Abstract
The aim was to investigate the effects of far infrared (FIR) ray emitting clothes on indirect markers of exercise-induced muscle damage and physical performance recovery after a plyometric bout applied to soccer players. Twenty-one male players (18.9±0.6 years; 70.8±5.01 kg; 178.3±0.06 cm) performed 100 drop-jumps. Six hours after the bout, athletes put on FIR clothes (FIR) (density of 225 g·m-2, 88% far infrared rays emitting polyamide 66 Emana yarn (PA66) fibre, 12% Spandex, emissivity of 0.88 and power emitted of 341 W/m2µm at 37°C in the 5-20 µm wavelength range, patent WO 2009/077834 A2) (N = 10) or placebo clothes (PLA) (N = 11). Mid-thigh circumferences, creatine kinase (CK), and delayed-onset muscle soreness (DOMS) were assessed before, immediately after and 24, 48, and 72 h after the bout. Squat (SJ) and countermovement jump (CMJ) heights were measured before and at 24, 48, and 72 h after, while 1RM leg press (maximum strength) was measured before and at 72 h after the plyometrics. No differences between groups were found in mid-thigh circumferences, SJ, CMJ or 1RM. CK increased significantly 24 h after the plyometrics in comparison to before (p < 0.05) in both groups. PLA showed significant DOMS increases at 24, 48, and 72 h, while FIR showed significant increases at 24 and 48 h (p < 0.05). DOMS effect sizes were greater in FIR (moderate at 48 h, ES = 0.737 and large at 72 h, ES = 0.844), suggesting that FIR clothes may reduce perceived DOMS after an intense plyometric session performed by soccer players.
Keywords: Delayed-onset muscle soreness\power, Football, Sports recovery
INTRODUCTION
It is well established that lower limb muscle power plays an important role in soccer players’ performances [1, 2]. Motor tasks such as kicking, jumping, sprinting, and changing direction are crucial during a match [3]. A traditional way to improve muscle power production capacity is the use of plyometric training [4, 5], which is characterized by a combination of fast eccentric contractions followed by explosive concentric muscle actions. However, the eccentric contraction content of plyometrics may result in exercise-induced muscle damage (EIMD) [6–11]. EIMD is associated with reduced levels of muscle strength and power for several days after the damaging exercise [7, 12]. Considering that elite soccer players are often submitted to high eccentric actions during matches (i.e., landing, accelerating, and changing direction) and training sessions [13], it is necessary to investigate recovery strategies to attenuate the specific effects of EIMD.
In this regard, nutritional supplementation, cryotherapy, hydrotherapy, active recovery, stretching, compression garments, and electrical stimulation have been used by conditioning coaches and trainers in an attempt to accelerate players’ recovery [11, 14–20]. Although these strategies have already demonstrated some degree of effectiveness, there is no consensus on the most suitable method. Indeed, some recovery strategies may be very uncomfortable, require specific and expensive equipment and not completely cover the damaged area.
Although not extensively explored, far infrared (FIR) ray therapy has been suggested as a post-exercise recovery method [17]. FIR consists of short electromagnetic waves with lengths within the infrared (IR) spectrum ranging from 5.6 to 1000 µm. They are able to penetrate almost 4 cm into human tissues, inducing biological effects such as accelerating recovery of skeletal muscle function after exercise, increasing the flow of blood and the lymphatic vessels, improving endothelial function and decreasing pain, inflammation and oxidative stress [17, 21–23].
FIR radiation therapy is usually applied through light-emitting devices or saunas [17, 21, 24, 25]. Recently, FIR-emitting polymers or ceramic nanoparticles have been incorporated into sports clothing fabric, which facilitates their application when compared to other methods. The FIR emitting clothes may be a suitable method to apply therapeutic radiation since electromagnetic energy is more uniformly delivered on the body's surface and presents the same wavelength range as that generated by lamps or saunas. The human body emits FIR (range from 3 to 30 µm), and this can be absorbed by the ceramic nanoparticles, allowing then to re-emit FIR on the surface of the body [23]. Regardless of the irradiation source, the thermal effects elicited by FIR, when absorbed by the skin surface, are not the main mechanism involved in the therapeutic effects of FIR [26]. FIR radiation can be absorbed by water molecules present in biological systems, thus influencing cell membrane potentials and mitochondrial metabolism. These effects include scavenging H2O2, blunted cyclooxygenase-2 activation, increased haem-oxygenase 1 expression and e NOS activation, triggering anti-inflammatory signalling and decreasing oxidative stress [23, 26–28]. Nevertheless, the effects of FIR-emitting clothes on post-exercise physical and physiological recovery remain unknown.
Therefore, the purpose of this study was to investigate the effects of FIR-emitting clothing on indirect markers of EIMD and physical performance recovery after an intense plyometric exercise bout applied to high-level soccer players.
MATERIALS AND METHODS
Subjects
Twenty-one male soccer players (19.5 ± 0.8 years; 70.8 ± 5.01 kg; 178.3 ± 0.06 cm; 6.6 ± 1.5 years of soccer training) participated in the study. They were competing in the national Brazilian under-20 tournament, and their team was ranked fifth in the country. All players trained twice a day (∼120 min per session), five days per week. Exclusion criteria included clinically detected injuries, use of anti-inflammatory drugs and/or use of nutritional/ergogenic supplements in the previous six months. In accordance with the Declaration of Helsinki, all athletes were informed of the experimental risks and benefits of participating in the study and signed an informed consent form prior to the investigation. The study was approved by a local Institutional Review Board for the use of human subjects. The players were randomly assigned into two groups: FIR-emitting clothes (FIR, n = 11) or placebo (PLA, n = 10). The FIR non-compressive pants were made from Emana specific material (density of 225 g m-2, 88% far infrared rays emitting polyamide 66 Emana yarn (PA66) fibre, 12% Spandex, emissivity of 0.88 and power emitted of 341 W/m2µm at 37°C in the 5-20 µm wavelength range, patent WO 2009/077834 A2). The PLA group wore clothes with the following characteristics: 225 g m-2, 88% PA66 fibre, 12% Spandex but with no far infrared ray emitting functionality. All subjects wore the clothes during their sleeping period over three successive nights, for at least 10 h per night (from 10:00 pm to 08:00 am).
Experimental design
The present study was a randomized, double-blind, placebo-controlled trial. The study was conducted within a 6-week preparatory training period, prior to a national tournament. All athletes were submitted to anthropometric measurements and asked to refrain from training for at least 48 h prior to the intense plyometric exercise bout. Left and right mid-thigh circumferences, plasma creatine kinase (CK) activity and delayed-onset muscle soreness (DOMS) were assessed before, immediately after and 24, 48, and 72 h after the plyometric bout. Squat and countermovement jumping height (SJ and CMJ, respectively) were measured before and 24, 48, and 72 h after, while leg press maximum dynamic strength (1RM) was measured before and 72 h after the plyometric exercise bout. Subjects were randomly assigned to two groups and received treatment 6 hours after the exercise bout. The FIR group wore FIR-emitting clothes throughout the sleeping period (from 10:00 pm to 08:00 am) and the placebo group (PLA) followed the same procedures but wore placebo clothes. The characteristics of the two groups are presented in Table 1.
TABLE 1.
Characteristics of subjects in the fir-emitting clothes (FIR) and placebo (PLA) groups.
| Variables | FIR | PLA | P |
|---|---|---|---|
| Age (years) | 19.4 ± 0.5 | 19.6 ± 1.0 | 0.57 |
| Body mass (kg) | 70.7 ± 4.0 | 71.1 ± 5.7 | 0.98 |
| Height (cm) | 177.2 ± 0.5 | 179.5 ± 0.5 | 0.34 |
| Duration of soccer practice (years) | 13.7 ± 4.8 | 11.9 ± 5.2 | 0.42 |
| Leg press 1RM (kg) | 167.4 ± 22.7 | 168.4 ± 18.5 | 0.92 |
Mid-thigh circumferences
Right and left mid-thigh circumferences were assessed as a measure of limb swelling (i.e., oedema) using a standard anthropometric tape (Cescorf, Porto Alegre, RS, Brazil). Both circumferences were measured at the mid-portion of each thigh (i.e., at 50% of the distance between the greater trochanter and the lateral epicondyle of the femur). The point of measurement was marked on each subject with semi-permanent ink. During the measurement, participants remained standing with their thigh muscles relaxed. The sum of both the right and left thigh circumferences was used for statistical analysis [29, 30].
Plasma CK activity
A 5 ml venous blood sample was collected from the antecubital vein using a standard venipuncture technique with an ethylene diamine tetra acetic acid (EDTA) tube. Samples were centrifuged for 10 minutes at 1,500g to obtain the plasma. Plasma CK activity was determined spectrophotometrically using a commercial kit (Cobas Mira Plus, Roche, Basel, Switzerland).
Delayed-onset muscle soreness (DOMS)
DOMS was evaluated on a visual analogue scale consisting of a 100mm line with “no soreness” at one end and “extremely painful” at the other [31–33]. Subjects were asked to mark their current level of pain felt in the thigh muscles.
Squat and countermovement jump test
Prior to the jump tests the subjects performed a warm-up comprising 5 minutes of moderate running, 5 minutes of lower limb light stretching exercises, and 20 countermovement (CMJ) submaximal jumps (i.e., 4 sets x 5 CMJ, 30-second interval between sets). Three minutes after the warm-up each subject performed three SJ and three CMJ attempts every 10 seconds with a 1-min interval between jumping techniques. The best jump was used for statistical purposes. All participants were familiarized with both jumping techniques. They were instructed to keep their hands on their waist during the jumps. The SJ was initiated from a static 90° knee angle position, while the CMJ was performed with a preliminary countermovement from 0 to 90° knee angle. All attempts were executed on a contact platform (Smart Jump, Fusion, Australia) and jumping height was calculated using the formula by Bosco et al. [34].
Maximum dynamic strength test (1RM)
Maximum dynamic strength was determined through a 1RM test in the leg press exercise. Subjects performed two familiarization sessions prior to the 1RM test. A 5-min warm-up was performed on a treadmill at 9 km · h-1 followed by 5 minutes of lower limb light stretching exercises. Subsequently, participants performed two leg press warm-up sets: in the first set, they performed 5 repetitions at 50% of the estimated 1RM, and in the second set, they executed 3 repetitions at 70% of the estimated 1RM. A 3-min rest interval was allowed between sets. Three minutes after the warm up, the athletes were allowed up to 5 attempts to obtain the 1RM load (i.e., the maximum weight that could be lifted once using the proper technique), with a 3-min interval between attempts. The 1RM test was performed on a leg press machine (Plyo Press, Athletic Republic, Park City, Utah, USA), and strong verbal encouragement was provided during all attempts.
Plyometric exercise bout
Forty-five minutes after the 1RM test subjects were required to perform 100 consecutive drop-jumps starting from a height of 45 cm [35–37]. A 6-s interval was allowed between jumps, and the participants were encouraged to jump as high as possible.
Statistical analysis
Normality was verified with the Shapiro-Wilk test. A two-way repeated measures ANOVA was used to test for differences between groups in all dependent variables (using IBM SPSS Statistics for Windows, version 20.0. Armonk, NY: IBM Corp.). The effect sizes (ES) were calculated and classified following the scale proposed by Cohen [38]: small (ES from 0.2 to 0.5), moderate (ES from 0.5 to 0.8), and large (ES ≥ 0.8). In the present study, we only reported ES values above 0.5. The significance level was set at p < 0.05. Data are presented as mean ± SD.
RESULTS
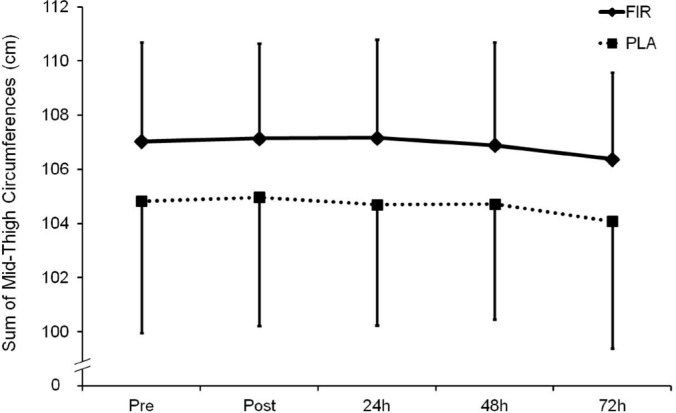
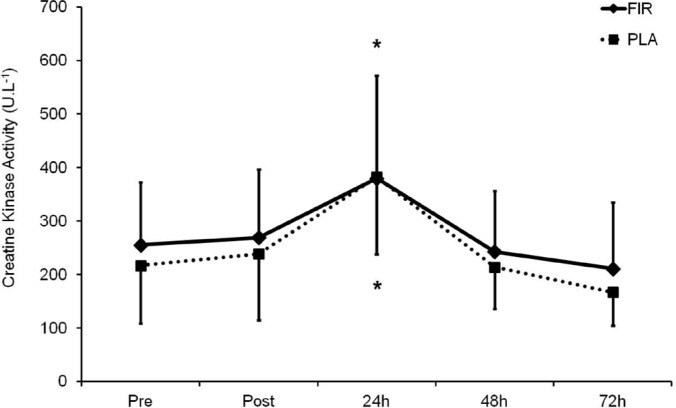
The sum of mid-thigh circumferences demonstrated neither a significant treatment effect nor a time x treatment interaction (Figure 1). Plasma CK activity presented a significant increase 24 h after the plyometric bout (p < 0.05) compared to before, with no significant differences between the groups. At 48 and 72 h the CK activity had returned to initial values (Figure 2).
FIG. 1.
Sum of left and right mid-thigh circumference values before, immediately after (Post), and 24, 48 and 72 h after the plyometric bout.
FIG. 2.
Plasma CK activity values before, immediately after (Post), and 24, 48 and 72 h after the plyometric bout.
Note: * p < 0.05 in relation to initial values.
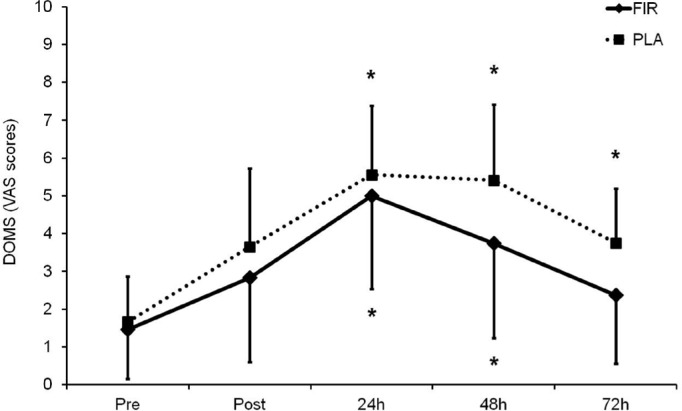
DOMS (VAS score) peaked at the 24 h time-point (Figure 3), without significant differences between groups. However, the PLA group showed significant increases at 24 h, 48 h, and 72 h compared to initial values (p < 0.05), while the FIR group showed significant increases only at 24 h and 48 h (p < 0.05) after the exercise bout. Despite the lack of significant differences between groups, the effect sizes were moderate at the 48 h and large at the 72 h time-points (ES = 0.737 and 0.844, respectively), favouring the FIR-emitting clothes (i.e., less perceived soreness).
FIG. 3.
DOMS (VAS score) before, immediately after (post), and 24, 48 and 72 h after the plyometric bout.
* p < 0.05 in relation to initial values.
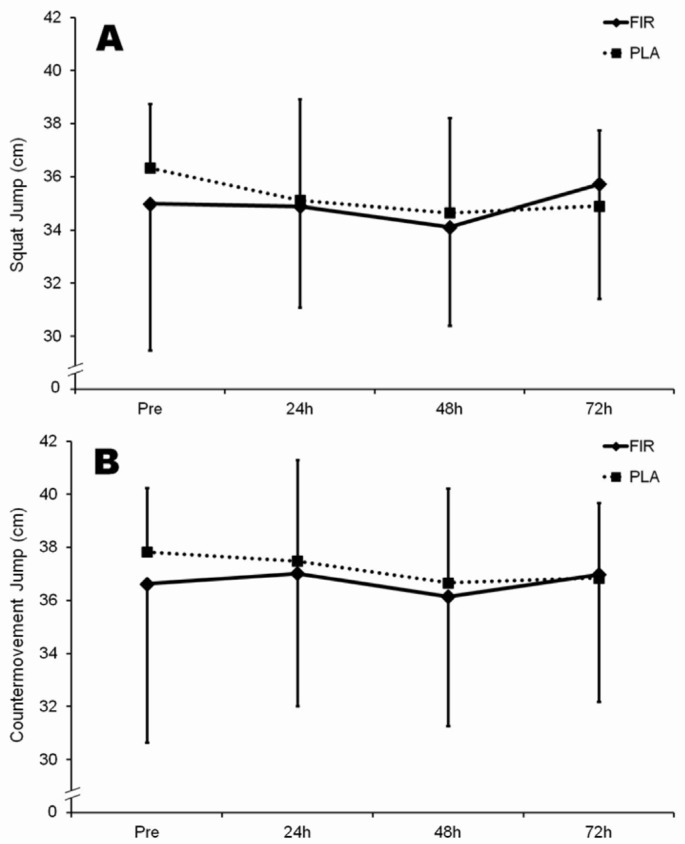
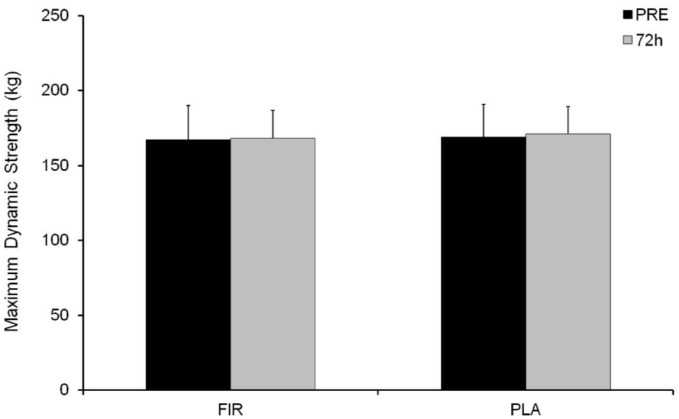
No differences were observed in SJ and CMJ tests or in leg press 1RM values throughout the experimental period (Figure 4A and 4B and Figure 5, respectively).
FIG. 4.
Squat jump (panel A) and countermovement jump (panel B) height values before, and 24, 48 and 72 h after the plyometric bout.
FIG. 5.
Leg press maximum dynamic strength (1RM) values before and 72 h after the plyometric bout.
DISCUSSION
To the best of our knowledge, this is the first study investigating the effectiveness of FIR-emitting clothes on EIMD indirect markers and physical performance recovery after the application of a plyometric exercise bout in highly trained subjects. The only EIMD marker which appeared to change due to the use of FIR-emitting clothes was DOMS at 48 h and 72 h (moderate to large ES, respectively) after the plyometric bout applied to elite soccer players. Indeed, neither isolated time and treatment effects nor time x treatment interactions were observed for vertical jumps (SJ and CMJ) or maximum dynamic strength (leg press 1RM) performances. Thus, it was not possible to identify the potential effect of FIR on players’ physical performance recovery. In addition, the plyometric bout did not cause significant changes in mid-thigh circumference at any time point in either group. The CK activity was also unaffected by the treatment, increasing only at 24 h after exercise. Hence, the actual effects of FIR may have been obscured by the lack of substantial changes in the markers of EIMD.
Conventional FIR treatment with small far-infrared radiators (e.g., lamps, ceramic disks, plaster, and pads) has been shown to be effective for reducing pain in clinical trials [2, 39–41]. For example, Bagnato et al. [39] demonstrated that FIR-emitting plaster applied to osteoarthritis patients 12 h per day resulted in a significant reduction in the VAS score after one week of intervention. Furthermore, Wong et al. [41] showed that 15 minutes of FIR exposure per day for five consecutive days was able to diminish subjective pain sensation and decrease serum interleukin-6 and endothelin-1 concentrations (which are biomarkers of pain) in subjects following total knee arthroplasty. On the other hand, a randomized double-blinded placebo clinical study observed that a neck device containing FIR ceramic particles incorporated into fibres was not more effective than a placebo device in decreasing chronic neck and shoulder muscle pain, after one week of daily treatment [42]. Hausswirth et al. [17] performed a running protocol to induce muscle damage in runners and applied FIR (lamps) immediately after and 24 h and 48 h after exercise. The authors observed that perception of muscle pain was significantly decreased after 48 h in relation to the non-treatment condition [17]. Although the findings of Hausswirth and colleagues [17] in relation to muscle pain may be partially biased due to the absence of blinding, our results suggest that FIR-emitting clothes may provide a faster decrease in pain perception over time, with possible relief from DOMS between 48 and 72 h. Hence, we confirm the results of Hausswirth et al. [17].
Recently, Hausswirth et al. [17] reported that highly trained runners exposed to 30 min of whole body far-infrared radiation were able to recover knee extensor maximum voluntary contractions 48 h after performing an EIMD protocol, comprised of 48 minutes of treadmill running with flat, downhill, and uphill runs. In contrast, we were not able to evaluate FIR effects on players’ physical performance recovery, since there was no significant deterioration in SJ, CMJ, or leg press 1RM performance after the plyometric exercise protocol. It should be mentioned that according to previous investigations [7, 30, 35], the exercise protocol adopted in our study, consisting of 100 vertical drop-jumps, is capable of inducing muscle damage and DOMS even in subjects familiarized with high-intensity activities, including eccentric contractions. Additionally, recreational athletes showed significant decreases in maximum voluntary contraction and increases in muscle oedema and plasma CK activity after performing a plyometric exercise protocol similar to the one adopted in the present study [7, 43]. According to Howatson et al. [7, 30], however, the exercise protocol encompassing 100 drop-jumps could evoke increased levels of CK at 24 h, peak muscle soreness at 48 h and decreased MVC after 24 h, in professional soccer and rugby athletes. However, no significant alteration in jump performance was detected in the present study. Therefore, athletes in our sample appeared to be well trained and adapted and hence resilient to the damaging effects of plyometric exercise.
In accordance with Howatson et al. [30], we also did not find significant differences in mid-thigh circumferences after performing 100 consecutive drop-jumps starting from a height of 45 cm. It is plausible again that the use of highly trained subjects in both studies was able to diminish the muscle damaging effects of the exercise bout. However, the increases in CK activity (24 h after) and DOMS (24, 48, and 72 h after) found in the present study may indicate a mild degree of muscle damage in both experimental groups. No effects on CK activity were observed in FIR-treated individuals. This is in accordance with a previous study which demonstrated that FIR (sauna) applied on three consecutive days did not decrease CK levels in highly-trained endurance runners [17].
One important aspect of the study was the time lapse between the end of the exercise protocol and wearing the FIR clothes. Since one of the main effects of FIR is to improve local microcirculation, we could expect that applying FIR immediately after exercise may increase blood flow and may prompt the development of oedema and leukocyte migration into tissues [44]. Indeed, an in vitro study demonstrated that irradiation of endothelial cells phosphorylated e-NOS and induced NO release after 30 minutes [45]. NO production via e-NOS may result in vasodilatation and a rise in tissue temperature, facilitating neutrophil migration into muscle and activation [46, 47]. The neutrophils are the first line of inflammatory cellular defence, migrating to skeletal muscle as early as 45 minutes after exercise, and are associated with oxidative stress in EIMD [40, 46–48]. To avoid acute effects of FIR in neutrophil migration and to make the treatment more comfortable than wearing clothes during daily activities, we decided to apply FIR during the sleeping period, under more controlled exposure time and ambient conditions.
The anti-inflammatory effects of FIR may be associated with production of NO and increased expression of haem oxygenase 1, resulting in decreased production free radicals and inflammatory mediators [28, 45, 49]. However, many of the anti-inflammatory effects of FIR irradiation may have been lost, since 6 hours after exercise some pro-inflammatory signalling pathways had probably already been activated and increased levels of pro-inflammatory mediators (such as interleukin-1) were expressed in muscle tissue [40, 48]. On the other hand, the time course of changes in inflammatory infiltration show a peak 24 h after EIMD [47]. Therefore applying FIR a few hours after exercise, during sleep, still could down-modulate production of inflammatory mediators which contribute to pain in DOMS. Although the anti-inflammatory properties of FIR may have decreased cell infiltration into muscle, we could not draw any conclusion about its effectiveness in this issue since no muscle biopsy was taken and muscle strength losses were not evident in our study. Finally, our study was limited by the sole use of the VAS scale to evaluate DOMS. Future studies should determine the effects of FIR-emitting clothes on muscle soreness using other methods (e.g., algometry) to confirm our results. Additionally, possible effects of using FIR-emitting clothes on other aspects of recovery (e.g., blood flow and “metabolic” recovery) should also be investigated in future studies, including the long-term use of FIR clothes on training-related adaptations.
CONCLUSIONS
In conclusion, it appears that elite soccer players are resilient to the effects of EIMD after 100 drop-jumps, since lower body strength and power performance were not negatively affected. The use of FIR-emitting clothes during a 10 h sleeping period over three successive nights may have contributed to reduced DOMS at 48 h and 72 h after (moderate and large effect sizes, respectively) the plyometric exercise bout. Therefore, we suggest that FIR clothes may be used to accelerate recovery of muscle pain after eccentrically-biased exercises in soccer players. Furthermore, the use of recovery clothes could be practical and advantageous for professional athletes, since this method does not affect the training routine and can be easily implemented. Pain reduction may be beneficial in improving training quality by means of increased loading and reduced injury risks. Future studies are needed to determine the possible effects of FIR clothes on the attenuation of EIMD markers in less trained subjects (including muscle pain) or in athletes with more pronounced loss of muscle function due to higher intensity/volume of eccentrically-biased exercises, using other quantitative measures of DOMS and metabolic/functional recovery.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Comfort P, Stewart A, Bloom L, Clarkson B. Relationships between strength, sprint, and jump performance in well-trained youth soccer players. J Strength Cond Res. 2014;28(1):173–177. doi: 10.1519/JSC.0b013e318291b8c7. [DOI] [PubMed] [Google Scholar]
- 2.Sander A, Keiner M, Wirth K, Schmidtbleicher D. Influence of a 2-year strength training programme on power performance in elite youth soccer players. Eur J Sport Sci. 2013;13(5):445–451. doi: 10.1080/17461391.2012.742572. [DOI] [PubMed] [Google Scholar]
- 3.Loturco I, Ugrinowitsch C, Tricoli V, Pivetti B, Roschel H. Different loading schemes in power training during the preseason promote similar performance improvements in Brazilian elite soccer players. J Strength Cond Res. 2013;27(7):1791–1797. doi: 10.1519/JSC.0b013e3182772da6. [DOI] [PubMed] [Google Scholar]
- 4.Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power:part 2 - training considerations for improving maximal power production. Sports Med. 2011;41(2):125–146. doi: 10.2165/11538500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Vaczi M, Tollar J, Meszler B, Juhasz I, Karsai I. Short-term high intensity plyometric training program improves strength, power and agility in male soccer players. J Hum Kinet. 2013;36:17–26. doi: 10.2478/hukin-2013-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hody S, Rogister B, Leprince P, Wang F, Croisier JL. Muscle fatigue experienced during maximal eccentric exercise is predictive of the plasma creatine kinase (CK) response. Scand J Med Sci Sports. 2013;23(4):501–507. doi: 10.1111/j.1600-0838.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 7.Howatson G, Goodall S, van Someren KA. The influence of cold water immersions on adaptation following a single bout of damaging exercise. Eur J Appl Physiol. 2009;105(4):615–621. doi: 10.1007/s00421-008-0941-1. [DOI] [PubMed] [Google Scholar]
- 8.Marklund P, Mattsson CM, Wahlin-Larsson B, Ponsot E, Lindvall B, Lindvall L, Ekblom B, Kadi F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol. 2013;114(1):66–72. doi: 10.1152/japplphysiol.01538.2011. [DOI] [PubMed] [Google Scholar]
- 9.Molina R, Denadai BS. Dissociated time course recovery between rate of force development and peak torque after eccentric exercise. Clin Physiol Funct Imaging. 2012;32(3):179–184. doi: 10.1111/j.1475-097X.2011.01074.x. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen G, Egner I, Raastad T, Reinholt F, Owe S, Lauritzen F, Brorson SH, Koskinen S. Inflammatory markers CD11b, CD16, CD66b, CD68, myeloperoxidase and neutrophil elastase in eccentric exercised human skeletal muscles. Histochem Cell Biol. 2013;139(5):691–715. doi: 10.1007/s00418-012-1061-x. [DOI] [PubMed] [Google Scholar]
- 11.Silva LA, Pinho CA, Silveira PC, Tuon T, De Souza CT, Dal-Pizzol F, Pinho RA. Vitamin E supplementation decreases muscular and oxidative damage but not inflammatory response induced by eccentric contraction. J Physiol Sci. 2010;60(1):51–57. doi: 10.1007/s12576-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan DL, Allen DG. Early events in stretch-induced muscle damage. J Appl Physiol. 1999;87(6):2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- 13.Reilly T, Atkinson G, Edwards B, Waterhouse J, Farrelly K, Fairhurst E. Diurnal variation in temperature, mental and physical performance, and tasks specifically related to football (soccer) Chronobiol Int. 2007;24(3):507–519. doi: 10.1080/07420520701420709. [DOI] [PubMed] [Google Scholar]
- 14.Andersson H, Raastad T, Nilsson J, Paulsen G, Garthe I, Kadi F. Neuromuscular fatigue and recovery in elite female soccer: effects of active recovery. Med Sci Sports Exerc. 2008;40(2):372–380. doi: 10.1249/mss.0b013e31815b8497. [DOI] [PubMed] [Google Scholar]
- 15.De Nardi M, La Torre A, Barassi A, Ricci C, Banfi G. Effects of cold-water immersion and contrast-water therapy after training in young soccer players. J Sports Med Phys Fitness. 2011;51(4):609–615. [PubMed] [Google Scholar]
- 16.Finberg M, Braham R, Goodman C, Gregory P, Peeling P. Effects of electrostimulation therapy on recovery from acute team-sport activity. Int J Sports Physiol Perform. 2013;8(3):293–299. doi: 10.1123/ijspp.8.3.293. [DOI] [PubMed] [Google Scholar]
- 17.Hausswirth C, Louis J, Bieuzen F, Pournot H, Fournier J, Filliard JR, Brisswalter J. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS One. 2011;6(12):e27749. doi: 10.1371/journal.pone.0027749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedelec M, McCall A, Carling C, Legall F, Berthoin S, Dupont G. Recovery in soccer:part ii-recovery strategies. Sports Med. 2013;43(1):9–22. doi: 10.1007/s40279-012-0002-0. [DOI] [PubMed] [Google Scholar]
- 19.Pruscino CL, Halson S, Hargreaves M. Effects of compression garments on recovery following intermittent exercise. Eur J Appl Physiol. 2013;113(6):1585–1596. doi: 10.1007/s00421-012-2576-5. [DOI] [PubMed] [Google Scholar]
- 20.Versey NG, Halson SL, Dawson BT. Water immersion recovery for athletes: effect on exercise performance and practical recommendations. Sports Med. 2013;43(11):1101–1130. doi: 10.1007/s40279-013-0063-8. [DOI] [PubMed] [Google Scholar]
- 21.Beever R. Far-infrared saunas for treatment of cardiovascular risk factors:summary of published evidence. Can Fam Physician. 2009;55(7):691–696. [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue S, Takemoto M, Chishaki A, Ide T, Nishizaka M, Miyazono M, Sawatari H, Sunagawa K. Leg heating using far infra-red radiation in patients with chronic heart failure acutely improves the hemodynamics, vascular endothelial function, and oxidative stress. Intern Med. 2012;51(17):2263–2270. doi: 10.2169/internalmedicine.51.7115. [DOI] [PubMed] [Google Scholar]
- 23.Vatansever F, Hamblin MR. Far infrared radiation (FIR):its biological effects and medical applications. Photonics Lasers Med. 2012;4:255–266. doi: 10.1515/plm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conrado LA, Munin E. Reduction in body measurements after use of a garment made with synthetic fibers embedded with ceramic nanoparticles. J Cosmet Dermatol. 2011;10(1):30–35. doi: 10.1111/j.1473-2165.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Ko GD, Berbrayer D. Effect of ceramic-impregnated „thermoflow” gloves on patients with Raynaud's syndrome: randomized, placebo-controlled study. Altern Med Rev. 2002;7(4):328–335. [PubMed] [Google Scholar]
- 26.Hsu YH, Chen YC, Chen TH, Sue YM, Cheng TH, Chen JR, Chen CH. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS One. 2012;7(1):e30674. doi: 10.1371/journal.pone.0030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung TK, Lee CM, Tsai SY, Chen YC, Chao JS. A Pilot Study of Ceramic Powder Far-Infrared Ray Irradiation (cFIR) on Physiology: Observation of Cell Cultures and Amphibian Skeletal Muscle. Chin J Physiol. 2011;54(4):247–254. doi: 10.4077/CJP.2011.AMM044. [DOI] [PubMed] [Google Scholar]
- 28.Tu YP, Chen SC, Liu YH, Chen CF, Hour TC. Postconditioning with far-infrared irradiation increases heme oxygenase-1 expression and protects against ischemia/reperfusion injury in rat testis. Life Sci. 2013;92(1):35–41. doi: 10.1016/j.lfs.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Goodall S, Howatson G. The effects of multiple cold water immersions on indices of muscle damage. J Sports Sci Med. 2008;7(2):235–241. [PMC free article] [PubMed] [Google Scholar]
- 30.Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids:a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr. 2012;9:20. doi: 10.1186/1550-2783-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br J Sports Med. 1992;26(4):267–272. doi: 10.1136/bjsm.26.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaile JM, Gill ND, Blazevich AJ. The effect of contrast water therapy on symptoms of delayed onset muscle soreness. J Strength Cond Res. 2007;21(3):697–702. doi: 10.1519/R-19355.1. [DOI] [PubMed] [Google Scholar]
- 33.Willoughby DS, McFarlin B, Bois C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24(1):15–21. doi: 10.1055/s-2003-37197. [DOI] [PubMed] [Google Scholar]
- 34.Bosco C, Tihanyi J, Komi PV, Fekete G, Apor P. Store and recoil of elastic energy in slow and fast types of human skeletal muscles. Acta Physiol Scand. 1982;116(4):343–349. doi: 10.1111/j.1748-1716.1982.tb07152.x. [DOI] [PubMed] [Google Scholar]
- 35.Miyama M, Nosaka K. Influence of surface on muscle damage and soreness induced by consecutive drop jumps. J Strength Cond Res. 2004;18(2):206–211. doi: 10.1519/R-13353.1. [DOI] [PubMed] [Google Scholar]
- 36.Nosaka K, Kuramata T. Muscle soreness and serum enzyme activity following consecutive drop jumps. J Sports Sci. 1991;9(2):213–220. doi: 10.1080/02640419108729882. [DOI] [PubMed] [Google Scholar]
- 37.Skurvydas A, Dudoniene V, Kalvenas A, Zuoza A. Skeletal muscle fatigue in long-distance runners, sprinters and untrained men after repeated drop jumps performed at maximal intensity. Scand J Med Sci Sports. 2002;12(1):34–39. doi: 10.1034/j.1600-0838.2002.120107.x. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 39.Bagnato GL, Miceli G, Atteritano M, Marino N, Bagnato GF. Far infrared emitting plaster in knee osteoarthritis:a single blinded, randomised clinical trial. Reumatismo. 2012;64(6):388–394. doi: 10.4081/reumatismo.2012.388. [DOI] [PubMed] [Google Scholar]
- 40.Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265(1 Pt. 2):R166–172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 41.Wong CH, Lin LC, Lee HH, Liu CF. The analgesic effect of thermal therapy after total knee arthroplasty. J Altern Complement Med. 2012;18(2):175–179. doi: 10.1089/acm.2010.0815. [DOI] [PubMed] [Google Scholar]
- 42.Lai CH, Leung TK, Peng CW, Chang KH, Lai MJ, Lai WF, Chen SC. Effects of far-infrared irradiation on myofascial neck pain: a randomized, double-blind, placebo-controlled pilot study. J Altern Complement Med. 2014;20(2):123–129. doi: 10.1089/acm.2013.0122. [DOI] [PubMed] [Google Scholar]
- 43.Skurvydas A, Brazaitis M, Venckunas T, Kamandulis S. Predictive value of strength loss as an indicator of muscle damage across multiple drop jumps. Appl Physiol Nutr Metab. 2011;36(3):353–360. doi: 10.1139/h11-023. [DOI] [PubMed] [Google Scholar]
- 44.Yang CS, Yeh CH, Tung CL, Chen MY, Jiang CH, Yeh ML. Impact of far-infrared ray exposure on the mechanical properties of unwounded skin of rats. Exp Biol Med (Maywood). 2010;235(8):952–956. doi: 10.1258/ebm.2010.009372. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Lee S, Cho DH, Park YM, Kang DH, Jo I. Far-infrared radiation acutely increases nitric oxide production by increasing Ca2+ mobilization and Ca2+/calmodulin-dependent protein kinase II-mediated phosphorylation of endothelial nitric oxide synthase at serine 1179. Biochem Biophys Res Commun. 2013;436(4):601–606. doi: 10.1016/j.bbrc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Kanda K, Sugama K, Hayashida H, Sakuma J, Kawakami Y, Miura S, Yoshioka H, Mori Y, Suzuki K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. 2013;19:72–85. [PubMed] [Google Scholar]
- 47.Paulsen G, Crameri R, Benestad HB, Fjeld JG, Morkrid L, Hallen J, Raastad T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc. 2010;42(1):75–85. doi: 10.1249/MSS.0b013e3181ac7adb. [DOI] [PubMed] [Google Scholar]
- 48.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells:what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 49.Lin CC, Liu XM, Peyton K, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol. 2008;28(4):739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]