD, EO
Writing the article: HA, VNN, JJ, PV, RMD, EO
Critical revision of the article: VNN, JJ, PV
REFERENCES
- 1.Myers EA, Feingold DL, Forde KA, Arnell T, Jang JH, Whelan RL. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions' experience. World J Gastroenterol. 2013;19(34):5651–7. doi: 10.3748/wjg.v19.i34.5651. doi: 10.3748/wjg.v19.i34.5651. PubMed PMID: 24039357; PMCID: 3769901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. doi: 10.3322/caac.20138. PubMed PMID: 22237781. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. doi: 10.3322/caac.21254. PubMed PMID: 25559415. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):104–17. doi: 10.3322/caac.21220. doi: 10.3322/caac.21220. PubMed PMID: 24639052. [DOI] [PubMed] [Google Scholar]
- 5.Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJL, Nfonsam VN. Is It Time to Lower the Recommended Screening Age for Colorectal Cancer? J Am Coll Surgeons. 2011;213(3):352–61. doi: 10.1016/j.jamcollsurg.2011.04.033. doi: DOI 10.1016/j.jamcollsurg.2011.04.033. PubMed PMID: WOS:000294670600003. [DOI] [PubMed] [Google Scholar]
- 6.Ahnen DJ, Wade SW, Jones WF, Sifri R, Silveiras JM, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin Proc. 2014;89(2):216–24. doi: 10.1016/j.mayocp.2013.09.006. doi: DOI 10.1016/j.mayocp.2013.09.006. PubMed PMID: WOS:000330581500016. [DOI] [PubMed] [Google Scholar]
- 7.You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch Intern Med. 2012;172(3):287–9. doi: 10.1001/archinternmed.2011.602. doi: DOI 10.1001/archinternmed.2011.602. PubMed PMID: WOS:000300223800027. [DOI] [PubMed] [Google Scholar]
- 8.Bleyer A. CAUTION! Consider cancer: common symptoms and signs for early detection of cancer in young adults. Semin Oncol. 2009;36(3):207–12. doi: 10.1053/j.seminoncol.2009.03.004. doi: 10.1053/j.seminoncol.2009.03.004. PubMed PMID: 19460578. [DOI] [PubMed] [Google Scholar]
- 9.Filik L. Is It Worthwhile to Check Colorectal Cancer in All Young Adults? Arch Intern Med. 2012;172(12):971. doi: 10.1001/archinternmed.2012.1555. PubMed PMID: WOS:000305594200027. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease C. Prevention. Cancer screening - United States, 2010. MMWR Morbidity and mortality weekly report. 2012;61(3):41–5. PubMed PMID: 22278157. [PubMed] [Google Scholar]
- 11.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. doi: 10.1002/cncr.24760. PubMed PMID: 19998273; PMCID: 3619726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe HL, Hinds RA, editors. NAACCR Annotated Bibliography of Research and Publications: Multi-registry Cancer Incidence and Mortality Data in the United States and Canada. North American Association of Central Cancer Registries; Springfield (IL): Dec, 2005. [Google Scholar]
- 13.Gingras D, Beliveau R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011;4(2):133–9. doi: 10.1007/s12307-010-0060-5. doi: 10.1007/s12307-010-0060-5. PubMed PMID: 21909875; PMCID: 3170421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–22. doi: 10.1007/s10552-013-0201-5. doi: 10.1007/s10552-013-0201-5. PubMed PMID: 23563998; PMCID: 4161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muc-Wierzgon M, Nowakowska-Zajdel E, Dziegielewska-Gesiak S, Kokot T, Klakla K, Fatyga E, Grochowska-Niedworok E, Waniczek D, Wierzgon J. Specific metabolic biomarkers as risk and prognostic factors in colorectal cancer. World J Gastroentero. 2014;20(29):9759–74. doi: 10.3748/wjg.v20.i29.9759. doi: DOI 10.3748/wjg.v20.i29.9759. PubMed PMID: WOS:000340730300010. [DOI] [PMC free article] [PubMed] [Google Scholar]