Abstract
Laboratory evidence supports the notion that dehydration degrades exercise performance and impairs certain cognitive processes. The purpose of this study is to examine the effect of a voluntary versus a dictated drinking condition on exercise and cognitive performance. The study used a double-blind and paired design. Twenty male and female college students (10 women, 10 men) participated in an exercise protocol consisting of 1 hr of treadmill running followed by a high intensity portion continuing until voluntary exhaustion. The dictated drinking condition consisted of 900 ml of water equally distributed in 4 pre-prepared opaque bottles. At 15 min intervals the subject was instructed to drink the entire contents until the end of the 1 hr treadmill protocol. The voluntary drinking condition consisted of 225 ml of water within arm's reach of the subjects while on the treadmill. Exercise performance was significantly better (longer duration and faster speed) in the voluntary condition compared with the dictated condition. Cognitive test outcomes were not significantly different between drinking conditions. A difference in fluid absorption is a potential source of exercise impairment seen in the dictated fluid condition. The higher fluid consumption rate presumably would cause greater gastric and esophageal distention resulting in the diversion of blood flow from working muscles to the gastrointestinal system. In situations where dehydration is likely, drinking to recommended guidelines may protect individuals from dehydration and its negative effects. However, when dehydration is not likely, allowing an individual to follow voluntary drinking behavior is preferable for exercise performance.
Keywords: Cognition, Dehydration, Drinking Behavior, Hot Temperature, Physiology
INTRODUCTION
Over the last 40 years dehydration has become a topic of attention for individuals at all fitness levels exercising at varied intensities and durations. Over this time laboratory studies have been conducted that seemingly support dehydration as a causative agent in aerobic exercise impairment [1–5]. This attention on fluid consumption and fluid replacement has presumably been prompted by the laboratory data that supports a loss of 2% body weight due to fluid loss associated with a decrement in aerobic performance. Because of this focus on hydration, athletic organizations, public health associations, educational institutions, and corporations have issued hydration recommendations. The recommendations often provide information on fluid consumption with respect to three different time points namely; pre-exercise, during exercise, and post-exercise. There is general uniformity on the inclusion of these three time points, however, some statements provide information on specific fluid amounts and specific timing, while others are vague and often disregard ambient conditions, current hydration status, and thirst. An example of hydration guidelines provided by Livestrong.com directs athletes to drink prior to and after exercising and that during exercise athletes should consume 1 cup of water (236 ml) every 15 minutes.
The majority of laboratory evidence supports the notion that dehydration degrades aerobic exercise performance [1–4, 6]. The evidence supports a dose-response relationship between the magnitude of fluid loss and performance impairment. This impairment has been described in multiple studies using various exercise modalities in both temperate and warm environments. Neilson et al. [7] reported lower total work performed on a cycle ergometer in dehydrated subjects compared to when euhydrated. The dehydrated condition for these subjects was achieved through heat exposure, prior exercise, or use of diuretics. In a study by Cheuvront et al. [4] subjects dehydrated (3% change in body mass) through heat exposure and prior exercise completed less work in a 30 min time trial on a cycle ergometer than when euhydrated.
Impairment attributed to dehydration is not limited to exercise performance. Studies examining cognitive changes in response to exercise interventions have also reported impairments when dehydration is a component of the study [8, 9]. Similar to exercise performance impairment, a loss of > ∼2% body mass is commonly reported as the threshold for this cognitive impairment [10]. A similar dose-response relationship has been observed. Cian et al. [9] examined the effect of dehydration, achieved through two different protocols on cognition. Both dehydration methods led to a significant decrease in a psychomotor tracking task and a test of short-term memory.
Observational studies, however, demonstrate that competing athletes may lose more than the 2% body mass and not suffer the deleterious effects reported in laboratory studies [11]. These observational studies also have reported on the apparent potential health risks of consuming too much fluid. This observation has led to a growing concern that athletes can drink excess fluid. Excessive drinking beyond or irrespective of thirst can have serious health consequences including coma and death. Excess fluid consumption has led people to suffer from exercise induced hyponatremia (EAH) [12]. The incidence of EAH is largely unknown; however, with athletes overly concerned with dehydration it is easy to speculate that over-zealous consumption of fluid is possible. In a case report by Dugas and Noakes [13] a female cyclist competing in a 109 km cycle tour consumed 735 ml · hr-1 of fluid. At the end of the event the cyclist's weight increased 2.4 kg and she complained of confusion and the inability to concentrate. Despite the data from these observational studies an athlete would be hard pressed to find recommendations that reflect these results. With little effort an individual can find drinking recommendations that ignore environmental conditions and provide vague fluid amounts and time course information and no mention of thirst.
The purpose of this study is to examine the effect of a voluntary drinking condition versus a dictated drinking condition on exercise and cognitive performance. Our study is a novel approach to hydration research, in that it is comparing voluntary drinking behaviour to a dictated drinking condition. This dictated drinking condition is given irrespective of thirst which corresponds with a prevailing omission in hydration recommendations for fluid consumption during exercise. While the dictated drinking amounts and timing of fluid consumption in this research do not follow any one specific guideline recommendation; they do fall within a wide range of recommendations that are readily available.
MATERIALS AND METHODS
Subjects
The study used a double-blind and paired design. The subjects were twenty 18-25 year old State University of New York at Fredonia male and female college students (10 women, 10 men). The research protocol was approved for human subjects by Fredonia Human Subject Review Board and the experiments reported were performed in accordance with the ethical standards of the Helsinki Declaration. Subjects were deemed "apparently healthy" when it was determined that they were free of signs and symptoms of cardiovascular and pulmonary disease and met the criteria for the American College of Sports Medicine (ACSM) low risk stratification for coronary artery disease [14]. Exclusionary criteria also included: diagnosed learning disability, a concussion in the preceding six months (since both learning disabilities and concussion could impact cognitive function), and lastly, the use of medication that could influence cognitive performance, including painkillers, antidepressants, etc. Female subjects were screened for pregnancy and excluded if tested positive. Females actively taking birth control were not excluded; menstrual cycle has been reported to have a minimal effect on cognition [15, 16].
Screening Laboratory Visit
After subjects read and signed the approved Human Subjects Consent Form a questionnaire was completed. Resting heart rate, resting blood pressure, height, and weight were measured. Body composition was assessed via 7-site skin caliper measurements [17], using Lange skin calipers (Beta Technology Inc., Santa Cruz, CA). Screening data was used to determine subject eligibility in meeting ACSM low risk stratification for coronary artery disease. Female subjects provided a urine sample for pregnancy screening (hCG ACON laboratories Inc., San Diego, CA).
After the screening process each subject completed a Modified Bruce Treadmill test in order to determine their maximal oxygen consumption [14]. Maximal oxygen consumption was determined in order to evaluate the subject's aerobic fitness. Throughout the treadmill protocol heart rate was continually monitored and recorded with a Polar heart rate monitor (Woodbury, NY). Borg Scale rate of perceived exertion was recorded at the end of each stage of the protocol [18].
Second and Third Laboratory Visits
Subjects were instructed (for both 2nd and 3rd visits) to refrain from eating, drinking or urinating prior to reporting to the lab on the morning of testing. Subjects were asked to supply a urine sample for specific gravity determination. Urine specific gravity and naked dry body weight data was collected both prior to and immediately after completion of the exercise protocol. Prior to arrival, subjects were randomly assigned a condition of voluntary drinking or dictated drinking. The water was measured and prepared in identical opaque bottles prior to the subject arriving at the lab and out of view of the principal investigator. The principal investigator was not allowed to handle the bottle.
Exercise Protocol
The exercise protocol consisted of 1hr of treadmill running. The male subjects ran at a speed of 8.9kph @ a 2% grade while the female subjects ran at 8.4 kph @ a 2% grade. At the completion of 60 minutes the subject started the high intensity/voluntary exhaustion portion of the protocol. This portion started at 12.1 kph for 90 s and increased in speed by 0.8 kph increments every additional 90 s until voluntary exhaustion was reached [19]. Elapsed time and terminal speed was recorded. Terminal speed was defined as the speed at which the subject reached at voluntary exhaustion irrespective of the duration of time running at that speed.
Drinking Conditions
The dictated drinking condition consisted of 225 ml of water in pre-prepared opaque bottles. At 15 min intervals the subject was handed a bottle and instructed to drink the entire contents. This process was repeated every 15 min until the end of the 1 hr treadmill protocol. The voluntary drinking condition consisted of available 225 ml of water in opaque bottles that were within arm's reach of the subjects while on the treadmill. The subject was not prompted to drink in this condition. At regular intervals, irrespective of fluid consumed, the bottles were replaced with new bottles in order to mimic the condition of bottle replacement seen in the dictated drinking condition. Subjects were blinded to the drinking conditions. In the dictated drinking condition subjects were handed newly prepped with 225ml for each time point and instructed to drink all the water in the bottle. The voluntary condition the subjects were told water was available and placed within reach of the subject. The bottles used were identical to the dictated drinking condition and prepped with 225ml of water.
Cognitive Testing
At 15, 30, 45 and 60 min time points (post drinking in the dictated condition) a matched and unmatched Stroop test was administered to the subjects while on the treadmill. The Stroop test was presented to the subjects via a projector located behind the subjects and projected on a screen placed in front of the treadmill. The matched Stroop test starts as a presentation of a 5x5 word grid where the color of the word is matched the denotation of the word. This was immediately followed by the unmatched Stroop test, which is a 5x5 word grid where the color of the word is not matched to its denotation. The Stroop test is a cognitive test of executive processes in cognition [20].
Statistics
Paired samples t-tests were used to compare exercise performance (elapsed time and terminal speed) and cognitive performance (Stroop test interference scores) between the drinking conditions. The interference score used for the Stroop test is the time to complete the unmatched test minus the time to complete matched test in seconds [21]. Urine specific gravity was evaluated for no change as treatments were not expected to effect a change. Urine specific gravity was not normally distributed; therefore a paired sign test was used to compare within (before and after exercise) and between drinking conditions. False discovery rate [22, 23] was used to determine the significance level for comparison correcting for multiple testing. Results are given as means and standard deviations.
RESULTS
Subject Demographics
The overall averages for the study population were 21.2 ± 1.2 years old, 172.9 ± 7.4 cm tall, 70.6 ± 7.5 kilograms, 12.1 ± 4.2 percent body fat, and an average VO2max of 58.2 ± 7.8 ml · kg-1 · min-1 (Table 1). The averages for the female subjects were 21.3 ± 1.4 years old, 168.4 ± 4.1 cm tall, 65.0 ±5.3 kilograms, 16.3 ± 3.3 percent body fat, and an average VO-2max of 54.7 ± 5.2 ml · kg-1 · min-1 (Table 1). The averages for the male subjects were 21.1 ± 2.0 years old, 177.3 ± 8.6 cm tall, 75.2 ± 6.0 kilograms, 10.9 ± 4.1 percent body fat, and an average VO2max of 61.4 ± 6.7 ml · kg-1 · min-1 (Table 1).
TABLE 1.
Subject demographic data.
| Variable | Combined | Female (n = 10) |
Male (n = 10) |
|---|---|---|---|
| Age (years) | 21.2 ± 1.2 | 21.3 ± 1.4 | 21.1 ± 2.0 |
| Height (cm) | 172.9 ± 7.4 | 168.4 ± 4.1 | 177.3 ± 8.6 |
| Weight (kg) | 70.6 ± 7.5 | 65.0 ± 5.3 | 75.2 ± 6.0 |
| Body Fat(%) | 12.1 ± 4.2 | 16.3 ± 3.3 | 10.9 ± 4.1 |
| VO2max (ml · kg-1 · min-1) | 58.2 ± 7.8 | 54.7 ± 5.2 | 61.4 ± 6.7 |
Note: Values expressed as means ± SD.
Urine Specific Gravity and Voluntary Drinking
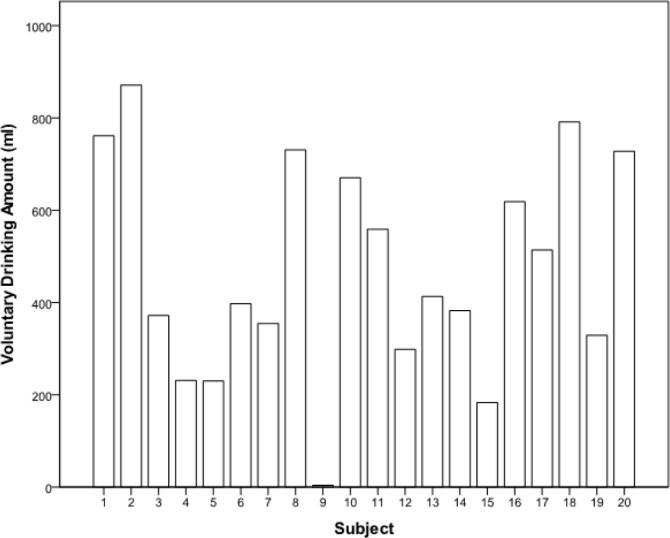
Urine specific gravity data was not different before or after exercise within drinking condition or between drinking conditions (Sign tests, P > 0.05). Voluntary drinking rates were highly variable (Fig 1). The mean voluntary drinking amount was 471.86 ml, standard deviation was 235.93 ml, and range was 867.50 ml.
FIG. 1.
B Voluntary drinking amounts in ml by subject number.
Cognition
Cognition measured by Stroop test interference score was not significantly different between dictated and voluntary drinking conditions (Paired samples t-test, t = 0.56, df = 19, P = 0.582). Stroop interference mean scores were 7.47 ± 2.02 s in dictated drinking and 7.25 ± 2.26 s in voluntary drinking conditions.
Exercise
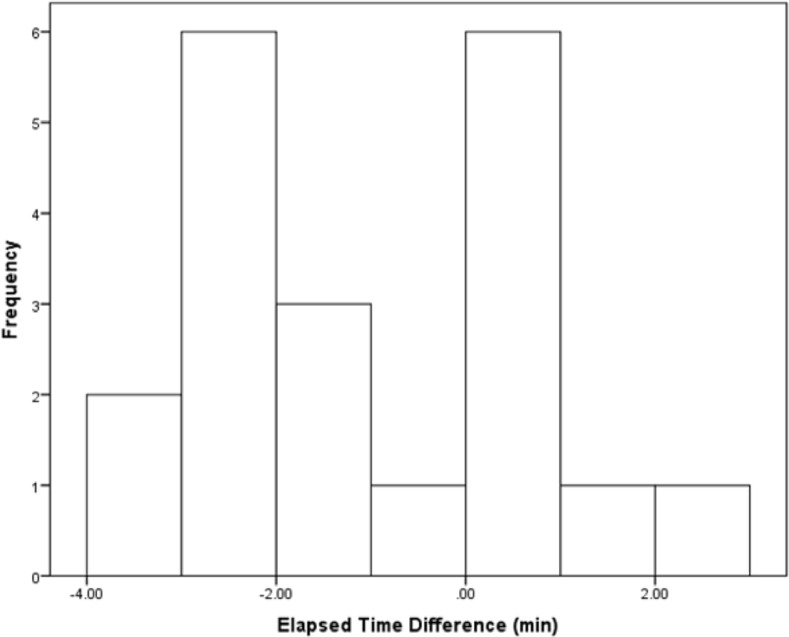
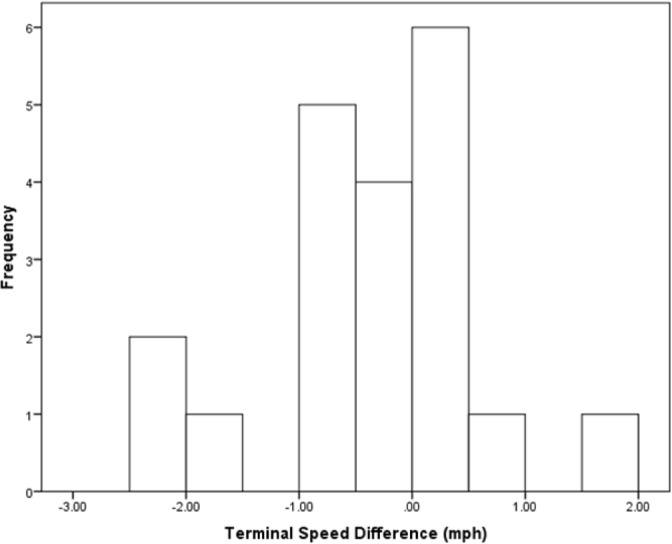
Exercise was negatively impacted by the dictated drinking condition. Elapsed time was significantly shorter in the dictated drinking condition (Paired samples t-test, t = -2.89, df = 19, P = 0.009). Elapsed mean times were 62.72 ± 2.91 min in dictated drinking and 63.80 ± 3.08 min in voluntary drinking conditions. Terminal speed was significantly slower in the dictated drinking condition (Paired samples t-test, t = -2.61, df = 19, P = 0.017). Terminal mean speeds were 7.61 ± 1.30 mph in dictated drinking and 8.19 ± 1.19 mph in voluntary drinking conditions. Histograms illustrate the significant difference (dictated drinking condition minus voluntary drinking condition) in elapsed mean times (Fig. 2) and terminal mean speeds (Fig. 3).
FIG. 2.
Elapsed mean time difference (min) of subjects. Difference values are elapsed mean time in the dictated drinking condition minus elapsed mean time in the voluntary drinking condition for each subject. The difference was significant (P = 0.009).
FIG. 3.
Terminal mean speed difference (mph) of subjects. Difference values are terminal mean speed in the dictated drinking condition minus terminal mean speed in the voluntary drinking condition. The difference was significant (P = 0.017).
DISCUSSION
Our study demonstrated that exercise performance was significantly better in the voluntary drinking condition compared with the dictated drinking condition. It is unclear what mechanism would result in better performance in the voluntary drinking condition or conversely impaired performance in the dictated drinking condition. The treadmill protocol used in this study included moderate and high intensity running that terminated at voluntary exhaustion. In the dictated drinking condition, the subjects voluntarily terminated exercise earlier at lower speeds.
It is possible that the increased weight of the ingested fluid influenced performance. The maximum possible added weight would be 0.9 kg if a subject ingested 0 ml of water in the voluntary condition and 900 ml in the dictated condition. Taking into account the average fluid consumed in the voluntary condition, 471.86 ml, and the fluid consumed in the dictated condition, 900 ml, the added mass based on these values would be 0.43 kg. This mass would have been added across the 60 minutes of the exercise protocol which makes it unlikely to be a factor in the exercise performance impairment.
Gastric discomfort could have led subjects to terminate exercise earlier and at lower speeds. A study by Backx, Van Someren, and Palmer [24] reported fluid consumption did not alter cycling performance in subjects consuming three different fluid volumes ranging from 40 to 300 ml despite subjects reporting different levels of "stomach fullness". It is possible that the exercise mode, running versus cycling, could result in a different level of gastric discomfort. A study by Shi [25] did find gastric discomfort with fluid consumption during intermittent running exercise. The fluid consumed in their study however was an 8% carbohydrate and electrolyte solution. The subjects in our study were given a gastrointestinal distress questionnaire; however, gastrointestinal distress was not reported by any subject.
A difference in fluid absorption by the small and large intestine between fluid conditions is a potential source of exercise impairment seen in the dictated fluid condition. Palma, Vidon et al. [26], reported maximum intestinal absorption rates of 600 ml · hr-1 of fluid and infused fluid rates higher than that value resulted in diarrhea. The dictated fluid condition exceeded this rate by 50% (900 ml · hr-1) whereas the mean voluntary volume was only 472 ml · hr-1. Furthermore, an observational study by Noakes et al. [27] found that athletes not directed to drink or follow drinking guidelines, drank at rates between 400 to 800 ml · hr-1. It is therefore notable that consuming 900 ml of fluid over a 1 hr period resulted in exercise terminating at lower speeds and shorter durations. The higher fluid consumption rate presumably would cause greater gastric and esophageal distention that would result in the diversion blood flow from working muscles to the gastrointestinal system. Also a recent study by Jeukendrup and Chambers [28] investigated the oral sensing of carbohydrates in order to provide an ergogenic exercise effect. This suggests a central nervous system role and not strictly a metabolic component. It is possible that the stretching of the esophagus and gastrointestinal organs in the dictated fluid condition involves the central nervous system that results in a cascade of physiological events that impacts exercise negatively.
Stroop test performance was not significantly different between drinking conditions. This is not a contradictory conclusion to other studies that have examined the relationship between dehydration and cognitive tests [29, 30]. Our subjects did not reach a clinical level of dehydration, so no cognitive impairment was expected. Studies that seemingly demonstrated a cognitive impairment related to dehydration may be confounded by the omission of a properly controlled voluntary drinking condition [31]. Furthermore, the range of variability in voluntary drinking amounts demonstrates that a rigid one size fits all type guidelines are not applicable to everyone and that allowing an individual to drink voluntarily is adequate to prevent impairment of cognitive function.
This study is a novel approach to drinking and dehydration studies because of its inclusion of the voluntary drinking condition. Few laboratory controlled studies have investigated the voluntary drinking behaviors of exercising athletes. Studies that have included a voluntary drinking condition have focused on making comparisons of taste (CHO and salt content) [32, 33]. Conversely typical hydration research compares drinking or hydrated athletes to either athletes drinking nothing or athletes already in a state of dehydration. The studies have employed the use of thermal stress, prior exercise, and /or diuretics to achieve the dehydrated condition [4]. Consequently, and predictably, these laboratory studies have demonstrated that dehydration can have a negative impact on both exercise performance and cognition when compared to a dictated drinking condition. Most of these laboratory dehydration studies, however, fail to include a voluntary drinking condition [34].
Few laboratory controlled studies have investigated the voluntary drinking behaviors of exercising athletes. Research has been done on the impact of athletes exercising while drinking dictated fluid volumes compared to either athletes drinking nothing or athletes already in a state of dehydration. These studies have employed the use of thermal stress, prior exercise, and /or diuretics to achieve the dehydrated condition. Consequently, and predictably, these laboratory studies have demonstrated that dehydration can have a negative impact on both exercise performance and cognition when compared to a dictated drinking condition. Most of these laboratory dehydration studies, however, fail to include a voluntary drinking condition. This study is a novel approach to drinking and dehydration studies because of its inclusion of the voluntary drinking condition.
Human drinking behavior is complex and multifaceted; therefore it is not surprising that voluntary drinking rates of the subjects were highly variable. It cannot be assumed that subjects engaged in voluntary drinking in response to the physiological stimulus of thirst. Thirst is a physiological drive that may be eliminated by the reversal of its original stimulus, such as an increase in plasma osmolality, however, other factors may also reduce or eliminate thirst. Factors such as moisture in the oral cavity, the physiological stress of exercise as well as esophageal and gastrointestinal distension from drinking may impact thirst and alter drinking behavior. All subjects in our study exercise regularly and therefore may have established drinking routines which must be considered as an influence on drinking behavior. Since a few subjects exhibited rates of voluntary fluid consumption that approached the dictated drinking condition, it can be speculated that these subjects engaged in habitual drinking. This habitual drinking behavior may be influenced by awareness of the aforementioned drinking guidelines or a personal preference based on their perceived fluid needs. In the scope of this study it was impossible to distinguish thirst from habitual drinking patterns.
Future research should examine the role the central nervous system plays in response to fluid consumption, including the stimulation oral sensory apparatus, gastric and esophageal distention, especially in exercise lasting approximately 1 hr. Despite none of the subjects reporting gastrointestinal distress in our study, it is likely that the dictated drinking condition imposed a distinct difference in gastric distention since this condition exceeded the upper limit of fluid absorption rate of the human gastrointestinal system as reported by Palma, Vidon, and Bernier (1981). Additional cognitive research should also include different types of cognitive tests as our results are limited to executive processes involved in Stroop test performance.
CONCLUSIONS
Our study is a novel in its approach to traditional fluid consumption and exercise performance studies because of the inclusion of a voluntary drinking condition. Voluntary drinking resulted in improved exercise performance and no significant difference in cognitive performance (based on Stroop test) compared to the dictated drinking condition. In situations where dehydration is likely, drinking to recommended guidelines may protect individuals from dehydration and its negative effects. However, when dehydration is not likely, allowing an individual to follow voluntary drinking behavior is preferable for exercise performance.
Acknowledgements
The authors thank the following Fredonia Exercise Science students for their research efforts: Andrew Danielson, Kyle Hunt, Julia Roszak, Jordan Simone, and Samantha Smithgall.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Barr SI, Costill DL, Fink WJ. Fluid replacement during prolonged exercise: the effects of water, saline or no fluid. Med Sci Sports Exerc. 1991;23:811–17. [PubMed] [Google Scholar]
- 2.Below PR, Mora-Rodriguez R, Gonzalez-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc. 1995;27:200–210. [PubMed] [Google Scholar]
- 3.Caldwell JE, Ahonen E, Nousiainen U. Differential effects of sauna-, diuretic-, and exercise induced hypohydration. J Appl Physiol. 1984;57:1018–23. doi: 10.1152/jappl.1984.57.4.1018. [DOI] [PubMed] [Google Scholar]
- 4.Cheuvront SN, Kenefick RW, Montain SJ, Sawka MN. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol. 2010;109:1989–95. doi: 10.1152/japplphysiol.00367.2010. [DOI] [PubMed] [Google Scholar]
- 5.Fallowfield JL, Williams C, Booth J, Choo BH. Effect of water ingestion on endurance capacity during prolonged running. J Sports Sci. 1996;14:497–502. doi: 10.1080/02640419608727736. [DOI] [PubMed] [Google Scholar]
- 6.Cheuvront SN, Carter R, Sawka MN. Hypohydration impairs endurance exercise performance temperate but not cold air. J Appl Physiol. 2005;99:1972–1976. doi: 10.1152/japplphysiol.00329.2005. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen B, Kubica A, Bonnesen A, Rasmussen IB, Stoklosa J, Wilk B. Physical work capacity after dehydration and hyperthermia. Scand J Sports Sci. 1981;3:2–10. [Google Scholar]
- 8.LoBue-Estes C, Horvath PJ, Burton H, Leddy J. Exhaustive exercise affects cognitive function in trained and untrained women. Percept Mot Skills. 2008;107:933–945. doi: 10.2466/pms.107.3.933-945. [DOI] [PubMed] [Google Scholar]
- 9.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 10.Cian C, Koulmann N, Barraud PA, Raphel C, Jimenez C, Melin B. Influence of variations in body hydration on cognitive function: Effect of hyperhydration, heat stress, and exercise- induced dehydration. J Psychophysiol. 2001;14:29–36. [Google Scholar]
- 11.Noakes TD, Myburgh KH, duPlessis J, Lang L, Lambert M, van der Reit C, et al. Metabolic rate, not percent dehydration, predicts rectal temperature in marathon runners. Med Sci Sports Exerc. 1991;23:443–449. [PubMed] [Google Scholar]
- 12.Speedy DB, Noakes TD, Rogers IR, Hellemans I, Kimber NE, Boswell DR, et al. A prospective study of exercise-associated hyponatremia in two ultradistance tri-athletes. Clin J Sport Med. 2000;10:136–141. doi: 10.1097/00042752-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dugas JP, Noakes TD. Hyponatraemic encephalopathy despite a modest rate of fluid intake during a 109 km cycle race. Br J Sports Med. 2005;39:e38. doi: 10.1136/bjsm.2005.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pescatello L. S, editor. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Baltimore (MD): Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 15.Epting LK, Overman WH. Sex-sensitive tasks in men and women: a search for performance fluctuations across the menstrual cycle. Behav Neurosci. 1998;112:1304–1317. doi: 10.1037//0735-7044.112.6.1304. [DOI] [PubMed] [Google Scholar]
- 16.Gordon HW, Corbin ED, Lee PA. Changes in specialized cognitive function following changes in hormone levels. Cortex. 1986;22(3):399–415. doi: 10.1016/s0010-9452(86)80004-1. [DOI] [PubMed] [Google Scholar]
- 17.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 18.Foss ML, Keteyian SJ. Fox's Physiological Basis for Exercise and Sport. 6th ed. Boston MA: McGraw-Hill; 1998. [Google Scholar]
- 19.Backes TP, Horvath PJ, Kazial KA. Salivary alpha amylase and salivary cortisol response to fluid consumption in exercising athletes. Biol Sport. 2015;32:275–280. doi: 10.5604/20831862.1163689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden CJ. Age. 1978. Stroop colour and word test. [Google Scholar]
- 21.Zysset S, Muller K, Lohman G, von Cramon Y. Color-word matching Stroop task: separating interference and response conflict. Neurolmage. 1999;13:29–36. doi: 10.1006/nimg.2000.0665. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 23.Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. [Google Scholar]
- 24.Backx K, Van Someren K, Palmer G. One hour cycling performance is not affected by ingested fluid volume. Int J Sport Nutr Exerc Metab. 2003;13:333–342. doi: 10.1123/ijsnem.13.3.333. [DOI] [PubMed] [Google Scholar]
- 25.Shi X, Horn MK, Osterberg KL, Stofan JR, Zachweija JJ, Horswill CA, et al. Gastrointestinal discomfort during intermittent high intensity exercise: Effect of carbohydrate-electrolyte beverage. Int J Sport Nutr Exerc Metab. 2004;14:673–683. doi: 10.1123/ijsnem.14.6.673. [DOI] [PubMed] [Google Scholar]
- 26.Palma R, Vidon N, Bernier JJ. Maximal capacity for fluid absorption in the human bowel. Dig Dis Sci. 1981;26:929–934. doi: 10.1007/BF01309499. [DOI] [PubMed] [Google Scholar]
- 27.Noakes TD, Sharwood K, Speedy D. Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2,135 weighed competitive athletic performances. Proc Natl Acad Sci. 2005;102:18550–18555. doi: 10.1073/pnas.0509096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeukendrup AE, Chambers ES. Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care. 2010;13(4):447–451. doi: 10.1097/MCO.0b013e328339de83. [DOI] [PubMed] [Google Scholar]
- 29.Benton D. Nutrients. 2011. Dehydration influences mood and cognition: a plausible hypothesis? pp. 555–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman HR. Hydration and human cognition. Nut Today. 2010;45:S33–S36. [Google Scholar]
- 31.Tomporowski PD, Beasman K, Ganio MS, Cureton K. Effects of dehydration and fluid ingestion on cognition. Int J Sports Med. 2007;28:891–897. doi: 10.1055/s-2007-965004. [DOI] [PubMed] [Google Scholar]
- 32.Wilk B, Bar-Or O. Effect of drink flavor and NaCl on voluntary drinking and hydration in boys exercising in the heat. J Appl Physiol. 1996;80:1112–7. doi: 10.1152/jappl.1996.80.4.1112. [DOI] [PubMed] [Google Scholar]
- 33.Horio T, Kawamura Y. Influence of physical exercise on human preferences for various taste solutions. Chem. senses. 1998;86:417–421. doi: 10.1093/chemse/23.4.417. [DOI] [PubMed] [Google Scholar]
- 34.Berkulo MA, Bol S, Levels K, Lamberts RP, Daanen HA, Noakes TD. Ad-libitum drinking and performance during a 40-km cycling time trial in the heat. Eur J Sport Sci. 2016;16:213–20. doi: 10.1080/17461391.2015.1009495. [DOI] [PubMed] [Google Scholar]