Abstract
What is the strongest acid? Can a simple Brønsted acid be prepared that can protonate an alkane at room temperature? Can that acid be free of the complicating effects of added Lewis acids that are typical of common, difficult-to-handle superacid mixtures? The carborane superacid H-(CHB11F11) is that acid. It is an extremely moisture-sensitive solid, prepared by treatment of anhydrous HCl with [Et3Si–H–SiEt3][CHB11F11]. It adds H2O to form [H3O][CHB11F11] and benzene to form the benzenium ion salt [C6H7][CHB11F11]. It reacts with butane to form a crystalline tBu+ salt and with n-hexane to form an isolable hexyl carbocation salt. Carbocations, which are thus no longer transient intermediates, react with NaH either by hydride addition to re-form an alkane or by deprotonation to form an alkene and H2. By protonating alkanes at room temperature, the reactivity of H(CHB11F11) opens up new opportunities for the easier study of acid-catalyzed hydrocarbon reforming.
Keywords: Brønsted acids, carbocations, carboranes, hydrocarbons
For the past decade, the strongest acid to be isolated and fully characterized has been the chlorinated carborane acid, H(CHB11Cl11).[1, 2] More recently, this has been equaled by the comparably strong, isoelectronic, all-boron diprotic superacid, H2(B12Cl12).[3] The superior strength of these Brønsted acids has been established in all phases. In solution, the fact that both readily protonate benzene (whereas oxyacids do not) places them ahead of HFSO3, the previous strongest pure acid measured on the Ho scale.[4] In the gas phase, the calculated[5] and measured[6] deprotonation enthalpy of H(CHB11Cl11) is the lowest of any available acid. The νNH anion basicity scale[7] indicates that carborane anions are less basic than the oxyanions of traditional acids. This same scale indicates that fluorinated carborane anions are less basic than chlorinated carborane anions, so the conjugate acid H-(CHB11F11) should be a stronger acid than H(CHB11Cl11). Calculated gas-phase deprotonation energies concur.[5] There is, of course, no guarantee that weaker anion basicity leads to an isolable stronger conjugate acid. The anion must also be chemically stable to H+.[8] However, the stability of fluorinated carborane anions[9] towards the potent Lewis acidity of trialkysilylium ions[10] augurs well for their stability towards H+.
Indeed, there was a preliminary report in 2007 of two fluorinated carborane acids, H(HCB11F11) and H(EtCB11F11).[10] As evidence of formulation, an IR spectrum of the latter was given. By analogy to H(CHB11Cl11), which is known from X-ray analysis to have a linear polymeric structure with H+ bridges between anion Cl atoms, and from IR spectroscopy to have low-barrier H-bonding,[11] the expected IR features for H(EtCB11F11) were present. These include two bands at about 1705 and 1620 cm−1 as candidates for νFHF and a band at about 920 cm−1 for δFHF, along with expected νBB and νBF bands from the anion at circa 1300 and 700 cm−1, respectively.
Given that there has been no follow-up to this preliminary report in the intervening six years, we were curious whether we could reproduce this work and prepare a fluorinated carborane acid in synthetic amounts for reactivity studies with hydrocarbons. We chose the non-alkylated anion CHB11F11− for our studies, based on concern for the chemical stability of the ethyl group in EtCB11F11−. From preliminary investigations into the reactivity of the chlorinated carborane acid H(CHB11Cl11) with alkanes at somewhat elevated temperatures, we had reason to believe that fluorinated carborane acids might react with alkanes at room temperature, thus giving H(EtCB11F11) the capability of reacting with its own ethyl group. An indication that this may be occurring is suggested by the IR spectrum reported for H(EtCB11F11)[10] where two of the three νCH bands of the ethyl group in the 2900–3020 cm−1 region are nearly absent in comparison to other EtCB11F11− salts (see Figure S8 in the Supporting Information of Ref. [10]).
The synthesis of H(CHB11F11) (Scheme 1) was eventually achieved using much the same procedure as that for its chlorinated analogue H(CHB11Cl11),[12] although the preparation of significant amounts of clean product eluded us for a long time. We now understand this difficulty in terms of H(CHB11F11) being a considerably stronger acid than H(CHB11Cl11), and thus capable of reacting rapidly with all organic species, and being the ultimate desiccant. So, purity of starting materials and the scrupulous exclusion of water were imperative. In particular, we found that the trityl salt of CHB11F11− must be carefully purified by successive recrystallizations from o-dichlorobenzene to remove occluded organic compounds. Another critical step was to perform the reaction of [Et3Si–H–SiEt3][CHB11F11] with HCl twice (sequentially) to completely remove silane byproducts. Once isolated, small quantities of H(CHB11F11) were obtained in high purity by sublimation under high vacuum at about 160 °C, a temperature similar to that used for the sublimation of H(CHB11Cl11). Otherwise, synthetic amounts were typically made in 100–200 mg batches and, under the best conditions, were estimated, from the virtual absence of νOH in the IR, to contain less than 2 % hydrated impurity. In our experience, samples can be stored for no more than a few days, even in a good dry box, before becoming significantly degraded by hydration and/or reaction with trace solvent vapors. Once hydrated, the acid cannot be dehydrated by vacuum sublimation.
Scheme 1.
Synthesis of the carborane acid HY (Y=CHB11F11−).
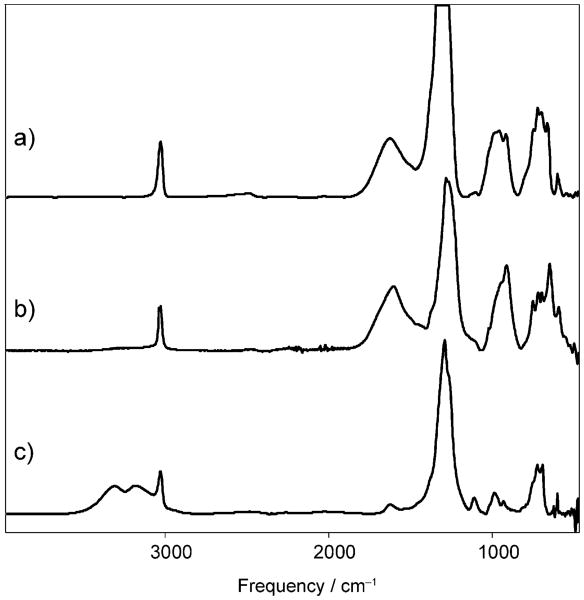
H(CHB11F11) is expected to be isostructural with H(CHB11Cl11) and have a linear polymeric structure with two-coordinate, low-barrier H+ bridging between F atoms. As shown in Figure 1a and b, the IR spectrum is somewhat related to that of the symmetrical bifluoride ion, FHF−,[13] and is diagnostic of low-barrier H-bonding.[14] Instead of νHF at about 2500 cm−1 as might be expected for a terminal H–F bond, H(CHB11F11) shows a broad band at about 1605 cm−1 assigned to νFHF and absorptions in the 1000–900 cm−1 region assigned to δFHF in a nearly symmetrical proton-bridged structure. The corresponding bands in H(CHB11Cl11) are at about 1100 and 615 cm−1, respectively.[11] Deuteration, achieved by stirring the protio acid in liquid DCl, led to the loss of both the 1605 and 1000–900 cm−1 bands. The corresponding νFDF and δFDF bands, expected at about 1140 and 710–640 cm−1 by harmonic oscillator calculations, are both largely masked by the very strong νBB and νBF bands of the anion. Otherwise, the IR spectrum of D(CHB11F11) shows only bands expected from the anion: νCH at 3030 cm−1 and multiple absorptions centered near 1300 and 700 cm−1 from νBB and νBF, respectively.
Figure 1.
IR spectra of a) H(CHB11F11) purified by sublimation, b) H-(CHB11F11) from bulk synthesis, and c) [H3O][CHB11F11] from minimal uptake of water. Spectra (a) and (c) were recorded in transmission mode, (b) in ATR mode.
Upon exposure to traces of water vapor, H(CHB11F11) is instantly hydrated. With minimal hydration, the IR spectrum (Figure 1c; Supporting Information, Figure S11) is consistent with the formation of an H3O+ salt. Broad νOH bands at about 3300 and 3175 cm−1 are assigned to νs and νas, respectively. Their frequencies are higher than those in the chlorinated analogue [H3O][CHB11Cl11] at 3225, 2910 cm−1, [15] indicating weaker H-bonding of the H3O+ ion to the less-basic fluorinated carborane anion. The band at about 1625 cm−1 is assigned to δ(H3O). The other major bands in the spectrum are νBB and νBF at circa 1300 and 700 cm−1, respectively.
As expected for such a strong acid, solid H(CHB11F11) reacts instantly on contact with benzene to give an isolable benzenium ion salt, [C6H7+ ][CHB11F11− ] (for the IR spectrum, see the Supporting Information, Figure S12). The circa 10 cm−1 increase in frequency observed for the ν(CC) + δ-(CCH) band near 1600 cm−1 relative to free benzene is diagnostic of the benzenium ion.[16] The νCH bands of the acidic sp3 CH2 group occur at low frequency (2818 and 2794 cm−1) owing to C–H⋯anion H-bonding.[17] Their frequencies are somewhat higher than that in [C6H7][CHB11Cl11] (2776 cm−1), consistent with weaker H-bonding to the less-basic fluorinated carborane anion relative to the chlorinated analogue. The structure and remarkable stability of these so-called Wheland intermediates of electrophilic aromatic substitution have been discussed previously.[16]
In a clear demonstration of the extremely high acid strength of this Brønsted-only acid, we find that H(CHB11F11) reacts with alkanes at room temperature, like mixed Brønsted/Lewis acids such as “magic acid” (HFSO3/SbF5).[18] When solid H(CHB11F11) is stirred in suspension with n-hexane, the appearance of a diagnostic low frequency νCH band at 2758 cm−1 in the IR spectrum of the product (the low frequency arising from hyperconjugation and C–H⋯anion hydrogen bonding)[17, 19] indicates that about 50% of the solid acid is converted into a microcrystalline carbocation salt within 2 h (Supporting Information, Figure S14). This is the expected outcome of protonation of an alkane and elimination of H2 [Eq. (1) ]:
| (1) |
Evolved H2 was detected by gas chromatography (Supporting Information, Figure S13). A similar protonation experiment with a suspension of H(CHB11F11) in liquefied n-butane indicates that the acid converts butane into a micro-crystalline salt of tert-butyl cation [Eq. (2) ]:
| (2) |
The distinctive low frequency and shape of the νCH absorptions in the IR spectrum of the product (Supporting Information, Figures S15–S17) closely matches that of known [tBu+ ][CHB11Cl11− ],[17] except that the νmax frequency at 2823 cm−1 of the fluorinated anion salt is higher than that of the chlorinated anion salt (2788 cm−1). This is readily understood in terms of weaker C–H⋯anion H-bonding of tBu+ to the fluorinated anion. The tertiary isomer of butyl cation is the expected result of facile 1,2 shifts from initially formed primary or secondary carbocation-like species.[20] The X-ray structures of two tert-butyl salts with carborane anions have been reported previously.[17, 21]
Additional characterization of these C4 and C6 carbocation salts was obtained from reactivity studies with NaH, anticipating two possible outcomes: 1) hydride transfer to reform an alkane; or b) hydride acting as a base, deprotonating the carbocation and forming an alkene.
When a sample of the presumed C6 carbocation salt, obtained as above from the protonation of n-hexane with H(CHB11F11), was treated with a NaH in liquid SO2, a single hydrocarbon product was detected by gas chromatographic analysis (Supporting Information, Figure S18). Its identity as a C6 alkene was shown by mass spectroscopy (m/z 84.086; see the Supporting Information, Figure S19). Electron impact fragments corresponding to loss of methyl, ethyl, and propyl groups were observed and comparison of this fragmentation pattern to those catalogued for C6 alkenes shows the closest match to that of 3,3-dimethyl-1-butene, although it is shares substantial similarity to those of 2-methyl-2-pentene, 2,3-dimethyl-1-butene, 2,3-dimethyl-2-butene, and 4-methyl-2-pentene (Supporting Information, Figure S20). Thus, a C6 carbocation was the product of protonation of n-hexane, most likely with a tertiary cationic center arising from the well-known rearrangement of initially formed primary or secondary carbocation-like species to a more stable tertiary cation by rapid 1,2 shifts.[20]
Under somewhat different experimental conditions, NaH acts as a hydride transfer agent rather than a base. Intimate co-grinding of the presumed C6 carbocation salt with NaH(s) led to evolution of four different C6 alkanes by GC analysis: 2,2-dimethylbutane, 2,3-dimethylbutane, 2-methylpentane, and 3-methylpentane (Supporting Information, Figure S21). This mixture presumably reflects the rapid rearrangement equilibria possible in all tertiary alkyl cations.[20] Similarly, intimate co-grinding with NaH(s) of the tBu+ salt derived from protonation of n-butane led to iso-butane as the major alkane detectable by GC/MS in the head space (Supporting Information, Figure S22). tert-Butyl cations produced in a different manner,[22] by protonation of butyl chloride with H(CHB11Cl11), behaved in identical fashion.
In contrast to these room-temperature reactions of H-(CHB11F11) with alkanes, the chlorinated carborane acid H(CHB11Cl11) scarcely reacts with n-hexane or butane under comparable conditions. Even after 24 h stirring as a suspension in n-hexane, less than 25 % conversion of H(CHB11Cl11) into carbocation salts was detected by IR spectroscopy. Thus, the fluorinated carborane acid is now the strongest pure acid known.
The existence of a Brønsted-only acid that can protonate alkanes at room temperature offers new opportunities for studying acid-catalyzed hydrocarbon chemistry related to hydrocarbon reforming on zeolites at high temperatures. Potential advantages are that a sublimable solid acid is easier to handle than a liquid acid and a Brønsted-only acid is free of the potentially complicating effects of a Lewis acid. Room-temperature access to alkane protonation chemistry should also allow the easier application of investigative techniques that are difficult to apply to zeolites;[23] the acidity of zeolites is much lower[24] and their reactions with alkanes must be carried out at high temperatures.[25] Such room-temperature techniques have already been applied with unexpected results to chloroalkanes, which are more easily protonated than alkanes. Peculiar stoichiometric relationships in reversible, carbocation-like oligomerization sequences were observed.[22]
Experimental Section
All manipulations were carried out under very dry conditions using Schlenkware or an inert atmosphere glovebox (H2O, O2 < 0.5 ppm). Solvents were dried by standard methods. HF was dried by condensing gaseous HF into a PFA vessel containing potassium hexafluor-onickelate. HCl was dried by passing it through a 25 cm column of P2O5 plugged at both ends with glass wool. 10% F2 in N2 was used without additional purification. 1H, 11B, and 19F spectra were obtained on a Bruker Avance 300 MHz spectrometer. Attenuated Reflectance spectra (ATR) IR spectra were run on ABB MB3000 spectrometer in the 525–4000 cm−1 frequency range using a diamond crystal. Mass spectra were collected using a Waters GCT GC/MS operating at 15 °C.
Cs(CHB11F11): Caution! HF and F2 are extremely toxic and are used under pressure. All reactions should be carried out with appropriate apparatus and safety precautions in a well-ventilated hood using full-body protective clothing and the “buddy” system. Adapting the method of Solntsev and Strauss,[9] a 300 mLTeflon-lined Monel bomb equipped with a Teflon stir bar was charged with 2 g of Cs(CHB11H11)[26] and placed in an oven at 80 °C for 4 h. It was then connected to a PFA vacuum manifold, evacuated, and cooled to dry ice temperature in a dry ice/methanol bath. Approximately 35 mL of high purity hydrogen fluoride was condensed into the reactor and the reaction was stirred at room temperature for 24 h. After cooling in a dry ice/methanol bath, the reactor was evacuated and then placed on a stainless steel vacuum manifold. 280 PSI (20 bar) of 10 % F2 in N2 was introduced into the bomb, sealed, and allowed to warm to room temperature. After stirring for 8 h, the bomb was cooled in a dry ice/methanol bath and the excess pressure vented. The process of adding fluorine, allowing it to react, and venting the reactor was repeated five times. Then, hydrogen fluoride was condensed out of the bomb and the reactor was carefully opened and allowed to stand in the air. After 1 hour, the contents were dissolved in 80 mL of dry acetonitrile and filtered through fine frit, to remove insoluble CsBF4. The filtrate was evaporated to dryness on a rotary evaporator, yielding a sticky yellow powder. This powder was placed in an inert atmosphere glove box, suspended in dry dichloromethane (100 mL), and stirred overnight. The suspension was filtered off to yield a white powder, which was dried under vacuum for 1 hour (2.74 g; 80%). 1H NMR ([D6]acetone): δ = 4.35 ppm (s, 1H, CH). 11B NMR ([D6]acetone, unreferenced): δ = −7.75 (s, 1B), 15.86 (s, 5B), 17.09 ppm (s, 5B) (SI, Figure S3). 19F NMR ([D6]acetone, unreferenced): δ = −251.59 (m, 1F), −255.63 ppm (m, 10F) (SI, Figure S2). The completeness of fluorination was initially judged by the absence of νBH bands near 2550 cm−1 in the IR spectrum of the product (SI, Figure S1). 19F and 11B NMR spectra were found to be less reliable indicators of completeness of fluorination. The most sensitive indicator of anion purity was negative ion electrospray mass spectrometry (SI, Figure S4).
Ag(CHB11F11)·2 C6H6: Cs(CHB11F11) (2.78 g, 5.87 mmol) and AgNO3 (4.98 g, 29.93 mmol) were suspended in dry benzene (300 mL) and stirred magnetically. After 24 h, the benzene was decanted and a fresh 300 mL aliquot added to the remaining solids. This process was repeated three times. The combined (3 × 300 mL) benzene fractions were filtered through a fine frit and evaporated to dryness to yield an off-white solid that was dried under vacuum for 1 hour (2.18 g, 83 %). 1H NMR: (300 MHz, CD2Cl2): δ = 3.946 (s, 1H, CH), 7.36 ppm (s, 6H, benzene) (SI, Figure S6). ATR-IR (SI, Figure S5).
[(C6H5)3C][CHB11F11]: In the glove box, Ag(CHB11F11)·2 C6H6 (2.48 g, 4.14 mmol) and (C6H5)3CBr (1.48 g, 4.55 mmol) were dissolved in 25 mL of dry dichloromethane and stirred overnight. The yellow precipitate of AgBr was filtered off with a fine frit, washing with 5 mL of dichloromethane. The filtrate was evaporated to dryness under vacuum, yielding a bright yellow waxy solid. This was recrystallized twice by layering 10 mL 1,2-dichlorobenzene solutions with 15 mL of hexane. The solid was filtered off and dried under vacuum for 1 h (2.2 g, 89 %). 1H NMR (300 MHz, CD2Cl2): δ = 2.776 (s, 1H, C-H), 7.705, 7.938, 8.325 ppm (phenyl groups) (SI, Figure S8). ATR-IR (SI, Figure S7).
[(Et3Si)2H][CHB11F11]: [(C6H5)3C][CHB11F11] (200 mg, 0.342 mmol) was covered with dry 1,2-dichlorobenzene (0.5 mL). To this suspension 1 mL of Et3SiH was added and the solution stirred for 0.5 h. Dry hexane (5 mL) was added gradually while stirring. The resulting precipitate was filtered and washed with dry hexanes (5 mL) (0.175 g, 89%). ATR-IR (SI, Figure S9). The broad band at 1873 cm−1 is diagnostic of the hydride-bridged disilyl cation.[27]
H(CHB11F11): Extra-dry HCl gas was condensed (ca. 2 mL) onto [(Et3Si)2H][CHB11F11] (200 mg) using liquid N2, and the mixture was stirred at 0°C for 0.5 hour in a sealed heavy-walled Schlenk tube having a wide bore Teflon stopcock below the joint. The HCl was removed under vacuum and a new portion of HCl (2 mL) was added, stirring for 0.5 hour at 0°C. The HCl allowed to escape anaerobically and the remnant solid was pumped under vacuum for 0.5 h (96 mg, 81%). The deuterated acid, D(CHB11F11), was prepared by stirring the protio acid in liquid DCl followed by evaporation of DCl/HCl and drying under vacuum. The IR spectrum is compared to the protio form in the Supporting Information, Figure S10.
Supplementary Material
Footnotes
We thank Prof. Dr. Pingyun Feng and Dr. Richard W. Kondrat for kind assistance with H2 detection and GC/MS, respectively. This work was supported by the National Science Foundation (CHE 1144838 and CHE 0742001).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201308586.
Contributor Information
Matthew Nava, Center for s and p Block Chemistry, Department of Chemistry University of California, Riverside, CA 92521 (USA).
Irina V. Stoyanova, Center for s and p Block Chemistry, Department of Chemistry University of California, Riverside, CA 92521 (USA)
Dr. Steven Cummings, Center for s and p Block Chemistry, Department of Chemistry University of California, Riverside, CA 92521 (USA)
Prof. Dr. Evgenii S. Stoyanov, Center for s and p Block Chemistry, Department of Chemistry University of California, Riverside, CA 92521 (USA)
Christopher A. Reed, Email: chris.reed@ucr.edu, Center for s and p Block Chemistry, Department of Chemistry University of California, Riverside, CA 92521 (USA), Homepage: http://reedgrouplab.ucr.edu/.
References
- 1.Juhasz M, Hoffmann SP, Stoyanov ES, Kim KC, Reed CA. Angew Chem. 2004;116:5466–5469. doi: 10.1002/anie.200460005. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:5352–5355. doi: 10.1002/anie.200460005. [DOI] [PubMed] [Google Scholar]
- 2.Reed CA. Chem N Z. 2011 Oct;:174–179. [Google Scholar]
- 3.Avelar A, Tham FS, Reed CA. Angew Chem. 2009;121:3543–3545. doi: 10.1002/anie.200900214. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:3491–3493. doi: 10.1002/anie.200900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie RJ. Acc Chem Res. 1968;1:202–209. [Google Scholar]
- 5.Lipping L, Leito I, Koppel I, Koppel IA. J Phys Chem A. 2009;113:12972–12978. doi: 10.1021/jp905449k. [DOI] [PubMed] [Google Scholar]
- 6.Meyer MM, Wang XB, Reed CA, Wang LS, Kass SR. J Am Chem Soc. 2009;131:18050–18051. doi: 10.1021/ja908964h. [DOI] [PubMed] [Google Scholar]
- 7.Stoyanov ES, Kim KC, Reed CA. J Am Chem Soc. 2006;128:8500–8508. doi: 10.1021/ja060714v. [DOI] [PubMed] [Google Scholar]
- 8.Reed CA. Chem Commun. 2005:1669–1677. doi: 10.1039/b415425h. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov SV, Rockwell JJ, Polyakov OG, Gaudinski CM, Anderson OP, Solntsev KA, Strauss SH. J Am Chem Soc. 1998;120:4224–4225. [Google Scholar]
- 10.Küppers TK, Bernhardt E, Eujen R, Willner H, Lehmann CW. Angew Chem. 2007;119:6462–6465. doi: 10.1002/anie.200701136. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:6346–6349. doi: 10.1002/anie.200701136. [DOI] [PubMed] [Google Scholar]
- 11.Stoyanov ES, Hoffmann SP, Juhasz M, Reed CA. J Am Chem Soc. 2006;128:3160–3161. doi: 10.1021/ja058581l. [DOI] [PubMed] [Google Scholar]
- 12.See the Supporting Information in: Reed CA. Acc Chem Res. 2010;43:121–128. doi: 10.1021/ar900159e.
- 13.Kawaguchi K, Hirota E. J Chem Phys. 1987;87:6838–6841. [Google Scholar]
- 14.Stasko D, Hoffmann SP, Kim KC, Fackler NLP, Larsen AS, Drovetskaya T, Tham FS, Reed CA, Rickard CEF, Boyd PDW, Stoyanov ES. J Am Chem Soc. 2002;124:13869–13876. doi: 10.1021/ja012671i. [DOI] [PubMed] [Google Scholar]
- 15.Stoyanov ES, Kim KC, Reed CA. J Am Chem Soc. 2006;128:1948–1958. doi: 10.1021/ja0551335. [DOI] [PubMed] [Google Scholar]
- 16.Reed CA, Kim KC, Stoyanov ES, Stasko D, Tham FS, Mueller LJ, Boyd PDW. J Am Chem Soc. 2003;125:1796–1804. doi: 10.1021/ja027336o. [DOI] [PubMed] [Google Scholar]
- 17.Stoyanov ES, Stoyanova IV, Tham FS, Reed CA. Angew Chem. 2012;124:9283–9285. doi: 10.1002/anie.201203958. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:9149–9151. doi: 10.1002/anie.201203958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olah GA, Molinár Á. Hydrocarbon Chemistry. Wiley; New York: 2003. [Google Scholar]
- 19.Reed CA, Stoyanov ES, Tham FS. Org Biomol Chem. 2013;11:3797–3802. doi: 10.1039/c3ob40737c. [DOI] [PubMed] [Google Scholar]
- 20.Saunders M, Kates MR. J Am Chem Soc. 1978;100:7082–7083. [Google Scholar]
- 21.Kato T, Reed CA. Angew Chem. 2004;116:2968–2971. [Google Scholar]; Angew Chem Int Ed. 2004;43:2908–2911. doi: 10.1002/anie.200453931. [DOI] [PubMed] [Google Scholar]
- 22.Stoyanov ES, Stoyanova IV, Reed CA. J Am Chem Soc. 2011;133:8452–8454. doi: 10.1021/ja201569p. [DOI] [PubMed] [Google Scholar]
- 23.Sani Souna Sido A, Barbiche J, Sommer J. Chem Commun. 2010;46:2913–2914. doi: 10.1039/c000513d. [DOI] [PubMed] [Google Scholar]
- 24.Haw JF. Phys Chem Chem Phys. 2002;4:5431–5441. [Google Scholar]
- 25.Haw JF, Nicholas JB, Xu T, Beck LW, Ferguson DB. Acc Chem Res. 1996;29:259–267. [Google Scholar]
- 26.Plešek J, Jelínek T, Drdáková E, Heřmánek S, Štíbr B. Collect Czech Chem Commun. 1984;49:1559–1562. [Google Scholar]
- 27.Hoffmann SP, Kato T, Tham FS, Reed CA. Chem Commun. 2006:767–769. doi: 10.1039/b511344j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.