Abstract
Oxidation of substrates to generate ATP in mitochondria is mediated by redox reactions of NADH and FADH2. Cardiac ischemia and reperfusion (IR) injury compromises mitochondrial oxidative phosphorylation. We hypothesize that IR alters the metabolic heterogeneity of mitochondrial redox state of the heart that is only evident in the 3-D optical cryoimaging of the perfused heart before, during, and after IR. The study involved four groups of hearts: time control (TC: heart perfusion without IR), global ischemia (Isch), global ischemia followed by reperfusion (IR) and TC with PCP (a mitochondrial uncoupler) perfusion. Mitochondrial NADH and FAD autofluorescence signals were recorded spectrofluorometrically online in guinea pig ex vivo-perfused hearts in the Langendorff mode. At the end of each specified protocol, hearts were rapidly removed and snap frozen in liquid N2 for later 3-D optical cryoimaging of the mitochondrial NADH, FAD, and NADH/FAD redox ratio (RR). The TC hearts revealed a heterogeneous spatial distribution of NADH, FAD, and RR. Ischemia and IR altered the spatial distribution and caused an overall increase and decrease in the RR by 55% and 64%, respectively. Uncoupling with PCP resulted in the lowest level of the RR (73% oxidation) compared with TC. The 3-D optical cryoimaging of the heart provides novel insights into the heterogeneous distribution of mitochondrial NADH, FAD, RR, and metabolism from the base to the apex during ischemia and IR. This 3-D information of the mitochondrial redox state in the normal and ischemic heart was not apparent in the dynamic spectrofluorometric data.
Keywords: Optical cryoimaging, mitochondria, ischemia and reperfusion, redox state, metabolism
Changes in the mitochondrial redox state are hallmarks of cardiac ischemia and reperfusion (IR) injury; hence, they are very relevant for our understanding of the metabolic alterations of oxygen deprivation, such as occurs during cardiac arrest. To determine how the global distribution of mitochondrial redox state changes during cardiac ischemia and IR, we characterized the spatiotemporal distribution of the redox state in the heart tissue using a novel 3D optical cryoimaging technique. This paper presents the visualization and quantification of the mitochondrial redox state during ischemia and IR injuries in ex vivo guinea pig perfused hearts. We also show quantitatively for the first time the regional and topographic distribution of mitochondrial redox state across the ventricles during and after ischemia, and validate the functional stability of the isolated perfused heart model.
I. Introduction
The heart is a complex organ with anatomical, metabolic, and physiological heterogeneity [1]–[5]. Local energy demand, and for that matter local metabolic state, show spatial variations in the heart, i.e. atria vs. ventricles [1]. The heart in the presence of O2 oxidizes the food-derived metabolites, the reducing equivalents nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), to produce the chemical energy ATP. The regulation of energy metabolism in the heart is also complex and it involves changes in mitochondrial redox state (i.e. NADH, FAD, NADH/FAD redox ratio (RR)). Therefore, the redox state is a key regulator of mitochondrial bioenergetics, and topographical variations of the redox state in the heart have been suggested [6], [7]. Furthermore, studies have shown that local hypoxic/ischemic conditions leads to pockets of patchy heterogeneous patterns of NADH autofluorescence in the heart [6].
Changes in mitochondrial redox state are hallmark of cardiac ischemia and reperfusion (IR) injury, and hence they are very relevant for our understanding of the metabolic alterations of O2 deprivation in a complex and heterogeneous organ like the heart. Indeed, NADH and FAD are sensitive indicators of tissue metabolic change in response to O2 availability. Ischemia and IR injuries are commonly encountered clinically in conditions such as heart transplantation, acute myocardial infarction, or crush injury to the chest [8]. Prolonged ischemia and subsequent reperfusion leads to irreversible mitochondrial dysfunction, cell death, and tissue damage [9].
Mitochondrial metabolic derangement and dysfunction are cardinal features in cardiac ischemia and IR injuries [10], [11]. The ventricles, being the main blood pumping chambers, are endowed with high density of mitochondria needed to provide the ATP necessary for the contractile force to effectively eject blood for the systemic and pulmonary circulation [12]–[14]. Cardiac tissue sensitivity to ischemia or IR injury is manifested in significant changes in mitochondrial redox state (i.e. NADH, FAD and NADH/FAD or RR) and altered ATP production. During cardiac ischemia, occlusion of blood flow leads to insufficient O2 delivery to mitochondria and reflow also leads to insufficient O2 delivery to mitochondria due to impaired O2 extraction [15]. Both conditions lead to differential effects on mitochondrial redox state and bioenergetics.
It is well known that NADH and FAD are two intrinsic flourophores in mitochondria that indicate mitochondrial redox state. The NADH/FAD RR provides information regarding the metabolic state as well as a quantitative marker of oxidative stress in metabolic active tissues like heart. We have reported on the dynamic changes in the mitochondrial redox state (NADH and FAD) and reactive oxygen species (ROS) production in the perfused ex vivo heart during ischemia and IR using spectrofluorometry. This approach involves collecting fluorescent signals from a narrow patch of tissue from the left ventricular free wall to represent the entire heart during the progression from ischemia to reperfusion. This approach is, however, limited in its scope, because the redox state distribution across the heart is complex and heterogeneous, and local metabolites accumulate in different degree in anoxic or ischemic condition [16]. Whether ischemia and IR alter the spatial distribution of the redox state across the heart is not well known.
To determine how the global distribution of mitochondrial redox state changes during cardiac ischemia and IR, we characterized the spatiotemporal distribution of the redox state in the heart tissue using a novel 3D optical cryoimaging technique. This approach enables us to visualize mitochondrial NADH and FAD autofluorescence (AF) signals in the ventricles in a spatiotemporal fashion. It also provides several advantages over the dynamic spectrofluorometry approach. It is able to construct 3D images of the redox state of the heart, which is important in elucidating the regional and global distribution of NADH and FAD AF intensities throughout the organ. It does this by the use of an optical cryoimaging, which at a low temperature (−40°C) elevates the quantum yield of desired fluorophores (NADH and FAD) to help us acquire a stronger signal when compared to room temperature [7], [17]. Higher signal acquisition leads to more precise measurement and a greater depth of the fluorescence signal. Lastly, rapid freezing of the tissue with liquid N2 provides a snapshot of its metabolism at a specific moment in space and time [18]–[22].
The objective of this study was therefore to provide a 3D visualization and quantification of mitochondrial redox state during ischemia and IR injuries in ex vivo guinea pig perfused hearts. We also showed quantitatively, the regional and topographic distribution of mitochondrial redox state across the ventricles, which, hitherto, have not been examined during and after ischemia.
II. Methods and Procedures
A. Langendorff Heart Preparations
All experiments conformed to the Guide for the Care and Use of Laboratory Animals, and were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Twenty adult male albino English shorthaired guinea pigs were sacrificed, and their hearts were extracted for ex vivo Langendorff perfusion studies as described previously [23]–[27]. The animal was given intra-peritoneal injection of ketamine for sedation along with heparin to prevent clotting. Following complete sedation, the animal was decapitated, the chest opened, and the heart was quickly cannulated and perfused with cold physiological saline, before it was removed from the chest cavity. The heart was transferred to a Langendorff apparatus and perfused at 55 mmHg via the aortic root with an online filtered Krebs Ringer solution (KR: in mM 138 Na+, 4.5 K+, 1.2 Mg2+, 2.5 Ca2+, 134 ClK−, 15 HCO3−, 1.2 H2PO4−, 11.5 glucose, 2 pyruvate, 16 mannitol, 0.05 EDTA, and 5 U/L insulin) gassed with 3% CO2 and 97% O2 at 37°C (pH 7.4). The heart was instrumented with a saline-filled balloon placed in the left ventricle (LV) via an opening in the left atrium, and attached to a pressure transducer to measure left ventricular pressure (LVP) isovolumetrically. The LV balloon volume was initially adjusted to a diastolic LVP of 0 mmHg, so that any subsequent increase in the diastolic LVP reflects left ventricular contracture and cardiac dysfunction. Characteristic data from LVP were systolic (sys/max), diastolic (dia/min), developed LVP (max-min LVP), and the maximal and minimal first derivatives of LVP (dLVP/dt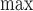 and dLVP/dt
and dLVP/dt , as indices of contractility and relaxation, respectively. Coronary flow (CF) was measured by an ultrasonic flowmeter (Transonic T106X; Ithaca, NY) placed directly in the aortic inflow line as described in our previous studies [23]–[28]. Coronary inflow and outflow of Na+, K+, Ca2+, PO2, pH, and PCO2 were monitored off-line with an intermittently self-calibrating analyzer system (Radiometer Copenhagen ABL 505; Copenhagen, Denmark). Following instrumentation, the heart was left to stabilize as determined by stable LVP and normal sinus rhythm. After stabilization, the experiment was initiated and physiological variables were recorded online in a time-dependent manner, as determined by the protocols.
, as indices of contractility and relaxation, respectively. Coronary flow (CF) was measured by an ultrasonic flowmeter (Transonic T106X; Ithaca, NY) placed directly in the aortic inflow line as described in our previous studies [23]–[28]. Coronary inflow and outflow of Na+, K+, Ca2+, PO2, pH, and PCO2 were monitored off-line with an intermittently self-calibrating analyzer system (Radiometer Copenhagen ABL 505; Copenhagen, Denmark). Following instrumentation, the heart was left to stabilize as determined by stable LVP and normal sinus rhythm. After stabilization, the experiment was initiated and physiological variables were recorded online in a time-dependent manner, as determined by the protocols.
B. Experimental Groups and Protocols
The study consisted of four groups of guinea pig hearts (n = 5/group): Time Control (TC), TC + pentachlorophenol (PCP), ischemia (Isch) only, and ischemia and reperfusion (IR). In the TC group, hearts were perfused with KR for the duration of the ischemia or IR protocols without ischemia (i.e. no obstruction of flow). This approach validates the functional stability of the isolated perfused heart model. In another TC group, PCP, a mitochondrial uncoupler, was added to the KR perfusate to record changes in cardiac function and mitochondrial redox state. In the ischemia (Isch) and IR groups, hearts were subjected to either 30 min no flow global ischemia only or 30 min ischemia and 60 min reperfusion, respectively. At the end of each experiment, the heart was snap frozen in liquid N2 to preserve its metabolic and structural integrity, as described in our recent studies [17]. Maintaining the heart structural integrity using this approach can be challenging. To minimize cracks and structural deformations caused by the liquid N2, the heart is first carefully immersed in very cold liquid isopentane before snap freezing in liquid N2. All hearts were stored in −80°C freezer for later optical cryoimaging (see sections 2.4 and 2.5).
C. Spectrofluorometric Measurements of Mitochondrial NADH and FAD in the Intact Beating Hearts
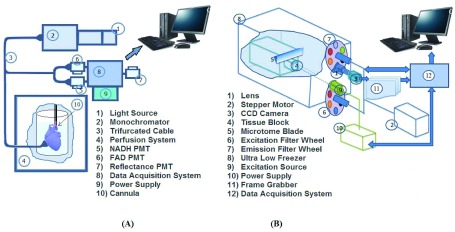
Cardiac mitochondrial NADH and FAD autofluorescence (AF) were measured online in the intact beating heart using fluorescence technique with a Photon Technology International (PTI) instrument (London, Canada) as described before [9], [28]–[30]. All experiments were conducted in a light-proofed Faraday cage to block all incident lights and minimize photo bleaching of fluorescent signals. The signals were acquired by placing a trifurcated fiber optic probe gently against the beating perfused heart. The distal end of the fiber optic probe was positioned against the LV free wall, and the two proximal ends of the probe connected to the PTI spectrofluorometer (Fig. 1A). The placement of the probe against the heart did not impede normal cardiac function, e.g. LVP.
FIGURE 1.
Schematic illustration of the PTI system (A) and the cryoimager system (B) employed in this study. Different components of each system are appropriately labeled and self-descriptive.
Cardiac mitochondrial NADH AF was assessed at 350 nm excitation with a xenon arc lamp at 75 W, filtered through a 350-nm monochrometer, and emission ratio of 450 nm/390 nm; FAD AF was assessed at 480 nm excitation and 540 nm emission [9], [28]–[31]. The arc lamp shutter was opened only for 2.5 s recording intervals to prevent photobleaching. Intensities were measured by photomultipliers (Photomultiplier Detection System 814, PTI) [31]. Each signal was digitized and recorded at 200 Hz on computers for later signal analyses [9], [23], [29]. The signal intensities were quantified in arbitrary fluorescence unit (a.f.u.).
D. 3D Optical Cryoimaging of Mitochondrial NADH and FAD in the Frozen Hearts
The cryopreserved hearts from the experiments were imaged at cryogenic temperatures by a custom-designed optical imaging system, a cryoimager, in the Biophotonics Laboratory at the University of Wisconsin-Milwaukee, WI. As previously described [17], the cryoimager (Fig. 1B) is an automated image acquisition instrument, which sequentially slices the tissue and is able to acquire images of up to five fluorophores [17], [18]. A mercury arc lamp (200 W, Oriel) is used as a light source, and desired wavelengths (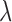 ) are selected by filtering the broadband light to excite a specific fluorophore from the surface of the frozen tissue block. The excitation filter for NADH was set at 350 nm (80-nm bandwidth, UV Pass Blacklite, HD Dichroic, Los Angeles, CA), and the corresponding FAD filter was set at 437 nm (20-nm bandwidth, 440QV21, Omega Optical, Brattleboro, VT), with respective emission filters at 460 nm (50-nm bandwidth, D460/50M, Chroma, Bellows Falls, VT) and 537 nm (50-nm bandwidth, QMAX EM 510-560, Omega Optical), for NADH and FAD, respectively. Filters for
) are selected by filtering the broadband light to excite a specific fluorophore from the surface of the frozen tissue block. The excitation filter for NADH was set at 350 nm (80-nm bandwidth, UV Pass Blacklite, HD Dichroic, Los Angeles, CA), and the corresponding FAD filter was set at 437 nm (20-nm bandwidth, 440QV21, Omega Optical, Brattleboro, VT), with respective emission filters at 460 nm (50-nm bandwidth, D460/50M, Chroma, Bellows Falls, VT) and 537 nm (50-nm bandwidth, QMAX EM 510-560, Omega Optical), for NADH and FAD, respectively. Filters for 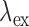 and
and 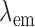 are controlled using two motorized filter wheels (Oriental Motor Vexta Step Motor PK268-01B), and images are captured using a charge-coupled device camera (QImaging, Rolera EM-C2, 14 bit) with 1,
are controlled using two motorized filter wheels (Oriental Motor Vexta Step Motor PK268-01B), and images are captured using a charge-coupled device camera (QImaging, Rolera EM-C2, 14 bit) with 1, ,002 pixel arrays (Fig. 1B). Prior to the imaging process, the frozen hearts were embedded in a black mounting medium that is fluorescence free. For this study, the NADH and FAD autofluorescence filtered images of each slice were acquired and saved for further processing. The resolution in x and y was
,002 pixel arrays (Fig. 1B). Prior to the imaging process, the frozen hearts were embedded in a black mounting medium that is fluorescence free. For this study, the NADH and FAD autofluorescence filtered images of each slice were acquired and saved for further processing. The resolution in x and y was 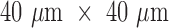 and the z resolution was
and the z resolution was 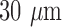 . The details of freezing, embedding and imaging has been described in a previous report [17], [18].
. The details of freezing, embedding and imaging has been described in a previous report [17], [18].
E. 3D Optical Cryoimage Processing
NADH and FAD autofluorescence images obtained from approximately 800 slices/heart from the four groups were analyzed using MATLAB (The MathWorks Inc., Natick, MA). As described previously [17], [18], calibration and preprocessing were done in order to eliminate day-to-day variations in light intensity, dark current noise, mirror angle, and non-uniformity of the illumination pattern. 3D representations of the hearts were rendered using all of the image slices for both NADH and FAD channels. The ratio of NADH and FAD signals, the redox ratio (RR), was calculated voxel by voxel, using MATLAB, according to equation (1) [32], [33]:
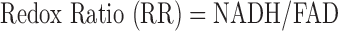 |
Subsequently, a histogram of the RR for each heart, which is a distribution of intensities throughout the volume, i.e. base to apex of the heart, was plotted and the corresponding mean value of each histogram was calculated according to equation (2):
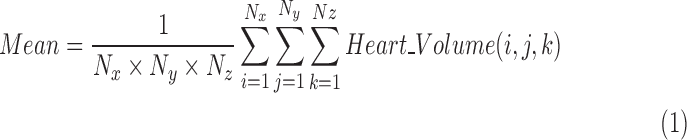 |
where 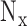 ,
, 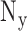 and
and 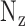 are the number of voxels in x, y and z directions, respectively.
are the number of voxels in x, y and z directions, respectively.
F. Statistical Evaluation of Data
Data are reported as mean ± SEM. NADH and FAD signals expressed in arbitrary fluorescence units (a.f.u.) are presented in a continuous timeline manner or as discrete data points at chosen time intervals, as summarized in the bar plots. Cardiac functional data are reported in Table 1, representing specific time intervals before (0 min), during (15 and 30 min) and after ischemia (5 and 60 min), which correspond with 0, 16, 36, 41, and 96 min of the timeline experimental protocol. Statistical differences were compared within and between groups at p < 0.05. Among groups (TC vs. Isch or IR) and within groups were compared using one-way ANOVA followed by Tukey’s post-hoc analysis to determine significant differences.
TABLE 1. Changes in Left Ventricular Pressure (LVP) and Coronary Flow (CF) at Specific Time Points of the Ischemia and Reperfusion (IR) Experimental Protocol: Baseline, 15 Min Ischemia (Isch15), 30 Min Ischemia (Isch30), 5 Min Reperfusion (IR5), 60 Min Reperfusion (IR60). Values are Mean ± SEM for Time Control (TC; 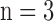 ) and IR Hearts (30 min and 60 min;
) and IR Hearts (30 min and 60 min; 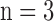 ).
).
| Baseline | Isch15 | lsch30 | IR5 | IR60 | ||
|---|---|---|---|---|---|---|
| CF (ml/min) | TC | 15.30 ± 0.80 | 15.54 ± 0.55 | 15.81 ± 0.76 | 15.54 ± 0.55 | 15.81 ± 0.76 |
| 30/60 | 14.37 ± 0.43 | 0.00 ± 0.00* | 0.00 ± 0.00* | 12.66 ± 0.39 | 6.98 ± 1.61*#^ | |
| dLVP(max-min) | TC | 105 ± 7.0 | 105.00 ±8.14 | 100.23 ± 7.66 | 99.55 ± 7.30 | 97.0 ± 8.14 |
| 30/60 | 98.33 ± 5.61 | 0.00 ± 0.00* | 0.00 ± 0.00* | 46.33 ± 6.44* | 55.67 ± 4.17* | |
| LVP min (diastolic) | TC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 30/60 | 0.00 ± 0.00 | 0.00 ± 0.00 | 15.67 ± 3.18* | 23.33 ± 9.28* | 13.67 ± 7.26* |
Table 1. Changes in left ventricular pressure (LVP) and coronary flow (CF) at specific time points of the ischemia and reperfusion (IR) experimental protocol: Baseline, 15 min ischemia (Isch15), 30 min ischemia (Isch30), 5 min reperfusion (IR5), 60 min reperfusion (IR60). Values are mean ± SEM for time control (TC; 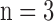 ) and IR hearts (30 min and 60 min;
) and IR hearts (30 min and 60 min; 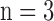 ).
).
P<0.05 respective ischemia and reperfusion period vs. baseline values;
P<0.05 reperfusion 5 min vs. reperfusion 60 min;
P<0.05 Ischemia 30 min vs. reperfusion 60 min.
III. Results
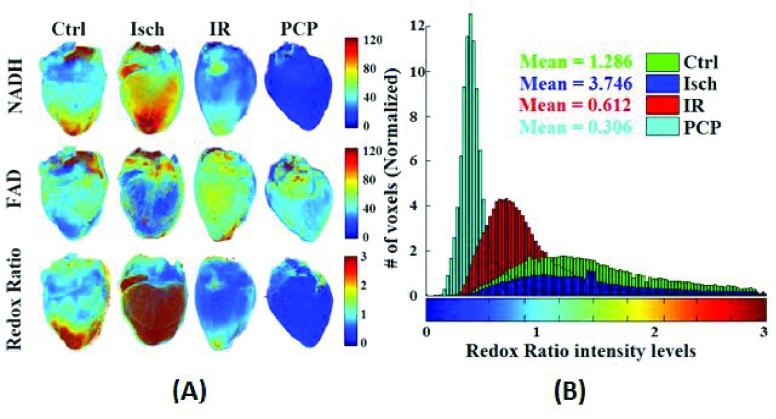
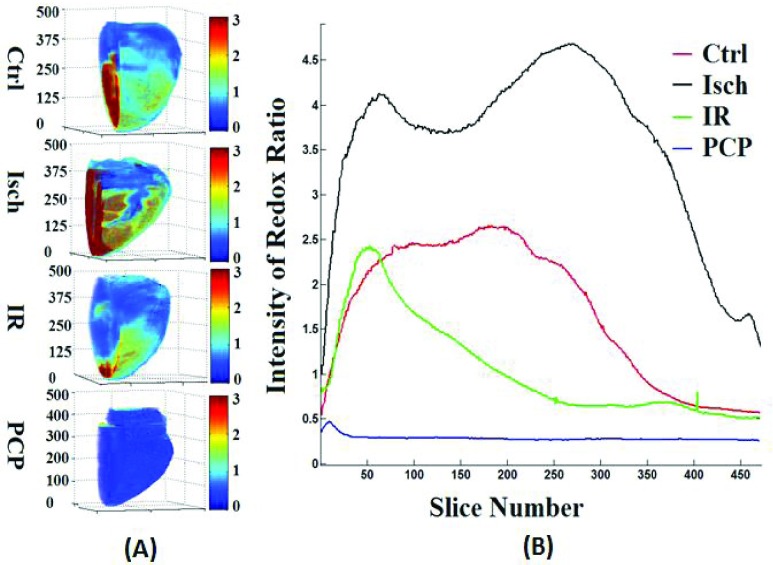
Figure 2A shows the 3D rendering of NADH and FAD AF signals and their ratio (RR = NADH/FAD) from representative hearts of each of the four groups (TC, TC + PCP, Isch, and IR). TC + PCP-treated hearts were maximally oxidized, which reflects decreased NADH and increased FAD signals, and concomitantly, a decreased RR when compared to the TC-treated hearts. Since mitochondria are the major source of NADH and FAD, complete oxidation of the RR by the uncoupler, PCP, ascertains that in the heart, mitochondria are the main determinants of the metabolic status, and the integrity of the electron transport chain (ETC) is critical. As expected, Isch-treated hearts showed relatively more reduced RR when compared to TC (Ctrl) alone, whereas IR-treated hearts were more oxidized when compared to the TC-treated hearts, but less oxidized when compared to the PCP-treated hearts. Figure 2B shows the corresponding summary of the cryoimages in histogram plots of RR for the four representative hearts shown in panel 2A. This figure displays a 3D RR intensity distribution throughout the hearts. For each histogram, the mean value of the RR intensity was calculated (see Materials and Methods). These mean values reflect the reduced vs. oxidized state of the heart. Large mean suggests more reduced and less oxidized state, while small mean suggests less reduced and more oxidized state. Therefore, the most oxidized mitochondrial RR was the TC + PCP-treated hearts, followed by the IR-treated hearts; the Isch-treated hearts were more reduced (i.e. less oxidized) compared to the TC-treated hearts.
FIGURE 2.
(A) Representative 3D reconstructions from the optical cryoimages of the mitochondrial NADH, FAD and NADH/FAD redox ratio (RR) in the frozen heart from each of the four groups (Ctrl: time control; Isch: ischemia; IR: ischemia and reperfusion; and PCP: a mitochondrial uncoupler). (B) Histogram distribution of the NADH/FAD RR for the four hearts exemplified in panel A.
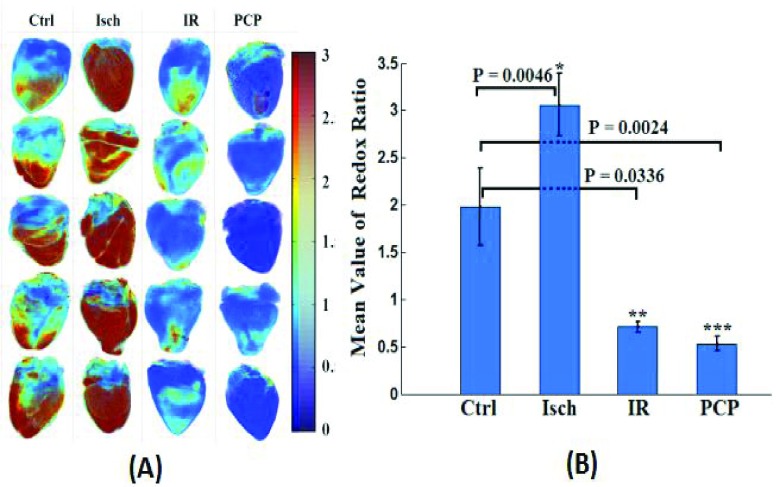
Figure 3A shows the 3D cryoimages of the mitochondrial RR (NADH/FAD) distribution of the individual hearts (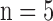 /group), which was used to generate the summarized bar graph shown in Figure 3B, for the various experimental groups described in Figure 2. The bar graph shows the mean ± SEM of the RR for all four groups.
/group), which was used to generate the summarized bar graph shown in Figure 3B, for the various experimental groups described in Figure 2. The bar graph shows the mean ± SEM of the RR for all four groups.
FIGURE 3.
(A) 3D optical cryoimages of the mitochondrial NADH/FAD redox ratio of the individual hearts (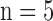 /group) in all four groups. (B) Bar graphs show the average values and standard errors of the means of the histogram redox ratios for each of the four groups of hearts in panel A. Statistical analysis using one-way ANOVA (
/group) in all four groups. (B) Bar graphs show the average values and standard errors of the means of the histogram redox ratios for each of the four groups of hearts in panel A. Statistical analysis using one-way ANOVA (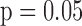 ) shows a significant difference between Ctrl (time control TC) vs. PCP, Ctrl vs. ischemia (Isch), and Ctrl (time control TC) vs. IR. The p-values are for Ischemia (Isch), IR and PCP compared to Ctrl (time control TC).
) shows a significant difference between Ctrl (time control TC) vs. PCP, Ctrl vs. ischemia (Isch), and Ctrl (time control TC) vs. IR. The p-values are for Ischemia (Isch), IR and PCP compared to Ctrl (time control TC).
Uncoupling of the ETC with PCP decreased the mean value of the RR by 73% compared to the TC (Ctrl) hearts. Ischemia (Isch) and IR treatments increased and decreased the mean values of the RR by 55% and 64%, respectively, compared to the TC-treated hearts. Based on statistical analysis, there was a significant difference between the three conditions compared to the TC group.
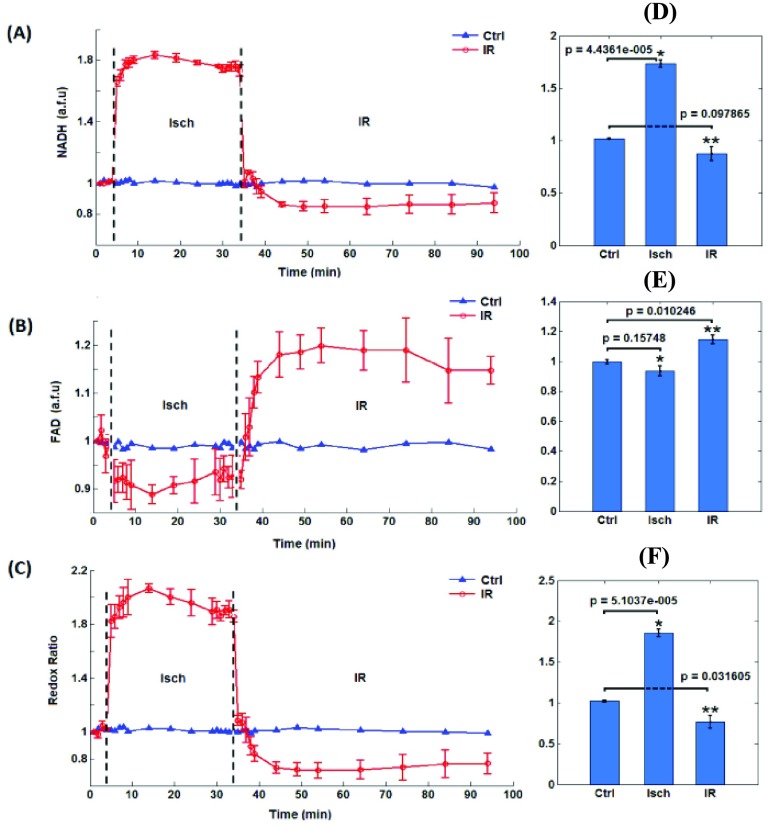
Figure 4 shows the corresponding time dependent changes in the mitochondrial redox state (NADH, FAD, and NADH/FAD RR) as measured online using the PTI spectrofluorometry in isolated ex vivo-perfused hearts. Figures 4A and 4B show the dynamic changes in NADH and FAD AF signals, respectively, during ischemia (Isch) and reperfusion (IR). Figure 4C represents the calculated RR for both ischemia (Isch) and ischemia followed by reperfusion (IR, red line) compared to TC (blue line). Baseline values were not different among the groups. The NADH and FAD AF signals remained unchanged in the TC (Ctrl) hearts, which signifies constancy in the functionality of our isolated heart preparations. Ischemia (Isch) caused an abrupt increase in NADH AF signal (Fig. 4A) and a reciprocal decrease in FAD AF signal (Fig. 4B), resulting in a sustained and significant increase in the RR during Isch (Fig. 4C). On initial reperfusion (IR), NADH and FAD AF signals returned to baseline values in the IR hearts. As reperfusion progressed, the NADH and FAD AF signals were significantly below and above the baseline AF signals, respectively. Consequently, the RR of IR-treated hearts decreased significantly during reperfusion when compared to the TC-treated hearts. This strongly suggests oxidation of the myocardium following reperfusion, which is consistent with the optical cryoimaging data (Figs. 2 and 3). The physiological implications of the altered mitochondrial bioenergetics following ischemic stress are summarized in Table 1.
FIGURE 4.
The left panels are the time courses of mitochondrial NADH (A), FAD (B), and NADH/FAD redox ratio (C) measured using the PTI spectrofluorometer for the Ctrl (time control TC) hearts and in hearts during ischemia (Isch) and during ischemia and reperfusion (IR). The right panels are the summary bar plots for NADH (D), FAD (E), and NADH/FAD redox ratio (F) during ischemia and reperfusion compared to the time control; statistical analysis is done based on one-way ANOVA.
Figure 5A shows the 3D spatial distribution of the mitochondrial redox state (NADH/FAD RR) in a mid-sagittal view of the myocardium from the apex (bottom) to the base (top). The hearts depicted are representatives from the four groups: TC (Ctrl), ischemia (Isch), IR and TC + PCP. Figure 5B shows the mean RR value of the slices of hearts through the z stack, which show heterogeneous distribution of RR (TC), from the apex to the base of the heart. The TC-treated hearts show a distribution of RR that is more robust in the midsection and toward the apical part of the heart. During ischemia (Isch), the RR regional distribution was similar to the TC (Ctrl) hearts, but intensity in RR increased markedly over the TC heart. This indicates a significantly higher reduced state when electron transfer along the ETC is impeded because of lack of O2, which is consistent with data shown in Figure 4C. PCP treatment, on the contrary, shows global homogeneous oxidation of the heart. In the IR-treated hearts, the RR intensity was less than the TC (Ctrl) hearts and the RR distribution skewed narrowly more towards the apical region of the heart and less in the mid-section and the base of the heart. This suggests that the mid and basal sections of the heart are more susceptible to IR injury, and that majority of the oxidation of the heart following IR (Fig. 4C) emanated from the mid-section and the base of the heart. This also suggests that the limited viable mitochondria in the apical region with reduced/normal redox state provided the ATP needed to maintain cardiac function on reperfusion (Table 1). Complete oxidation of mitochondria with PCP resulted in complete loss of contractile function (data not shown).
FIGURE 5.
(A) 3D distribution of mitochondrial redox state (NADH/FAD redox ratio) in a mid-sagittal view of the myocardium from the apex (bottom) to the base (top) of a representative heart during the Ctrl (time control TC), ischemia (Isch), ischemia and reperfusion (IR), and PCP treatments. (B) Plot of the volume-averaged redox ratio in approximately 800 different layers of the representative heart from the apex to the base.
Table 1 summarizes the functional data before, during, and after IR. TC (Ctrl) showed stable cardiac function throughout the perfusion period. This observation solidifies the notion made previously that the ex vivo perfused heart model is a stable and a reliable model to assess mitochondrial bioenergetics during IR. Coronary flow (CF) was only significantly reduced after 60 min (IR60, 96 min protocol time) reperfusion; developed left ventricular pressure (LVP) (max-min) was significantly blunted after 5 (IR5, 41 min protocol time) and 60 min (96 min protocol time) reperfusion.
The significant reduction on LVP corresponded with a significant decrease in the rates of contractility (dp/dtmax) and relaxation (dp/dtmin) (data not shown). The early display of LVP function suggests that the contractile apparatus show greater vulnerability to IR injury than the coronary vasculature. Diastolic (min) LVP was steady at 0 mmHg in the TC-treated hearts during the perfusion protocol. During ischemia, diastolic LVP, a marker for myocardial contracture or diastolic tone, increased significantly during late ischemia (30 min) and 5 min and 60 min reperfusion. The sustained contracture throughout reperfusion attests to damaged mitochondria and impaired ATP production. ATP is necessary to maintain cellular ionic homeostasis, and insufficient ATP production is likely to increase the susceptibility of post-ischemic hearts to contractile dysfunction [34].
IV. Discussion and Conclusion
Autofluorescence (AF) of the heart tissue obtained from the PTI spectrofluorometry and optical cryoimaging tools provides real-time and 3D volumetric mitochondrial redox state (NADH, FAD, and NADH/FAD redox ratio (RR)), respectively, during IR injury. The redox state marker RR is defined as the ratio of the two endogenous fluorophores, NADH and FAD. The results revealed that ischemic stress increased the heart RR, while IR stress resulted in a decrease in the heart RR caused by oxidative stress. The 3D optical cryoimaging of the heart tissue during ischemia and IR in the present study provides new evidence that: (i) the RR displayed different heterogeneity along the longitudinal axis of the heart, i.e., from the base to the apex, (ii) unlike ischemia, during reperfusion, the RR was more prominent in the apical region compared to the other parts of the heart, (iii) during ischemia and IR, the 3D cryoimages and the spectrofluorometric data overall presented similar RR changes with the 3D cryoimaging showing additional insights that may relate to the viability of mitochondria (improved RR) in the region of the heart responsible for producing the ATP necessary for cardiac function on reperfusion. Indeed, unrelated studies have revealed a causal link between enhanced mitochondrial function and improved muscle contractility [35].
In the current study, we used 3D optical cryoimaging to measure the mitochondrial redox state (NADH, FAD and NADH/FAD RR) distribution across the heart during global ischemia without reperfusion and during reperfusion following global ischemia (IR). The major source of the redox state in the heart is mitochondria, with negligible contribution from cytoplasmic sources and minimal impact from NADPH [36]–[38]. FAD AF signal originates only from mitochondria [38], [39]. Our study shows that IR stress alters the distribution of the NADH/FAD RR across the longitudinal axis, from the base to the apex of the heart. In our previous studies, using the spectrofluorometric technique (PTI) [9], [28], [30], [31], we reported that ischemia led to maximal reduction of mitochondria while ischemia followed by reperfusion led to overall oxidation when compared to TC (Ctrl) hearts, i.e. no IR. The current spectrofluorometric dynamic data (Fig. 4) recapitulates our previous observations [9], [28], [30], [31]. However, unlike the dynamic spectrofluorometric data, the 3D cryoimaging data show that mitochondrial redox state distribution in the heart is not uniform. In the TC (Ctrl) group, the basal level of the redox state showed a relatively normal distribution of a reduced redox state from the base to the apex of the heart. The RR seems to peak in middle of the heart. During ischemia alone, the RR maintained the same distribution but with significantly higher fluorescence intensity than the TC group. The RR increased markedly in the ischemia (alone) group because the limiting O2 supplies during myocardial ischemia prevent the oxidation of NADH by mitochondria ETC, and thus NADH builds up. This is consistent with the increased NADH that we observed using the PTI spectrofluorometric data (Fig. 4). In contrast, during IR, the RR fluorescence decreased with an altered distribution that shifted the reduced redox state more towards the apical part of the heart while most of the basal region is maximally oxidized, i.e. minimal RR. The corresponding spectrofluorometric data from the PTI also shows the heart is oxidized following reperfusion, but the data is limited in the information it provides. Nonetheless, it is worth noting that the 3D cryoimaging data validates the strategic placement of the fiberoptic probe to monitor dynamic changes in the global redox state in the heart during IR.
Mitochondria are the lynchpin of myocardial tissue energy and redox homeostasis, and hence, deterioration in mitochondrial function leads to cardiomyocytes and endothelial cell death and subsequent cardiovascular dysfunction. Since mitochondria are responsible for the production of the bulk of ATP in the heart, any perturbations that interfere with the biochemical machinery of oxidative phosphorylation (OxPhos) are expected to impair the ATP production. IR causes oxidative/nitrosative stress [40]–[42] that leads to damage of mitochondrial proteins (TCA cycle enzymes) and impaired electron transfer (defective ETC complexes) [25]. IR could also lead to damage mitochondria that contribute to lesser reducing equivalents (NADH and FADH2) for OxPhos. Therefore, 3D cryoimaging of the redox state strongly suggests that the high RR in the apical region during reperfusion provided the reducing equivalents for the ETC necessary for ATP production and to sustain post-ischemic contractile function. In the highly oxidized basal regions of the heart (lower RR), deficiency in ATP production is likely to increase the susceptibility of post-infarct hearts to cardiomyocyte death and contractile dysfunction. These two opposing scenarios together may contribute to the diminished functional return we observed during reperfusion (Table 1).
An alternative interpretation of our data as it pertains to the energetic state of mitochondria during reperfusion following ischemia is that the lower RR in the basal region could reflect higher ATP production because of the increased oxidation of the NADH and FADH2 at the time of the metabolic arrest in liquid N2. Conversely, the relative increase in RR in the apical region during IR could be attributed to impaired ATP production due to damage in the OxPhos process or defective electron transfer along the ETC. We believe, however, that our previous explanation (see above paragraph) seems more plausible based on our PCP data that show complete mitochondrial oxidation (discussed further in the next paragraph). Future experiments involving mitochondria or cardiomyocytes isolated from the apical and basal regions of the heart following time control (TC), ischemia (Isch) or IR are warranted to ascertain the regional differences in the RR, and the bioenergetics viability of the different populations of mitochondria during reperfusion. In addition, alternative experiments using cryoimaging to monitor reactive oxygen species (ROS) distribution throughout the heart under similar conditions (i.e. TC, Isch, IR) would be helpful to correlate the reduced RR with higher ROS emission (oxidative stress) or vice versa, following IR.
The heart requires a constant supply of ATP in order to maintain contractility. Following IR, depressed LVP and increased diastolic LVP signify insufficient ATP production [34]. Decrease ATP production also contributes to loss of cellular ionic homeostasis including, cytosolic Ca2+ overload due to activation of the Na+/H+ and Na+/Ca2+ exchangers [40], [43]. These effects culminate in mitochondrial Ca2+ overload, mitochondrial swelling and bursting, apoptosis/necrosis and ultimately cell death and infarction [9], [29]. The rupture of swollen mitochondria may also contribute to the loss of NADH and FADH2, and ultimately a lower RR. Increased necrosis and diminished RR of the affected areas, in this case the basal and mid-region of the heart, leads to impaired ETC function and ATP production. In support of this concept, it has been shown that improved mitochondrial ETC activity increased ATP production and enhanced contractile function in the aged heart [44]. Furthermore, uncoupling of the ETC from OxPhos (i.e. impede ATP production) with PCP dissipates the proton gradient across the inner mitochondrial membrane resulting in maximal oxidation of mitochondria (maximum RR; Figs. 2, 3, 5) resulting in complete block of cardiac contractility.
What is the relevance of this study? Ischemia and reperfusion injury is a critical cardiovascular complication that occurs after an acute myocardial infarct or during heart transplant. Ischemic heart disease is a common malady that afflicts millions of people worldwide. The mechanism of cardiac IR injury has been explored in numerous animal models and mitochondria have been implicated as critical in the etiology of the disease. These experiments might have some clinical implications. We have provided a 3D technique to monitor the distribution of mitochondrial redox state (RR), a hallmark of the metabolic state across the surface of the functioning myocardium. Therefore, a potentially important clinical application of optical fluorescence techniques includes delineation of the border zone of complete myocardial infarcts or in hypoperfused myocardium during incomplete coronary artery obstruction. This border zone detection would be highly valuable to clinicians in tailoring therapy to patients. That is, a mitochondria targeted-therapy could improve the efficacy of protecting tissue from further IR injury if the affected mitochondria could be delineated in the heart tissue. A catheter-based fluorometer can be applied via standard percutaneous venous or arterial techniques to assess the myocardial injury, in vivo. Thus understanding the cause of the underlying heterogeneity of cardiac metabolism and the role of mitochondria in IR injury, and other pathologies such as non-ischemic heart failure, could provide an opportunity for better therapeutic approaches to alleviate heart diseases.
In summary, we have reported two different approaches, an online dynamic (PTI) spectrofluorometric approach and a 3D optical cryoimaging approach, to monitor changes in cardiac mitochondrial redox state (RR) and energy metabolism under normal and pathological conditions (i.e. ischemia and IR). The quantitative optical cryoimaging data and the PTI spectrofluorometric data show overall similar patterns in the redox state of the heart during ischemia and ischemia followed by reperfusion (IR). This observation validates our previous findings of the redox state changes during IR using the fiber optic probe placed against the LV free wall. The 3D cryoimaging provided additional information regarding the heterogeneous spatial distribution of the metabolic state across the heart before, during and after ischemia and reperfusion. The 3D cryoimaging also provided cardiac mitochondrial energy metabolism of greater resolution, with unique insights into mitochondrial redox state of the guinea pig heart. The high RR in the apical region could be responsible for producing the ATP necessary to maintain the modicum contractility on reperfusion. However, alternative interpretations are also possible, and future experiments involving isolated mitochondria, isolated cardiomyocytes, or monitoring 3D distribution of ROS throughout the myocardium will be necessary to correlate the regional differences in the RR with the mitochondrial bioenergetics, ROS production and cardiac recovery during reperfusion. The potential translational significance of this study has been suggested and further studies are required to ascertain the clinical implications.
Limitations of the Study: There are other potential endogenous fluorophores in cardiac tissue, such as NADPH, collagen and elastin, which may contribute to the NADH autofluorescence (AF) signal [45]. However, metabolic perturbations mostly affect NADH when compared to NADPH [46]–[48]. Furthermore, NADPH has significantly lower tissue concentrations and quantum yields, and as a result, significantly lower AF signal when compared to NADH. In addition, unlike NADH, collagen and elastin contribution to AF signal is negligible and is not expected to change with variations in mitochondrial redox state [45], [49]. Thus, we believe the majority of the NADH AF signal in this study reflects mitochondrial energy metabolism.
Biographies

Mahsa Ranji received the Ph.D. degree from the University of Pennsylvania. She received the post-doctoral training from the Sanford-Burnham Medical Research Institute. She is currently an Associate Professor with the Department of Electrical Engineering, University of Wisconsin–Milwaukee, WI, USA, and the Director of the Biophotonics Laboratory with research focus on optical imaging and image processing of tissue metabolism. Her group has implemented optical devices to study cardiopulmonary injuries.

Mohammad Masoudi Motlagh received the bachelor’s degree in controls and instrumentation from Shiraz University, Iran, and the M.Sc. degree from the University of Wisconsin–Milwaukee in 2014. He is currently a Design Bioengineer with Greater Milwaukee Area, WI.

Fahimeh Salehpour received the B.S. degree in physics from the University of Isfahan, Isfahan, Iran, the M.S. degree in photonics from Shahid Beheshti University, Tehran, Iran, and the M.S. degree from the University of Wisconsin–Milwaukee with a concentration in biophotonics. She is currently as an Optical Engineer with Medici Technologies, Inc., Albuquerque, NM.

Reyhaneh Sepehr received the B.S. and M.S. degrees in biomedical engineering from the Amir Kabir University of Technology, Tehran, Iran, and the Ph.D. degree in electrical engineering from the University of Wisconsin–Milwaukee (UWM). She is currently a Post-Doctoral Scholar with the Physics Department, UWM.

James S. Heisner received the B.Sc. degree from UW-Oshkosh. He has worked in our laboratory for over 20 years. He is a Research Associate and Lab Manager of the Stowe/Camara Laboratory with the Department of Anesthesiology, Medical College of Wisconsin. He has coauthored numerous articles with members of the lab. His areas of expertise include small animal surgeries and isolated ex vivo and in vivo cardiac perfusion techniques.

Ranjan K. Dash received the Ph.D. degree in applied mathematics, specializing in computational biofluid dynamics, from IIT Delhi in 1998, before moving to the USA for postdoctoral studies. He is an Associate Professor with the Department of Physiology and Biotechnology and Bioengineering Center, Medical College of Wisconsin. He has authored over 65 papers in peer-reviewed scientific journals covering a wide range of research areas. His current research interests are focused on developing computational models in conjunction with experimental data to understand the underlying molecular mechanisms of cardiac ischemia and reperfusion injury and related pathologies.

Amadou K. S. Camara received the Ph.D. degree in renal and cardiovascular physiology from the Department of Physiology, Medical College of Wisconsin (MCW), in 1995. He is a Professor with the Department of Anesthesiology, MCW. After his post-doctoral training in cardiac electrophysiology with the Department of Anesthesiology, MCW, in 1999, he joined as a Faculty member and shortly became interested in mitochondrial biology. He has authored over 85 articles in peer-reviewed scientific journals and several book chapters, with the vast majority of his publications focused on mitochondrial biology and cardiac pathophysiology. Today, he continues to devote much of his research on mitochondria and its role in the etiology of heart disease, specifically, delving into the molecular mechanisms of changes in mitochondrial protein functions during ischemic stress and the impact on organ dysfunction.
Funding Statement
The work of M. Ranji was supported by UWM RGI, 101 290. The work of R. K. Dash was supported by the National Institutes of Health under Grant P01-GM066730. The work of R. K. DASH and A. K. S. Camara was supported by the National Institutes of Health under Grant R01-HL095122.
290. The work of R. K. Dash was supported by the National Institutes of Health under Grant P01-GM066730. The work of R. K. DASH and A. K. S. Camara was supported by the National Institutes of Health under Grant R01-HL095122.
Contributor Information
Mahsa Ranji, Email: ranji@uwm.edu.
Amadou K. S. Camara, Email: aksc@mcw.edu.
References
- [1].Zimmer H.-G., “Some aspects of cardiac heterogeneity,” Basic Res. Cardiol., vol. 89, no. , pp. 101–117, Mar-Apr 1994. [DOI] [PubMed] [Google Scholar]
- [2].Pries A. R. and Secomb T. W., “Origins of heterogeneity in tissue perfusion and metabolism,” Cardiovascular Res., vol. 81, pp. 328–335, Feb. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Balaban R. S. and Arai A., “Function, metabolic, and flow heterogeneity of the heart the view is getting better,” Circ Res, vol. 88, pp. 265–267, Feb. 2001. [DOI] [PubMed] [Google Scholar]
- [4].Deussen A., Lauer T., Loncar R., and Kropp J., “Heterogeneity of metabolic parameters in the left ventricular myocardium and its relation to local blood flow,” Basic Res. Cardiol., vol. 96, pp. 564–574, Nov. 2001. [DOI] [PubMed] [Google Scholar]
- [5].Decking U. K. M. and Schrader J., “Spatial heterogeneity of myocardial perfusion and metabolism,” Basic Res. Cardiol., vol. 93, pp. 439–445, Dec. 1998. [DOI] [PubMed] [Google Scholar]
- [6].Vetterlein F., Prange M., Tran P.-N., and Schmidt G., “Method for simultaneous determination of the distribution of capillary plasma flow and myocyte redox state (NADH-fluorescence) in the hypoperfused myocardium of the anesthetized rat,” in Oxygen Transport to Tissue XV, vol. 345 New York, NY, USA: Plenum Publishing Corp., 1994, pp. 271–273. [DOI] [PubMed] [Google Scholar]
- [7].Xu H. N., Zhou R., Moon L., Feng M., and Li L. Z., “3D imaging of the mitochondrial redox state of rat hearts under normal and fasting conditions,” J. Innov. Opt. Health Sci., vol. 7, p. 1350045, Mar. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ng C. S. H., Wan S., and Yim A. P. C., “Pulmonary ischaemia–reperfusion injury: Role of apoptosis,” Eur. Respiratory J., vol. 25, pp. 356–363, Feb. 2005. [DOI] [PubMed] [Google Scholar]
- [9].Aldakkak M., Camara A. K. S., Heisner J. S., Yang M., and Stowe D. F., “Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts,” Pharmacol. Res., vol. 64, pp. 381–392, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ali I., et al. , “Protection by 20-5,14-HEDGE against surgically induced ischemia reperfusion lung injury in rats,” Ann. Thoracic Surgery, vol. 93, no. 1, pp. 282–288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tambo A. L., Jacobs E., Medhora M., Gruenloh S., Gao Y., and Ali I., “The role of TLR4 and HMGB1 in the ischemia/reperfusion-mediated mitochondrial dysfunction,” Chest, vol. 140, no. 4, p. 914A, 2011. [Google Scholar]
- [12].Staniszewski K., Audi S. H., Sepehr R., Jacobs E. R., and Ranji M., “Surface fluorescence studies of tissue mitochondrial redox state in isolated perfused rat lungs,” Ann. Biomed. Eng., vol. 41, pp. 827–836, Apr. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Else P. L. and Hulbert A. J., “Mammals: An allometric study of metabolism at tissue and mitochondrial level,” Amer. J. Physiol., vol. 248, pp. R415–R421, Apr. 1985. [DOI] [PubMed] [Google Scholar]
- [14].Gan Z., Audi S. H., Bongard R. D., Gauthier K. M., and Merker M. P., “Quantifying mitochondrial and plasma membrane potentials in intact pulmonary arterial endothelial cells based on extracellular disposition of rhodamine dyes,” Amer. J. Physiol.-Lung Cellular Molecular Physiol., vol. 300, pp. L762–L772, May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kane J. J., Murphy M. L., Bisset J. K., deSoyza N., Doherty J. E., and Straub K. D., “Mitochondrial function, oxygen extraction, epicardial S-T segment changes and tritiated digoxin distribution after reperfusion of ischemic myocardium,” Amer. J. Cardiol., vol. 36, pp. 218–224, Aug. 1975. [DOI] [PubMed] [Google Scholar]
- [16].Vetterlein F., Prange M., Lubrich D., Pedina J., Neckel M., and Schmidt G., “Capillary perfusion pattern and microvascular geometry in heterogeneous hypoxic areas of hypoperfused rat myocardium,” Amer. J. Physiol., vol. 268, pp. H2183–H2194, Jun. 1995. [DOI] [PubMed] [Google Scholar]
- [17].Sepehr R., Staniszewski K., Maleki S., Jacobs E. R., Audi S., and Ranji M., “Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress,” J. Biomed. Opt., vol. 17, p. 046010, Apr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
[18].Salehpour F., et al. , “Effects of p
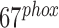 on the mitochondrial oxidative state in the kidney of Dahl salt-sensitive rats: Optical fluorescence 3-D cryoimaging,” Amer. J. Physiol.-Renal Physiol., vol. 309, pp. F377–F382, Jun.
2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
on the mitochondrial oxidative state in the kidney of Dahl salt-sensitive rats: Optical fluorescence 3-D cryoimaging,” Amer. J. Physiol.-Renal Physiol., vol. 309, pp. F377–F382, Jun.
2015. [DOI] [PMC free article] [PubMed] [Google Scholar] - [19].MasoudiMotlagh M., Sepehr R., Sheibani N., Sorenson C. M., and Ranji M., “Optical cryoimaging of mitochondrial redox state in bronchopulmonary-dysplasia injury models in mice lungs,” Quant. Imag. Med. Surgery, vol. 5, pp. 159–162, Feb. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maleki S., et al. , “Optical imaging of mitochondrial redox state in rodent model of retinitis pigmentosa,” J. Biomed. Opt., vol. 18, p. 016004, Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ghanian Z., et al. , “Optical imaging of mitochondrial redox state in rodent models with 3-iodothyronamine,” Experim. Biol. Med., vol. 239, pp. 151–158, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghanian Z., Maleki S., Park S., Sorenson C. M., Sheibani N., and Ranji M., “Organ specific optical imaging of mitochondrial redox state in a rodent model of hereditary hemorrhagic telangiectasia-1,” J. Biophoton., vol. 7, pp. 799–809, Oct. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aldakkak M., Stowe D. F., Heisner J. S., Spence M., and Camara A. K. S., “Enhanced Na+/H+ exchange during ischemia and reperfusion impairs mitochondrial bioenergetics and myocardial function,” J. Cardiovascular Pharmacol., vol. 52, pp. 236–244, Sep. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rhodes S. S., Camara A. K. S., Heisner J. S., Riess M. L., Aldakkak M., and Stowe D. F., “Reduced mitochondrial Ca2+ loading and improved functional recovery after ischemia-reperfusion injury in old vs. young guinea pig hearts,” Amer. J. Physiol.-Heart Circulatory Physiol., vol. 302, pp. H855–H863, Feb. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gadicherla A. K., Stowe D. F., Antholine W. E., Yang M., and Camara A. K. S., “Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine,” Biochim. Biophys. Acta, vol. 1817, no. 3, pp. 419–429, Mar. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riess M. L., et al. , “Preconditioning with sevoflurane reduces changes in nicotinamide adenine dinucleotide during ischemia-reperfusion in isolated hearts: Reversal by 5-hydroxydecanoic acid,” Anesthesiology, vol. 98, pp. 387–395, Feb. 2003. [DOI] [PubMed] [Google Scholar]
- [27].Riess M. L., Camara A. K. S., Kevin L. G., An J., and Stowe D. F., “Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17 °C ischemia in intact hearts,” Cardiovascular Res., vol. 61, pp. 580–590, Feb. 15 2004. [DOI] [PubMed] [Google Scholar]
- [28].Camara A. K. S., et al. , “ROS scavenging before 27 °C ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state,” Amer. J. Physiol.-Cell Physiol., vol. 292, pp. C2021–C2031, Jun. 2007. [DOI] [PubMed] [Google Scholar]
- [29].Aldakkak M., Stowe D. F., Chen Q., Lesnefsky E. J., and Camara A. K. S., “Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release,” Cardiovascular Res., vol. 77, pp. 406–415, Jan. 2008. [DOI] [PubMed] [Google Scholar]
- [30].An J., Camara A. K. S., Rhodes S. S., Riess M. L., and Stowe D. F., “Warm ischemic preconditioning improves mitochondrial redox balance during and after mild hypothermic ischemia in guinea pig isolated hearts,” Amer. J. Physiol.-Heart Circulatory Physiol., vol. 288, pp. H2620–H2627, Jun. 2005. [DOI] [PubMed] [Google Scholar]
- [31].Riess M. L., Camara A. K. S., Chen Q., Novalija E., Rhodes S. S., and Stowe D. F., “Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts,” Amer. J. Physiol.-Heart Circulatory Physiol, vol. 283, pp. H53–H60, Jul. 2002. [DOI] [PubMed] [Google Scholar]
- [32].Chance B. and Baltscheffsky H., “Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide,” J. Biol. Chem., vol. 233, pp. 736–739, Sep. 1958. [PubMed] [Google Scholar]
- [33].Chance B., Lee C. P., and Schoener B., “High and low energy states of cytochromes. II. In submitochondrial particles,” J. Biol. Chem., vol. 241, pp. 4574–4576, Oct. 1966. [PubMed] [Google Scholar]
- [34].Tanno M. and Kuno A., “Reversal of metabolic shift in post-infarct-remodelled hearts: Possible novel therapeutic approach,” Cardiovascular Res., vol. 97, pp. 195–196, Feb. 2013. [DOI] [PubMed] [Google Scholar]
- [35].Nogueira L., et al. , “(−)-epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle,” J. Physiol., vol. 589, pp. 4615–4631, Sep. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Katz L. A., Koretsky A. P., and Balaban R. S., “Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study,” FEBS Lett, vol. 221, pp. 270–276, Sep. 1987. [DOI] [PubMed] [Google Scholar]
- [37].Estabrook R. W., “Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles,” Anal. Biochem., vol. 4, pp. 231–245, Sep. 1962. [DOI] [PubMed] [Google Scholar]
- [38].Nuutinen E. M., “Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart,” Basic Res. Cardiol., vol. 79, pp. 49–58, Jan-Feb 1984. [DOI] [PubMed] [Google Scholar]
- [39].Brandes R. and Bers D. M., “Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery,” Biophys. J., vol. 71, pp. 1024–1035, Aug. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Camara A. K., Bienengraeber M., and Stowe D. F., “Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury,” Front Physiol., vol. 2, p. 13, Apr. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Camara A. K. S., Lesnefsky E. J., and Stowe D. F., “Potential therapeutic benefits of strategies directed to mitochondria,” Antioxidants Redox Signaling, vol. 13, pp. 279–347, Aug. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stowe D. F. and Camara A. K. S., “Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function,” Antioxidants Redox Signaling, vol. 11, pp. 1373–1414, Jun. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Camara A. K. S., et al. , “Na+/H+ exchange inhibition with cardioplegia reduces cytosolic [Ca2+] and myocardial damage after cold ischemia,” J. Cardiovascular Pharmacol., vol. 41, pp. 686–698, May 2003. [DOI] [PubMed] [Google Scholar]
- [44].Chen Q., Camara A. K. S., Stowe D. F., Hoppel C. L., and Lesnefsky E. J., “Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion,” Amer. J. Physiol.-Cell Physiol., vol. 292, pp. C137–C147, Jan. 2007. [DOI] [PubMed] [Google Scholar]
- [45].Georgakoudi I., et al. , “NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes,” Cancer Res., vol. 62, pp. 682–687, Feb. 2002. [PubMed] [Google Scholar]
- [46].Avi-Dor Y., Olson J. M., Doherty M. D., and Kaplan N. O., “Fluorescence of pyridine nucleotides in mitochondria,” Biol. Chem., vol. 237, pp. 2377–2383, Jul. 1962. [Google Scholar]
- [47].Klaidman L. K., Leung A. C., and Adams J. D., “High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions,” Anal. Biochem., vol. 228, pp. 312–317, Jul. 1995. [DOI] [PubMed] [Google Scholar]
- [48].O’Connor M., et al. , “Origin of labile NADH tissue fluorescence,” Oxygen Physiol. Funct., p. 10, 1977.
- [49].Ramanujam N., et al. , “In vivo diagnosis of cervical intraepithelial neoplasia using 337-nm-excited laser-induced fluorescence,” Proc. Nat. Acad. Sci. USA, vol. 91, pp. 10193–10197, Oct. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]