Abstract
Background
Cognitive impairment in persons with heart failure is common. Theories of cognitive reserve suggest that premorbid factors, such as intellectual ability, may provide a buffer against cognitive impairment due to neuropathological insult. No study has examined the influence of cognitive reserve on cognitive functioning in older adults with heart failure.
Aim
This study examined whether cognitive reserve moderates the relationship between heart failure severity and cognitive function.
Methods
A total of 157 persons with heart failure (69.26 ± 9.26 years; 39% female) completed neuropsychological testing and a brief fitness assessment. Cognitive reserve was operationalized using estimated premorbid intellect on American National Adult Reading Test (AMNART).
Results
A moderation analysis was performed using a hierarchical regression models for each cognitive domain. An interaction term between the AMNART and 2-minute step test was created and entered into the final block of the model, with demographic, psychosocial, and heart failure severity entered in the previous blocks. The interaction term was significant for attention (t(155) = −2.54, p = .012), executive function (t(155) = −3.30, p = .001), and language (t(155) = −2.83, p = .005) domains.
Conclusion
The current findings suggest that cognitive reserve moderates the association between heart failure severity and cognitive function in multiple cognitive domains. Further work is needed to clarify the mechanisms by which cognitive reserve attenuates cognitive impairment in this population.
Keywords: heart failure, cognitive reserve, cognitive function, cardiovascular disease, neuropsychology
1. Introduction
Recent research has demonstrated the adverse impact of cardiovascular disease on cognitive function, and vascular disease is now recognized as the second largest risk factor for dementia (Bowler, 2005). Among the cardiovascular diseases, heart failure already affects more than 5.7 million Americans in the United States alone (Lloyed-Jones et al., 2009) and it has become the most common reason for rehospitalization, accounting for an estimated $17.4 billion in Medicare costs per year (Jencks, Williams, & Coleman, 2009). Cardiovascular disease is the leading cause of morbidity, and mortality in the United States, and will likely remain so, as 78% of the United States population currently exhibits one or more cardiovascular disease risk factor (Kahn, Robertson, Smith, & Eddy, 2008; Lloyd-Jones et al., 2009; National Heart, Lung, and Blood Institute, 2007). Furthermore, a future trend of increased burden of cardiovascular disease seems inevitable due to recent increases in obesity, hypertension, and type 2 diabetes mellitus (Lloyd-Jones et al., 2009),
A likely contributor to elevated rates of hospital readmissions in persons with heart failure is cognitive impairment. Cognitive dysfunction secondary to vascular disease, often termed vascular cognitive impairment (VCI) (Hachinski et al., 2006), impacts both quality of life and functional independence (Leblanc, Meschia, Stuss, & Hachinski, 2006; Cohen et al., 1999; Rockwood et al., 2000; Bennett, Cordes, Westmoreland, Castro, & Donnelly, 2000). Past research examining adults with heart failure demonstrates that cognitive impairment negatively impacts instrumental activities of daily living, including poor medication compliance (Zuccala et al., 2001; Sloan & Pressler, 2009), and increases risk of mortality (Putzke, Williams, Daniel, Bourge, & Boll, 2000; Zuccala et al., 2003). This is perhaps not surprising, as up to 75% of persons with heart failure exhibit cognitive dysfunction on testing (Vogels, Scheltens, Schroeder-Tanka, & Weinstein, 2007).
Models of cognitive reserve suggest that premorbid factors, such as greater intellectual ability, may provide a buffer against cognitive impairment and thus contribute to the observed cognitive variability in individuals with the same neuropathological insult (Stern, 2009). Cognitive reserve is known to moderate cognitive impairment in a wide range of conditions, including Alzheimer’s disease (Stern, 2009), traumatic brain injury (Kesler, Adams, Blasey, & Bigler, 2003), HIV (Farinpour et al., 2003), and neuropsychiatric disorders (Barnett, Salmond, Jone, & Sahakian, 2006). Similarly, cognitive reserve also moderates cognitive impairment seen in populations with significant white matter involvement, including multiple sclerosis, and stroke (Brickman et al., 2009; Elkins et al., 2006; Sumowski, Chiaravalloti, & DeLuca, 2009). These findings are noteworthy, as white matter ischemia is common in persons with HF, and likely contributes to the cognitive dysfunction seen in this population (Almeida et al., 2005; Paul et al., 2005). Regardless of the medical population, the above findings are consistent with the notion that cognitive reserve may moderate cognitive function in almost any disorder with neurocognitive impairment (Stern, 2009).
The current study examined whether cognitive reserve, operationalized by an estimate of permorbid intelligence, moderates the relationship between heart failure severity and cognitive function. Based on findings in other populations, we expected that greater cognitive reserve would attenuate the cognitive deficits typically found in patients with heart failure.
2. Methods
2.1 Participants
The current sample included 157 consecutive persons with heart failure selected from a database of a large-scale NIH-funded research study examining cognitive function in persons with heart failure. Participants were recruited from Summa Health System in Akron, Ohio and reflect the heart failure population receiving treatment at that facility. For inclusion, participants were between the ages of 50-85 years of age, English-speaking, and had a previous diagnosis of New York Heart Association (NYHA) class II or III at the time of enrollment. Potential participants were excluded for history of significant neurological disorder (e.g. dementia, stroke), head injury with >10 minutes loss of consciousness, severe psychiatric disorder (e.g. schizophrenia, bipolar disorder), substance use, renal failure, and sleep apnea. Participants averaged 69.26 ± 9.26 years of age, were 38.9% female, 11.8% African-American, and 2.6% Native American. See Table 1 for participant demographic and medical information.
Table 1.
Demographic and Clinical Characteristics of 157 Older Adults with Heart Failure.
| Demographic Characteristics | |
| N | 157 |
| Age, mean (SD) | 69.26 (9.26) |
| Sex (% Women) | 38.8 |
| Race (% Caucasian) | 85.6 |
| Education, mean (SD) | 13.35 (2.77) |
| Medical Characteristics (%) | |
| CABG/Bypass Surgery | 34.4 |
| Diabetes | 35.0 |
| Hypertension | 72.0 |
| Elevated Total Cholesterol | 67.5 |
| MI | 55.4 |
Note. Means and Standard Deviations of Test Performance Scores are Raw Scores CABG = Coronary artery bypass graft; MI = Myocardial Infarction
2.2 Measures
2.2.1 Cognitive Reserve
The current study used an estimate of premorbid intelligence to measure cognitive reserve. Premorbid intelligence is well documented as a robust measure of cognitive reserve (Albert & Teresi, 1999; Alexander et al., 1997). To assess premorbid intelligence, the American National Adult Reading Test (AMNART) was administered to all participants. The AMNART asks individuals to read a list of irregularly pronounced words. The AMNART is a reliable estimate of intelligence in medical populations (Blair & Spreen, 1989; Friend & Grattan, 1998; Uttl, 2002).
2.2.2 HF Severity
The 2-minute step test (2MST) is an assessment of cardiovascular endurance and was used to serve as an estimate of heart failure severity (Jones & Rikli, 2002). The 2MST requires the patient to march in place for 2 minutes. The patient is asked to bring each knee up to a marked target set on the wall at the individual’s own midpoint between the kneecap and crest of the iliac. The number of times the right knee met the marked target was counted. Increased step count within the 2-minutes was reflective of greater cardiovascular fitness. The 2-minute step test has been suggested to be an alternative to the more cumbersome 6-minute walk test, which has been shown to be associated with functional work capacity (r = .64), maximal oxygen uptake (r = .70) (Nixon, Joswiak, & Fricker, 1996), and is a known predictor of poor prognosis in persons with HF (Bittner et al., 1993).
2.2.2 Neuropsychological Measures
All neuropsychological tests used in the current study demonstrate strong psychometric properties including reliability and discriminant and construct validity. The domains and neuropsychological tests administered are as follows:
Attention: Trail Making Test A (Spreen & Strauss, 1991), Digit Symbol Coding (Smith, 1983), and Letter Number Sequencing (LNS) (Wechsler, 1997), and the Adaptive Rate Continuous Performance Test Inter Stimulus Interval score (the average interval between stimulus items) (Cohen, 1993).
Executive function: Trail Making Test B (Dikmen, Heaton, Gran & Temkin, 1999), and the Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000)
Memory: The California Verbal Learning Test-II (CVLT-II) total learning, short delay free recall, long delay free recall, and total hits (Delis, Kramer, Kaplan & Ober, 2000).
Language: Boston Naming Test (BNT) (Hawkins et al., 1993), and Animal Fluency (Morris et al., 1989)
2.2.3 Depressive Symptoms
The BDI-II is a commonly used measure of depressive symptoms with excellent psychometric properties in persons with medical conditions (Amau, Meagher, Norris, & Bramson, 2001). Higher scores on the BDI-II reflect more severe depressive symptomatology.
2.3 Procedures
The local Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. Participants completed demographic, medical and psychosocial self-report measures. A brief neuropsychological examination was then administered to all heart failure participants to assess attention, executive function, memory, and language. Individuals then completed the 2MST under supervision.
2.4 Statistical Analyses
To facilitate clinical interpretation all raw scores of the neuropsychological measures assessing cognitive function were transformed to t-scores (a distribution with a mean of 50, and a standard deviation of 10) using existing normative data correcting for age. Gender was also corrected for on measures of memory functioning. An average composite score for the cognitive domains of attention, executive function, memory, and language were calculated by averaging the t-scores of the measures within each domain. Consistent with clinical interpretation, impairment in these domains for the current study was defined as a t-score of 1.5 standard deviations below the mean (t < 35).
Moderation analyses using a multiple linear hierarchical regression model was performed for each cognitive domain. All continuous predictor variables were transformed to z-scores, and gender was dummy coded as 1 for females, and 0 for males. For analyses examining attention, executive function, and language as the dependent variable, demographic and psychosocial predictors, which include participant gender, and depressive symptomatology (as assessed by the BDI-II) were entered into the first block of the model. Only depressive symptomatology was entered in the first block of the model that examined memory as the dependent variable. For all analyses, the 2MST was entered into the second block and AMNART in the third block. The cross product of the AMNART (moderator) and 2MST (independent variable) was generated and entered into the fourth block.
3. Results
Cognitive Impairment is Prevalent in an Older Adult HF population
Cognitive impairment in the domains of attention and executive function was prevalent in the sample, with many participants exhibiting t-scores <35 on composite scores for these domains. Specifically, 27.4% showed significant impairments in attention, and 21.7% of the sample showed impairments in executive function. Impairment in memory (10.2%) and language functioning (8.3%) were less common. See Table 2.
Table 2.
Descriptive statistics of cognitive tests (means ± standard deviations)
| ESTIMATED PREMORBID INTELLIGENCE | |
| AMNART | 111.19(10.85) |
| ATTENTION | |
| Digit-Symbol Substitution | 49.11(14.82) |
| TMTA | 42.79(17.94) |
| LNS | 8.75(2.58) |
| ARCPT ISI | 126.43(105.88) |
| EXECUTIVE FUNCTION | |
| FAB | 15.64(2.59) |
| TMTB | 114.09(56.58) |
| MEMORY | |
| CVLT Total | 39.15(11.09) |
| CVLT SDFR | 7.41(3.43) |
| CVLT LDFR | 7.91(3.46) |
| CVLT Hits | 13.41(2.41) |
| LANGUAGE | |
| Boston Naming Test | 53.25(6.39) |
| Animals | 19.17(5.06) |
| DEPRESSION | |
| Beck Depression Inventory-II | 8.22(7.83) |
| PHYSICAL FITNESS | |
| 2 Minute Step-test | 58.83(22.80) |
TMTA = Trail Making Test A; LNS = Letter Number Sequencing; ARCPT = Adaptive Rate Continuous Performance Test; FAB = Frontal Assessment Battery; TMTB = Trail Making Test B; CVLT = California Verbal Learning Test; SDFR = Short Delay Free Recall; LDFR = Long Delay Free Recall;
The 2MST is an Independent Predictor of Cognitive Function
We conducted a series of hierarchical regressions to identify predictors of cognitive function. In the model examining the main effects of gender and depression (as assessed by the BDI-II total score) were unrelated to cognitive function in attention, executive function, and language (all p > .05). Depression was also unrelated to memory functioning (p > .05). However, when the index of heart failure severity (2MST) was introduced into the models, it showed significant association with attention (ΔF(1,153) = 18.78, ΔR2 = .11, p < .001), executive function (ΔF (1, 153) = 19.32, ΔR2 = .11, p < .001), and language (ΔF(1,153) = 25.96, ΔR2 = .14, p < .001), after accounting for gender and depression. Better fitness (i.e. greater step count on the 2MST) was associated with better cognitive function in these domains. No such pattern emerged for memory (ΔF (1, 154) = 1.62, ΔR2 = .01, p = .221) after accounting for depression.
Premorbid intelligence is an independent predictor of performance in specific cognitive domains
The current sample exhibited a high estimated premorbid intelligence with a mean of 111.19 ± 10.85 on the AMNART. Hierarchical regression analyses revealed that after accounting for demographic variables and the 2MST performance, the AMNART showed significant association with attention (ΔF(1,152) = 25.43, ΔR2 = .13, p < .001), executive function (ΔF (1,152) = 35.84, ΔR2 = .17, p < .001), memory (ΔF(1,152) = 5.56, ΔR2 = .03, p = .020), and language (ΔF(1,152) = 90.27, ΔR2 = .31, p < .001). In each case, higher score on the AMNART was associated with better performance in a specific cognitive domain. See Table 3.
Table 3.
Association of the AMNART with Cognitive Functions in Older Adults with Heart Failure (N = 157): A summary of hierarchical regressions.
| Variable |
Attention
b(SE b) |
Executive Function
b(SE b) |
Memory
b(SE b) |
Language
b(SE b) |
|---|---|---|---|---|
| Gender | .34 (3.20) | −1.87 (2.84) | . _ | −.53 (1.38) |
| BDI-II | −.44 (1.51) | −.93 (1.34) | −.82 (.72) | −1.36 (.65)* |
| 2MST | 3.96 (1.66)* | 3.26 (1.47)* | .22 (.80) | 1.65 (.72)* |
| AMNART | 8.21 (1.63)** | 8.64 (1.44)** | 1.85 (.79)* | 6.70 (.71)** |
| R2 | .24** | .29** | .05* | .47** |
| F for ΔR2 | 25.43** | 35.84** | 5.56* | 90.27** |
Note.
denotes p < 0.05;
denotes p < .001
Abbreviations: b – unstandardized regression coefficients, SE – standard error, BDI-II = Beck Depression Inventory-II; 2MST = 2-minute step test; AMNART = American National Adult Reading Test
Premorbid Intelligence Moderates the Relationship between HF Severity and Cognitive Function
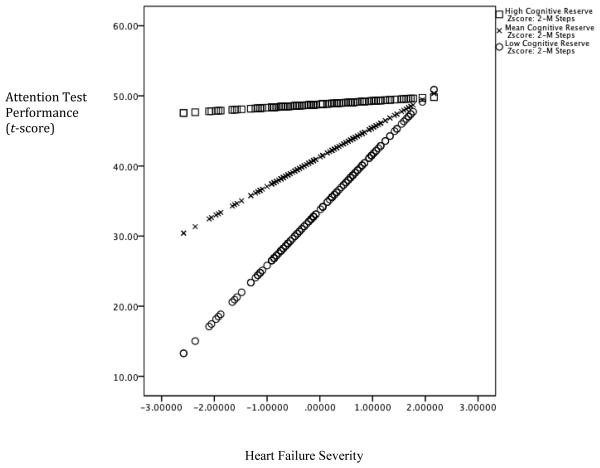
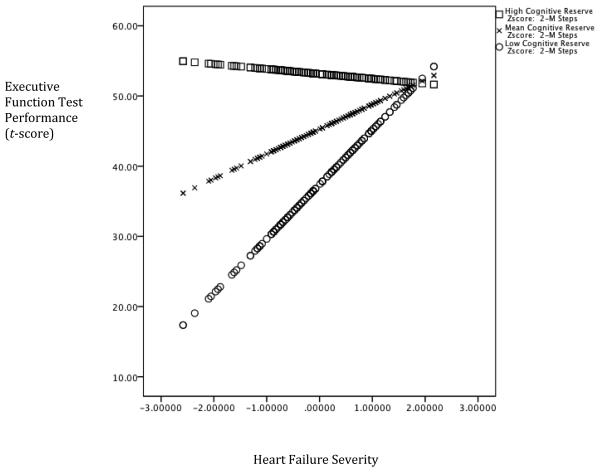
The interaction between the AMNART and the 2MST was significant, after controlling for the demographic variables, the 2MST, and the AMNART, in the domains of attention (ΔF(1,151) = 6.45, ΔR2 = .03, p = .012), executive function (ΔF(1,151) = 10.89, ΔR2 = .05, p = .001), and language (ΔF(1,151) = 7.98, ΔR2 = .03, p = .005), but not memory (ΔF(1,151) = .820, ΔR2 = .005, p = .367). Furthermore, the AMNART moderated the relationship between the 2MST, and cognitive function as the interaction term was a significant predictor in the model for attention (t(155) = −2.54, p = .012), executive function (t(155) = −3.30, p =.001), and language (t(155) = −2.83, p = .005). In each case, higher estimated premorbid intelligence attenuated the adverse impact of heart failure on cognitive function. See Figures 1-3.
Figure 1. Cognitive Reserve moderates the Association between Heart Failure Severity and Attention.
Note. Lower scores on the x-axis is reflective of worse heart failure severity and higher scores on the y-axis represents better test performance
Figure 3. Cognitive Reserve moderates the association between Heart Failure Severity and Language.
Note. Lower scores on the x-axis is reflective of worse heart failure severity and higher scores on the y-axis represents better test performance
4. Discussion
The main finding of this study is that although older adults with heart failure exhibited deficits in multiple cognitive domains, cognitive reserve moderated the negative impact of heart failure on cognitive function. The observed cognitive deficits are commonly reported in the extant studies of heart failure and are linked to elevated risk for Alzheimer’s disease (Qiu et al., 2006; Acanfora et al., 1996; Tilvis et al., 2004; Almeida et al., 2005). Such pattern of poor performance on tests of attention, executive function, and memory may reflect adverse impact of cerebral hypoperfusion and inadequate oxygenation, particularly on frontal and temporal regions (Vogels, et al., 2007; Woo, Macey, Fonarow, Hamilton, & Harper, 2003; Rains, 2002; Pressler et al., 2010). Moreover, in various patient populations, the described pattern of deficits is associated with significant personal burden, including reduced day-to-day functions and poorer quality of life (Cahn-Weiner, Boyle, & Malloy, 2002; Kalmar, Gaudino, Moore, Halper, & DeLuca, 2008; Pressler et al., 2010). Recent work shows that cognitive impairment is an independent predictor of instrumental activities of daily living and self-care behaviors in persons with heart failure (Alosco et al., in press; Pressler et al., 2010) and it is important to clarify the contribution of cognitive dysfunction to mortality and disability in this population (Zuccala et al., 2003; Zuccala et al., 2001). Past work has suggested that exercise may improve cognitive function in persons with heart failure (Stanek et al., 2011; Tanne et al., 2005; Stern, 2009), and future studies should explore a possibility that the extent of such benefits may depend on cognitive reserve.
The current findings indicate that cognitive reserve moderates the relationship between heart failure severity and performance on tests of attention, executive function, and language. Cognitive reserve moderates cognitive declines in Alzheimer’s disease (Stern, 2009), as patients with high cognitive reserve reveal more advanced brain pathology than their low-reserve counterparts, while displaying no differences in symptoms severity (Stern, Alexander, Prohovnik, & Mayeux, 1992; Stern, 2009). A growing literature demonstrates cognitive reserve provides benefits across various neurological populations. Work in Alzheimer disease patients has found cognitive reserve to attenuate the impact of pathology including β-amyloid (Roe et al., 2008), neuritic plaques, and diffuse plaques (Bennett et al., 2003) on cognitive function. Similar findings emerge in patients with multiple sclerosis, a disease known to predominately affect the white matter of the brain (Benedict et al., 2010; Sumowski, Chiaravalloti, & DeLuca, 2009; Sepulcre et al., 2009). Perhaps more directly related to the current sample, increased cognitive reserve mitigates the adverse effect of white matter hyperintensities on cognitive function in a sample of neurologically healthy older adults (Brickman et al., 2009). Future studies should determine the degree to which cognitive reserve moderates different mechanisms for cognitive impairment in persons with HF, including white matter hyperintensities.
The current study identifies cognitive reserve as an important contributor to cognitive function in older adults with HF. However, it may also be possible that psychosocial and medical interventions may provide cognitive benefits in this population. Physical activity (Stanek et al., 2011; Tanne et al., 2005; Stern, 2009), cognitive stimulation (Jedrziewski, Ewbank, Wang & Trojanowski, 2010), and environmental enrichment (Brown et al., 2003), have been proposed as interventions that could alleviate the effects of neuropathology on cognitive performance and daily functions in a variety of patient samples. Whether cognitive deficits due to heart failure can be alleviated by the same techniques awaits empirical test.
Interestingly, in this study, cognitive reserve did not moderate the association between heart failure and memory performance, despite its beneficial influence on other cognitive domains. A likely explanation for this finding is the low rates of impairments in the memory domain among the current sample. Despite these low rates, assessment of memory functioning in this population remains important, as HF patients have been shown to have poorer memory when compared to a healthy population (Pressler et al., 2010). Future studies should examine cognitive function in HF persons longitudinally, as HF patients are at risk for developing Alzheimer’s disease over time (Qiu et al., 2006). Another potential explanation for this finding may involve pathology threshold effects. Past work in persons with AD shows that patients with higher education levels receive the diagnosis later but decline more rapidly than persons with lower educational attainment (Stern et al., 1995; Stern, Albert, Tang & Tsai, 1999). Thus, it is possible that in heart failure, the high level of cognitive reserve is protective until much later in the disease process, at which time cognitive impairment rapidly declined due to the high level of pathology, as proposed in the case of AD (Stern et al., 1995; Stern et al., 1999).
Limitations of the current findings warrant brief review. No gold standard for assessing cognitive reserve exists in the literature and future studies might benefit from comparing different operational definitions of that construct. Specifically, studies should examine the potential influence of occupational attainment, as previous work has shown it to be a contributor to cognitive reserve in patients with Alzheimer’s disease (Stern et al., 1994). Prospective studies examining the influence of cognitive reserve on cognitive decline in heart failure are also much needed, particularly those employing neuroimaging. For example, brain reserve, which includes neural compensatory processes, could be an alternate explanation of our findings, as it is known to mitigate impairment in persons with Alzheimer’s disease (Serra et al., 2011). Prospective studies should examine the possible contribution of neural reserve and neural compensation, as research suggests that higher cognitive reserve may be associated with increased neural networks and pathways (Stern, 2009).
In summary, the current findings indicate that cognitive reserve moderates cognitive function in older adults with heart failure. Future studies are needed to clarify the physiological mechanisms for this phenomenon and potential clinical benefits of assessing premorbid ability levels.
Figure 2. Cognitive Reserve moderates the association between Heart Failure Severity and Executive Function.
Note. Lower scores on the x-axis is reflective of worse heart failure severity and higher scores on the y-axis represents better test performance
References
- 1.Acanfora D, Trojano L, Iannuzzi GI, Furgi G, Picone C, Rengo C, et al. The brain in congestive heart failure. Archives of Gerontology and Geriatrics. 1996;23:247–256. doi: 10.1016/s0167-4943(96)00733-9. [DOI] [PubMed] [Google Scholar]
- 2.Albert SM, Teresi JA. Reading ability, education, and cognitive status assessment among older adults in Harlem, New York City. American Journal of Public Health. 1999;89:95–97. doi: 10.2105/ajph.89.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander GE, Furey ML, Grady CL, Pietrini P, Mentis MJ, Schapiro MB. Association of premorbid function with cerebral metabolism in Alzheimer's disease: Implications for the reserve hypothesis. American Journal of Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- 4.Almeida JR, Alves TC, Wajngarten M, Rays J, Castro CC, Cordeiro Q, et al. Late-life depression, heart failure and frontal white matter hyperintensity: A structural magnetic resonance imaging study. Brazilian Journal of Medical and Biological Research. 2005;38:431–436. doi: 10.1590/s0100-879x2005000300014. [DOI] [PubMed] [Google Scholar]
- 5.Alosco ML, Spitznagel MB, Cohen R, Lawrence S, Colbert LH, Josephson R, Waechter D, et al. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. Journal of Cardiovascular Nursing. doi: 10.1097/JCN.0b013e318216a6cd. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnau R, Meagher M, Norris M, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychology. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 7.Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychological Medicine. 2006;36:1053–1064. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- 8.Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, Schretlen DJ. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. Journal of the International Neuropsychological Society. 2010;36:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- 9.Bennett SJ, Cordes DK, Westmoreland G, Castro R, Donnelly E. Self-care strategies for symptom management in patients with chronic heart failure. Nursing Research. 2000;36:139–145. doi: 10.1097/00006199-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;36:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 11.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. The Journal of the American Medical Association. 1993;36:1702–1707. [PubMed] [Google Scholar]
- 12.Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- 13.Bowler JV, Vascular Cognitive Impairment Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(suppl V):v35–v44. doi: 10.1136/jnnp.2005.082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J, Cooper-Kuh CM, Kemperman G, van Praag H, Winkler J, Gage FH. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;36:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 16.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Applied Neuropsychology. 2002;36:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 17.Cohen R. The Neuropsychology of Attention. Springer; New York: 1993. [Google Scholar]
- 18.Cohen RA, Moser DJ, Clark MM, Aloia MS, Cargil BR, Stefanik S, et al. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. American Journal of Cardiology. 1999;36:1374–1378. doi: 10.1016/s0002-9149(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 19.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition: Adult Version. Manual. Psychological Corporation. 2000 [Google Scholar]
- 20.Dikmen S, Heaton R, Grant I, Temkin N. Test-retest reliability of the Expanded Halstead Reitan Neuropsychological Test Battery. Journal of the International Neuropsychological Society. 1999;36:346–356. [PubMed] [Google Scholar]
- 21.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 22.Elkins JS, Longstreth WT, Jr., Manolio TA, Newman AB, Bhadelia RA, Johnston SC. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology. 2006;36:435–440. doi: 10.1212/01.wnl.0000228246.89109.98. [DOI] [PubMed] [Google Scholar]
- 23.Farinpour R, Miller EN, Satz P, Selnes OA, Cohen BA, Becker JT, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS) Journal of Clinical and Experimental Neuropsychology. 2003;25:654–670. doi: 10.1076/jcen.25.5.654.14577. [DOI] [PubMed] [Google Scholar]
- 24.Friend L, Grattan K. Use of the North American Adult Reading Test to estimate premorbid intellectual functioning in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 1998;6:846–851. doi: 10.1076/jcen.20.6.846.1110. [DOI] [PubMed] [Google Scholar]
- 25.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;36:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins KA, Sledge WH, Orlean JE, Quinlan DM, Rakfeldt J, Huffman RE. Normative implications of the relationship between reading vocabulary and Boston Naming Test performance. Archives of Clinical Neuropsychology. 1993;36:525–537. [PubMed] [Google Scholar]
- 27.Jedrziewski MK, Ewbank DC, Wang H, Trojanowski JQ. Exercise and cognition: Results from the National Long Term Care Survey. Azheimer’s & Dementia. 2010;36:448–455. doi: 10.1016/j.jalz.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in Medicare fee-for-service program. New England Journal of Medicine. 2009;36:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 29.Jones CJ, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging. March April. 2002:24–30. [Google Scholar]
- 30.Kahn R, Robertson RM, Smith R, Eddy D. The Impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;188:576–585. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- 31.Kalmar JH, Gaudino EA, Moore NB, Halper J, DeLuca J. The relationship between cognitive deficits and everyday functional activities in multiple sclerosis. Neuropsychology. 2008;22:442–449. doi: 10.1037/0894-4105.22.4.442. [DOI] [PubMed] [Google Scholar]
- 32.Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: An investigation of the cognitive reserve hypothesis. Applied Neuropsychology. 2003;36:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- 33.Leblanc GG, Meschia JF, Stuss DT, Hachinski V. Genetics of vascular cognitive impairment: The opportunity and the challenges. Stroke. 2006;36:248–255. doi: 10.1161/01.STR.0000195177.61184.49. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 35.Morris J, Heyman A, Mohs R, Hughes JP, van Belle G, Fillenbaum G, et al. Part I. Clinical and neuropsychological assessment of Alzheimers disease. Neurology. 1989;36:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 36.National Heart, Lung, and Blood Institute . Morbidity & Mortality: 2007 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: 2007. [Google Scholar]
- 37.Nixon PA, Joswiak ML, Fricker FJ. A six-minute walk test for assessing exercise tolerance in severly ill children. The Journal of Pediatrics. 1996;36:362–366. doi: 10.1016/s0022-3476(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 38.Paul RH, Gunstad J, Poppas A, Tate DF, Foreman D, Brickman AM, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovascular Diseases. 2005;36:129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits in chronic heart failure. Nursing Research. 2010;36:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putzke JD, Williams MA, Daniel FJ, Bourge RC, Boll TJ. Activities of daily living among heart transplant candidates: Neuropsychological and cardiac function predictors. The Journal of Heart and Lung Transplantation. 2000;19:995–1006. doi: 10.1016/s1053-2498(00)00183-2. [DOI] [PubMed] [Google Scholar]
- 41.Qiu C, Winblad B, Marengoni A, Klarin I, Fastborn J, Fratiglioni L. Archives of Internal Medicine. 2006;36:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 42.Rains GD. Principles of human neuropsychology. McGraw-Hill; Boston: 2002. [Google Scholar]
- 43.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology. 2000;36:447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- 44.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve. Archives of Neurology. 2008;36:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sepulcre J, Goni J, Masdeu JC, Bejarano B, Velez de Medizabal N, Toledo JB, et al. Contribution of white matter lesions to gray matter atrophy in multiple sclerosis. Archives of Neurology. 2009;36:173–179. doi: 10.1001/archneurol.2008.562. [DOI] [PubMed] [Google Scholar]
- 46.Serra L, Cercignani Ma., Petrosini L, Basile B, Perri R, Fadda L, et al. Neuroanatomical correlates of cognitive reserve in Alzheimer Disease. Rejuvenation Research. 2011;14:1–9. doi: 10.1089/rej.2010.1103. [DOI] [PubMed] [Google Scholar]
- 47.Sloan RS, Pressler SJ. Cognitive deficits in heart failure: Re-cognition of vulnerability as a strange new world. Journal of Cardiovascular Nursing. 2009;36:241–248. doi: 10.1097/JCN.0b013e3181a00284. [DOI] [PubMed] [Google Scholar]
- 48.Smith A. Clinical psychological practice and principals of neuropsychological assessment. In: Walker C, editor. Handbook of clinical psychology: Theory, Research, and practice. Dorsey Press; Homewood, IL: 1983. [Google Scholar]
- 49.Spreen O, Strauss E. A compendium of Neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- 50.Stanek KM, Gunstad J, Spitznagel MB, Waechter D, Hughes JW, Luyster F, et al. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. International Journal of Neuroscience. 2011;36:86–93. doi: 10.3109/00207454.2010.531893. [DOI] [PubMed] [Google Scholar]
- 51.Stern Y. Cognitive Reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1957. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 53.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Annals of Neurology. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 54.Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, et al. Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;36:55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- 55.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. Journal of the American Medical Association. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 56.Sumowki JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 2009;36:913–926. doi: 10.1080/13803390902740643. [DOI] [PubMed] [Google Scholar]
- 57.Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. International Journal of Cardiology. 2005;36:145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 58.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala JH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology. Series A Biological Sciences, and Medical Sciences. 2004;36:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 59.Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- 60.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. European Journal of Heart Failure. 2007;36:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Third Edition. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 62.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. Journal of Applied Physiology. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 63.Zuccala G, Onder G, Pedone C, Cocchi A, Carosella L, Cattel C, et al. Cognitive dysfunction as a major determinant of disability in patients with heart failure: results from a multicentre survey. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;36:109–112. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. American Journal of Medicine. 2003;36:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]