Abstract
Shifting from the historical TNM paradigm to the determination of molecular and genetic subtypes of tumors has been a major improvement to better picture cancerous diseases. The sharper the picture is, the better will be the possibility to develop subsequent strategies, thus achieving higher efficacy and prolonged survival eventually. Recent studies suggest that urokinase-type plasminogen activator (uPA), uPA Receptor (uPAR), and plasmino-gen activator inhibitor-1 (PAI-1) may play a critical role in cancer invasion and metastasis. Consistent with their role in cancer dissemination, high levels of uPA, PAI-1, and uPAR in multiple cancer types correlate with dismal prognosis. In this respect, upfront determination of uPA and PAI-1 as invasion markers has further opened up the possibilities for individualized therapy of breast cancer. Indeed, uPA and PAI-1 could help to classify patients on their risk for metastatic spreading and subsequent relapse, thus helping clinicians in their decision-making process to propose, or not propose, adjuvant therapy. This review covers the implications for cancer diagnosis, prognosis, and therapy of uPA and PAI-1, and therefore how they could be major actors in the development of a precision medicine in breast cancer.
Keywords: urokinase plasminogen activator, breast cancer, therapy decision, precision medicine
Introduction
Breast cancer (BC) is one of the most frequent cancers that occur in women. Worldwide, each year, the incidence and mortality have increased to over 1,050,000 newly diagnosed cases and 400,000 deaths. The progress made in terms of screening, treatment, and care support has contributed to improved relative survival (over 85% of BC five-year survivors after diagnosis).1,2 A significant improvement in survival correlates with early diagnosis and use of adjuvant therapy. A major step toward optimal care had been to define BC diversity and to accurately categorize patient sub-populations on a genomic and molecular basis, beyond standard histopathological TNM classifications.3,4 Biomarkers can be combined with clinicopathological characteristics to estimate recurrence risk (ie, prognostic value) and to predict therapy efficacy (ie, predictive value). Identifying patients with high risk for recurrence and administering optimal therapies while avoiding over-treating low-risk patients are major issues in BC management, because of the number of patients newly diagnosed each year. In addition, resistance to therapy and containment of metastatic spreading need to be effectively addressed for prolonging the survival.5
In this context, two major compounds of the plasminogen activator system, urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1), have been found to play possibly a key role in individualizing breast cancer treatment. The predictive value of the combinational use of these two biological markers for discriminating the risk group in both nodes positive and notably node-negative breast cancer patients has been subsequently established and validated.
Moreover, the combined ability of uPA/PAI-1 to predict both outcome and response/resistance to specific therapies should further lead to individualized management of patients with breast cancer, thus helping clinicians to predict treatment efficacy.6 With the rise in personalized medicine, studying uPA/PAI-1 could be key to refine the therapeutic strategies in breast cancer. This review presents the biology, roles, and the putative role that uPA/PAI-1 could play in precision medicine in oncology.
Biology of the uPA System
The uPA system is a conversion system from plasminogen into plasmin, which plays important physiological roles. It also plays a key role in cancer invasion and metastasis dissemination by allowing malignant cells to invade the tumor site locally and spread to distant sites.7,8 This system includes the serine protease, uPA, membrane-linked receptor uPAR, and two serpin inhibitors, PAI-1 and PAI-2.
uPA is a 53-kDa serine protease initially synthesized as a catalytically inactive single chain polypeptide. pro-uPA whose proteolytic cleavage – mediated by different proteases including plasmin, cathepsin B, cathepsin L, and human kallikrein type 2 – gives the mature form that is a two-chain protein linked by a single disulfide bond. uPA can be structurally divided into three domains, an amino-terminal domain, which contains the binding site for uPAR, a carboxy terminal sequence containing the catalytic site, and a kringle domain of a yet unknown function.7,8
Unlike most serine proteases, uPA has two notable particularities. First, in contrast to many other proteases, uPA restricts substrate specificity, since it only converts inactive plasminogen to the enzymatically active form, plasmin, which is also a serine protease but has broad substrate specificity. Plasmin can particularly degrade several extracellular matrix (ECM) components such as laminin, fibronectin, and osteopontin.9,10 By cleaving these proteins, plasmin can activate growth factors and liberate them from ECM sequestration. The binding of these activated growth factors to their cognate receptors can cause increased proliferation, migration, invasion, and metastasis eventually. In addition, plasmin is able to activate precursor forms of a number of specific matrix metalloproteases, inter alia the precursor form of uPA, pro-uPA. These activated metalloproteases can then degrade the diverse forms of collagens, kallikrein-related peptidases, and other proteins in the ECM.11
Thus, plasmin can play a critical role during multiple steps of cancer invasion and metastasis, by inducing the degradation of a number of ECM proteins and activating certain growth factors leading to aggressive cancers.12,13 The second characteristic of uPA is that both the proenzyme form (pro-uPA) and the active form of uPA bind with high affinity to their specific receptor.14 uPAR is formed by a three-domain glycoprotein and because of lack of a transmembrane domain, uPAR will be linked to the cell membrane via a glycosyl phosphatidylinositol anchor.15
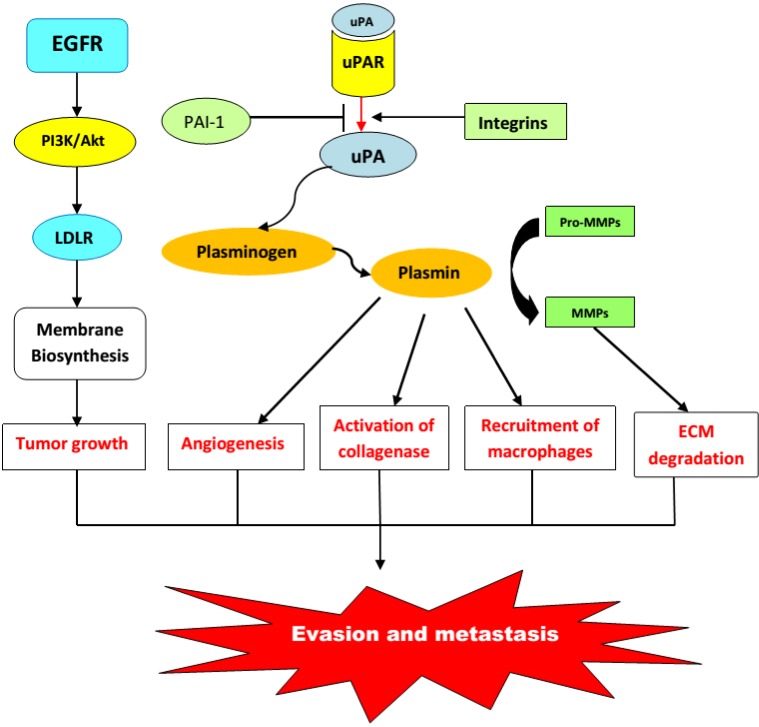
To initiate signaling, uPAR must interact with molecules such as epidermal growth factor receptor, platelet-derived growth factor receptor, specific integrins, or low-density lipoprotein receptor-related (LDLR) proteins16–19 (Fig. 1). Then, the active complex uPA–uPAR initiates different signaling pathways, that is, the MAPK, Jak-Stat, and focal adhesion kinase systems.20 All these signaling systems stimulate cell proliferation, enhance cell migration, and modulate cell adhesion.8,21 Thus, uPA may trigger cell signaling and trafficking by two distinct ways: directly by binding to its receptor uPAR and indirectly by the activation of plasmin (Fig. 3).
Figure 1.
Urokinase plasminogen activation system: content and functions.
Abbreviations: MMPs, Metalloproteases; ECM, Extracellular matrix; uPA, urokinase plasminogen activator; uPAR, Urokinase receptor; PAI-1, Plasminogen activator inhibitor type 1; EGFR, Epidermal growth factor receptor; LDLR, Low-density lipoprotein receptor; Akt, Alpha serine/threonine-protein kinase; PI3K, Phosphatidylinositol-3-kinases.
Figure 3.
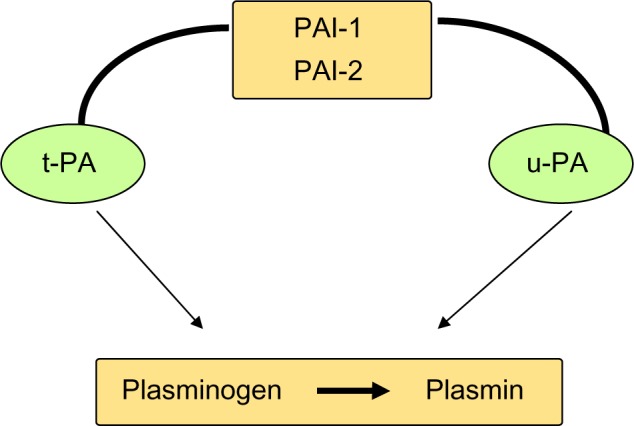
t-PA, u-PA and their inhibitors.
In order to limit its proteolytic action, uPA can be inhibited by two protease inhibitors PAI-1 and PAI-2. PAI-1 is the primary physiological inhibitor of uPA, formed by a single-chain glycoprotein of 43 kDa. It reacts with UPA to form a stable complex.7,8 PAI-1 is also able to induce the internalization and degradation of uPAR-bound uPA; then, uPAR is itself endocytosed and recycled back to the cell surface.22
There are 2 forms of PAI-2; a 47-kDa intracellular non-glycosylated form and a 60-kDa extracellular glycosylated form.23 PAI-2 reacts in the same way but more slowly than PAI-1, and it has been shown that its overexpression inhibits apoptosis and therefore fosters cancer development.24 Numerous studies indicate that the uPA system is involved in cancer.7,25,26 uPAR and uPA are overexpressed with remarkable consistency in a variety of human tumors, including leukemia and breast, lung, bladder, colon, liver, pleura, pancreas, and brain cancers.27–37 (Fig. 2).
Figure 2.
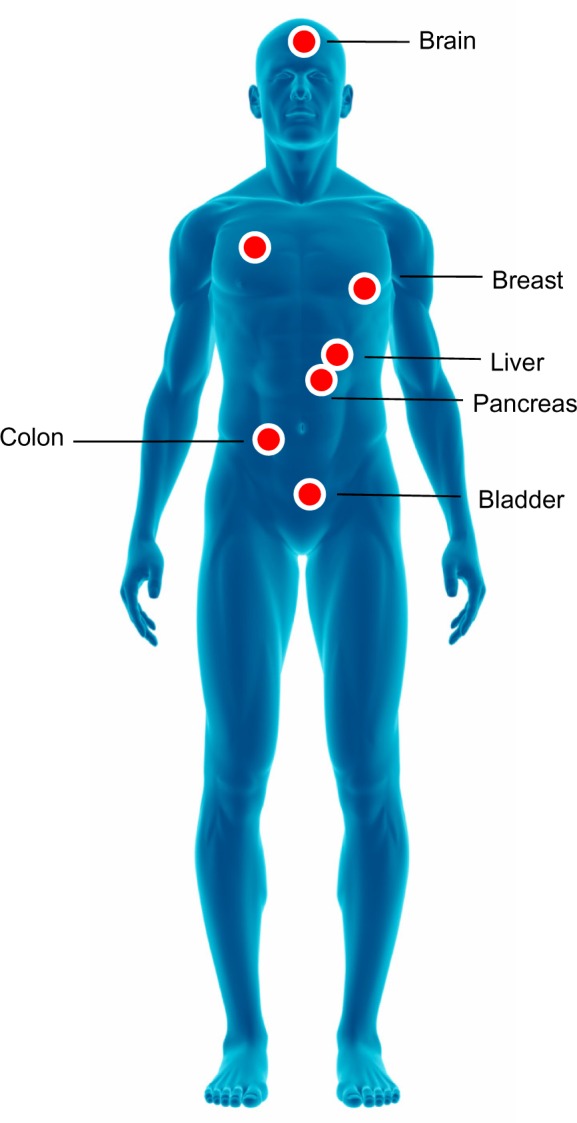
Solid tumors overexpressing uPA and uPAR.
Role of the uPA System in Breast Cancer
The results of experiments conducted on model systems have shown that uPA plays a major role in cancer invasion and metastasis.38 Indeed, first reports have shown that there are correlations between the concentrations of uPA and the metastatic potential of various cell lines, and that using antagonist antibodies or small interfering RNA (SiRNA) against uPA tends to prevent or reduce metastases.8,39 Another confirmation of the role of uPA in metastatic spreading was obtained with uPA or plasminogen-deficient mice. Several studies reported that a deficiency in plasminogen in breast cancer mouse models reduced the formation of lung and lymph node metastasis without affecting main tumor growth.40
It was originally believed that uPA promoted the spread of cancer simply by degrading the ECM, thus allowing invasion and metastasis. It is now widely acknowledged that there are additional activities supporting its role in the proliferation and spreading of cancer.6,41
During cancer invasion and metastasis, malignant cells must penetrate and remodel the ECM on several occasions. uPA has a limited specificity toward the ECM components and to date, it has only been shown to directly degrade fibronectin.41 However, plasmin can digest most of the non-collagenous proteins including fibrin, laminin, fibronectin, and perlecan (a heparan sulfate proteoglycan). 6,41–43 Plasmin can also contribute to the dissolution of the ECM by activating the precursor forms of certain MMPs. MMPs shown to be activated by plasmin include MMP-3, MMP-9, MMP-12, and MMP-13.12 Clearly, therefore, regulated and focused proteolysis is necessary for promoting invasion. For uPA, degradation is likely to be achieved by binding to its receptor, while excessive proteolysis can be modulated by the endogenous inhibitors, that is, PAI-I and PAI-2.8
In vitro studies have provided direct evidence that the uPA system is capable of stimulating mitogenesis. In some cell types, such as epidermal tumor lines (CCL.20.2) and melanoma cells,44,45 the mitogenic activity of uPA required both binding to uPAR and catalytic activity. On the other hand, with the human ovarian cancer cell line OV-MZ-6, only binding to the receptor was necessary for induction of proliferation.46 Activation or release of a positive growth stimulation factor could also lead to a higher mitogenesis. Specific growth factors that are activated by plasmin and that stimulate cellular proliferation include FGF2, VEGF, IGF-1, and HGF.47,48
FGF2 and VEGF are well-known growth promoters of endothelial cells and therefore play a major role in angiogenesis, while IGF-1 and HGF stimulate the growth of epithelial cells.49–51 Angiogenesis is required for tumor growth, invasion, and metastasis. uPA acting through its receptor plays a key role in the multi-step mode. This role is likely to include both the ECM remodeling, allowing endothelial cells to invade the tumor stroma and the activation/release of pro-angiogenic factors such as FGF2, TGFb, and VEGF13 (Fig. 4).
Figure 4.
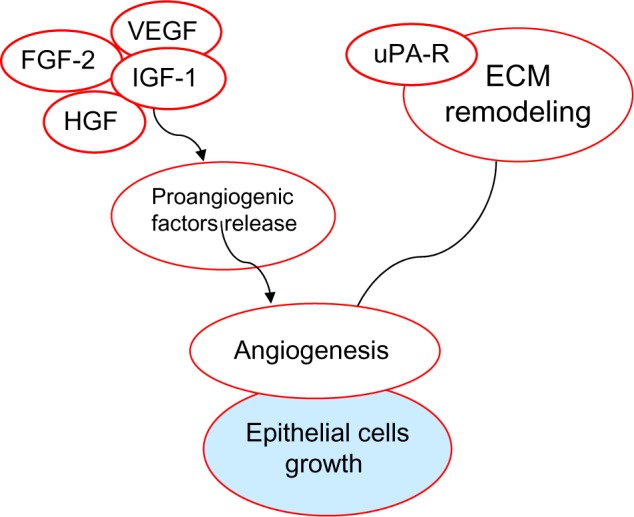
The role of uPA-R and other effectors in the growth of epithelial cells.
Abbreviations: VEGF, Vascular endothelial growth factor; FGF-2, Fibroblast growth factor 2; IGF-1, Insulin-like growth factor 1; HGF, Hepatocyte growth factor.
Because uPA promotes angiogenesis, we can assume that PAI-1 inhibits the process. Indeed, the different effects of PAI-1 on angiogenesis seem to be related to its concentration. Remarkably, in a recent study, PAI-1 was found to be pro- angiogenic at nanomolar concentrations corresponding to normal concentrations in the mouse plasma, but anti-angiogenic at micromolar concentrations.52
To generate metastasis, malignant cells must migrate from their primary site to a distant location. Using both MCF-7 breast cancer cells and HT1080 fibrosarcoma, it was shown that uPA-enhanced cell migration required co-operation between the Ras-Erk and Rho-Rho kinase pathways.53 It is therefore not surprising that in addition to enhancing cell migration, uPA may also stimulate cell adhesion. Attaching uPA modifies uPAR conformation receptor, which increases its affinity for vitronectin. These events, however, occur only when uPA is present in excess as compared with PAI-1.54 Few studies have attempted to study the epigenetics of the uPA/PAI-1 system and it was demonstrated that uPA is hypomethylated and methylation of PAI-1 gene has been suggested as one of the molecular mechanisms involved in breast cancer associated with the downregulation of the expression of PAI-1.55,56
Recently, uPA was also shown to be able to prevent apoptosis. The inhibition of apoptosis could thus increase the survival potential of malignant cells during the metastatic process, therefore increasing the possibility for the establishment of secondary lesions. In addition, it could help tumor cells to acquire resistant phenotype in stress conditions, that is, after treatment. The ability for uPA to signal through uPAR will maintain an elevated basal level of activated ERK while inhibiting apoptosis, thus representing a novel mechanism by which the uPA–uPAR system may affect breast cancer progression in vivo.57 This multifunctional capacity can explain why the uPA system is a powerful mediator of cancer spreading, and it has been identified as both a predictive and prognostic marker in breast cancer.
Predictive Value of uPA System as a Response Marker
Several studies have tried to establish whether uPA status could be associated with antitumor efficacy of several regimens, especially cytotoxic chemotherapy.
The predictive value of uPA/PAI-1 to forecast response to CMF regimen (ie, cyclophosphamide, methotrexate, and fluorouracil) has shown an intermediate level of evidence (ie, LOE II) and therefore remains to be confirmed in further randomized studies.58 In high-risk patients (ie, high levels of uPA and/or PAI-1), a better response was achieved, especially in grade 2 patients, thus suggesting that chemotherapy was beneficial eventually. The CMF protocol is nevertheless the therapeutic standard, irrespective of the uPA PAI-1status. The results of the European trial NNBC-3 (“Node Negative Breast Cancer 3-Europe”), using uPA/PAI-1 as a discriminating factor to predict response to anthracyclines and taxanes in patients with no lymph node metastasis, has shown a high risk of relapse in patients with high uPA and PAI-1 values compared to clinical-pathological factors.59 In addition to the trial by Janicke et al (2004), by using uPA and PAI-1 it has been shown that it is possible to classify about half of the patients with negative lymph node breast cancer as low risk, for whom chemotherapy in adjuvant conditions can be avoided, and half as high risk, who appear to benefit from adjuvant chemotherapy.60
In the multicentric prospective study conducted by the AGO group including patients without lymph node metastasis, high uPA and PAI-1 patients (ie, 182/315) were further randomized between chemotherapy (CMF: n = 88) or observation (n = 94).60
Among the 241 patients in the low-risk group, relapse-free survival at three years was 93.3% (P = 0.006) and the relapse rate was 6.7%. Before any treatment, in patients with high values of uPA and/or PAI-1 (n = 315), disease-free survival at three years was 85.3% and the relapse rate was 14.7%. These data confirm the previous findings in the literature as reviewed by Prechtl et al (2000) and led the Ethics Committee to validate this test, allowing better individualization of adjuvant treatment for patients with high levels of uPA and/or PAI-1.61 In pN0 patients, uPA/PAI-1 reached the highest level of evidence (LOE I) for the prognostic value of disease-free survival at 10 years. For Oncotype DXTM and MammaPrint®, as on date, the prognostic and predictive values have not reached the level of evidence LOE I, which clearly shows the interest of uPA/PAI-1 in predicting the response to neoadjuvant chemotherapy.62
Complementary analyses showed that adjuvant chemotherapy reduced the risk of relapse to 43.8% but over a short follow-up period only (three years).63 Similarly, when the global population (ie, with or without lymph node metastasis) was studied, response to chemotherapy was higher in patients with elevated levels of uPA/PAI-1.64,65
The clinical application of these findings would be to provide additional information that could facilitate the selection for chemotherapy. However, for definitive conclusions about the predictive value of uPA/PAI-1 as a response marker with systemic therapies in various different molecular subtypes of breast cancer, large prospective trials are warranted.
Implementing uPA/PAI-1 Determination as Part of Precision Medicine in Breast Cancer
Modern oncology has entered the era of precision medicine, a generic term that encapsulates all the effort to shift from standard to customized treatment. Individualized medicine is mostly based on huge resources to identify markers that are likely to predict the level of aggressiveness of a newly diagnosed cancer, and next to search for predictive markers of response to a given therapy. Such preliminary information can be used subsequently to help clinicians to select the best strategies (ie, administrating systemic adjuvant therapy or not) and the best drug or drugs combination to be given, should they be given.66,67 Avoiding unnecessary treatments is now a critical issue in oncology, both because of the cost of the drugs (ie, with targeted therapies) and the possible treatment-related severe toxicities (ie, with cytotoxics), thereby negatively impacting the quality of life and efficacy–toxicity balance eventually. In this respect, using reliable markers that are likely to predict a risk for relapse or metastatic spreading is a promising strategy to implement precision medicine at the bedside. Several studies have tried to develop dedicated mathematical models as in silico tools for such a purpose.68,69
From a bioguided-medicine standpoint, as a follow-up to studies performed on patients with primary breast cancer, the Receptor and Biomarker Group of the European Organization for Research and Treatment of Cancer (EORTC) has validated the prognostic role that uPA and PAI-1 could play for this kind of cancer.70 According to uPA/PAI-1 levels, therapeutic management has therefore changed.71,72 In particular, uPA and PAI-1 are critical in lymph node–negative patients because it can reduce significantly the over-treatment rate in patients with little risk of metastatic relapse.73
In accordance with the St. Gallen Consensus guidelines,73 more than 90% of node-negative patients today receive adjuvant systemic therapy, even though only 30% of these patients will eventually relapse. The low-risk group identified by uPA/PAI-1, representing about 50% of node-negative breast cancer patients, has a low risk for disease recurrence and usually excellent prognosis. This group has 10 years of disease-free survival (DFS) and overall survival (OS) of 87% and 90%, respectively, without any adjuvant systemic therapy.74,75 With today’s standards of endocrine therapy, these patients would have a 10-year OS over 90%, with much milder treatment- related toxicities as compared with chemotherapy. Such encouraging results can, therefore, be used to support individualized therapy decisions.76
Recent observations suggest that only patients considered high risk based on their values of uPA/PAI-1 shall benefit from adjuvant chemotherapy.71,72 Consequently, associating chemotherapy to novel targeted agents is justified with respect to the uPA/uPA-R-values.77 Indeed, along with canonical nodal status, uPA and PAI-1 seem to be strong predictive factors for both DFS and OS.
Conclusions and Perspectives
Refining treatment modalities is now a major trend in clinical oncology. Bioguided medicine is largely implemented in several settings (lungs, digestive, melanoma), mostly as part of the upfront determination of biomarkers predictive of response to targeted therapies. Breast cancer has pioneered the field of personalized medicine through molecular and genomic determination of cancer sub-types, supplanting historical TNM classification. uPA/PAI-1 could be an emerging, new powerful marker to further refine the picture of the disease. In addition to selecting the best treatments and strategies to be undertaken in breast cancer patients, avoiding unnecessary adjuvant therapy is now a critical issue indeed. Along with other approaches, using upfront uPA/PAI-1status determination as a marker of risk for metastatic relapse could help to better select BC patients requiring a systemic therapy. In addition, uPA/PAI-1 could help as well to forecast treatment efficacy, thus potentially being part of a bioguided therapy.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 810 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the review: AG, KE, SB. Analyzed the data: AG, KE, AD. Wrote the first draft of the manuscript: AG, AD, KE, NL, AA. Contributed to the writing of the manuscript: JC, MC, GM. Agree with manuscript results and conclusions: AG, GM, JC, MC. Jointly developed the structure and arguments for the paper: AG, KE. Made critical revisions and approved final version: AG, KE, GM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Engstrøm MJ, Opdahl S, Hagen AI, et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140(3):463–73. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12(4):207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013;5:61–70. doi: 10.4137/BIC.S9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luporsi E, Bellocq JP, Barrière J, et al. uPA/PAI-1, Oncotype DX™, MammaPrint(®) Prognosis and predictive values for clinical utility in breast cancer management. Bull Cancer. 2015;102(9):719–29. doi: 10.1016/j.bulcan.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004;10:39–49. doi: 10.2174/1381612043453559. [DOI] [PubMed] [Google Scholar]
- 8.Deryugina EI, Quigley JP. Cell surface remodeling by plasmin: a new function for an old enzyme. J Biomed Biotechnol. 2012;5:642–59. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjwa M, Moura R, Moons L, et al. Fibrinolysis-independent role of plasmin and its activators in the haematopoietic recovery after myeloablation. J Cell Mol Med. 2009;13:4587–95. doi: 10.1111/j.1582-4934.2008.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaufort N, Plaza K, Utzschneider D, et al. Interdependence of kallikrein- related peptidases in proteolytic networks. Biol Chem. 2010;391:581–7. doi: 10.1515/BC.2010.055. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Moons L, Lijnen R, et al. Urokinase-generated plasmin activates matrix metalloproteinase during aneurysm formation. Nat Genet. 1997;17:439–44. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 12.Rifkin DB. Cross-talk among proteases and matrix in the control of growth factor action. Fibrinolysis Proteolysis. 1997;11:3–9. [Google Scholar]
- 13.Ferraris GM, Sidenius N. Urokinase plasminogen activator receptor: a functional integrator of extracellular proteolysis, cell adhesion, and signal transduction. Semin Thromb Hemost. 2013;39:347–55. doi: 10.1055/s-0033-1334485. [DOI] [PubMed] [Google Scholar]
- 14.Blaisi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 15.Jo M, Thomas KS, Marozkina N, et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280:17449–57. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- 16.Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase-induced signaling in human vascular smooth muscle cells is mediated by PDGFR-beta. EMBO J. 2005;24:1787–97. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montuori N, Cosimato V, Rinaldi L, Rea VE, Alfano D, Ragno P. uPAR regulates pericellular proteolysis through a mechanism involving integrins and fMLF-receptors. Thromb Haemost. 2013;109:309–18. doi: 10.1160/TH12-08-0546. [DOI] [PubMed] [Google Scholar]
- 18.Conese M, Nykjaer A, Petersen CM, et al. Alpha-2 macroglobulin receptor/Ldl receptor-related protein (Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–22. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Alessio S, Blasi F. The urokinase receptor as an entertainer of signal transduction. Front Biosci. 2009;14:4575–87. doi: 10.2741/3550. [DOI] [PubMed] [Google Scholar]
- 20.Sidenius N, Andolfo A, Fesce R, Blasi F. Urokinase regulates vitronectin binding in vitro and in vivo by controlling receptor oligomerization. J Biol Chem. 2002;277:27982–90. doi: 10.1074/jbc.M111736200. [DOI] [PubMed] [Google Scholar]
- 21.Nykjaer A, Petersen CM, Moller B, et al. Purified alpha2-macroglobulin receptor/LDL receptor-related protein binds plasminogen activator inhibitor type-1 complex. Evidence that the alpha2- macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem. 1992;267:14543–6. [PubMed] [Google Scholar]
- 22.Kruithof EKO, Baker MS, Bunn CL. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood. 1995;86:4007–24. [PubMed] [Google Scholar]
- 23.Zhou H-M, Bolon I, Nichols A, et al. Over expression of plasminogen activator inhibitor type 2 in basal keratinocytes enhances papilloma formation in transgenic mice. Cancer Res. 2002;61:970–6. [PubMed] [Google Scholar]
- 24.Dano K, Romer J, Nielsen BS, et al. Cancer invasion and tissue remodeling –cooperation of protease systems and cell types. APMIS. 1999;107:120–7. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnsen M, Lund LR, Romer J, Almholt K, Dano K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–71. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 26.Plesner T, Ralfkiaer E, Wittrup M, et al. expression of the receptor for urokinase- type plasminogen activator in normal and neoplastic blood cells and hematopoietic tissue. Am J Clin Pathol. 1994;102:835–41. doi: 10.1093/ajcp/102.6.835. [DOI] [PubMed] [Google Scholar]
- 27.Mustjoki S, Alitalo R, Stephens RW, Vaheri A. Blast cell surface and plasma soluble urokinase receptor in acute leukemia patients: relationship to classification and response to therapy. Thromb Haemost. 1999;81:705–10. [PubMed] [Google Scholar]
- 28.Carriero MV, Del Vecchio S, Franco P, et al. Vitronectin binding to urokinase receptor in human breast cancer. Clin Cancer Res. 1997;3:1299–308. [PubMed] [Google Scholar]
- 29.Morita S, Sato A, Hayakawa H, et al. Cancer cells over express mRNA of urokinase-type plasminogen activator, its receptor and inhibitors in human non-small-cell lung cancer tissue: analysis by Northern blotting and in situ hybridization. Int J Cancer. 1998;78:286–92. doi: 10.1002/(SICI)1097-0215(19981029)78:3<286::AID-IJC4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 30.Hudson MA, McReynolds LM. Urokinase and the urokinase receptor: association with in vitro invasiveness of human bladder cancer cell lines. J Natl Cancer Inst. 1997;89:709–17. doi: 10.1093/jnci/89.10.709. [DOI] [PubMed] [Google Scholar]
- 31.Pyke C, Kristensen P, Ralfkiaer E, et al. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991;138:1059–67. [PMC free article] [PubMed] [Google Scholar]
- 32.De Petro G, Tavian D, Copeta A, Portolani N, Giulini SM, Barlati S. Expression of urokinase-type plasminogen activator (u-PA), u-PA receptor, and tissue-type PA messenger RNAs in human hepatocellular carcinoma. Cancer Res. 1998;58:2234–9. [PubMed] [Google Scholar]
- 33.Shetty S, Idell S. A urokinase receptor mRNA binding protein-mRNA interaction regulates receptor expression and function in human pleural mesothelioma cells. Arch Biochem Biophys. 1998;356:265–79. doi: 10.1006/abbi.1998.0789. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi T, Kakkar AK, Tuddenham EG, Williamson RC, Lemoine NR. Enhanced expression of urokinase receptor induced through the tissue factor-factor VII a pathway in human pancreatic cancer. Cancer Res. 1998;58:4461–7. [PubMed] [Google Scholar]
- 35.Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994;54:5016–20. [PubMed] [Google Scholar]
- 36.Yamamoto M, Sawaya R, Mohanam S, et al. Activities, localizations, and roles of serine proteases and their inhibitors in human brain tumor progression. J Neurooncol. 1994;22:139–51. doi: 10.1007/BF01052889. [DOI] [PubMed] [Google Scholar]
- 37.Duffy MJ. The biochemistry of metastasis. Adv Clin Chem. 1996;32:135–66. doi: 10.1016/s0065-2423(08)60427-8. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt M, Harbeck N, Brünner N, et al. Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers Upa and its inhibitor PAI-1. Expert Rev Mol Diagn. 2011;11:617–34. doi: 10.1586/erm.11.47. [DOI] [PubMed] [Google Scholar]
- 39.Bugge TH, Lund LR, Kombrinck KK, et al. Reduced metastasis of Polyomavirus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene. 1998;16:3097–104. doi: 10.1038/sj.onc.1201869. [DOI] [PubMed] [Google Scholar]
- 40.Almholt K, Lund LR, Rygaard J, et al. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer. 2004;113:525–32. doi: 10.1002/ijc.20631. [DOI] [PubMed] [Google Scholar]
- 41.Gold LL, Schwimmer R, Quigley J. Human plasma fibronectin as a substrate for human urokinase. Biochem J. 1989;262:529–34. doi: 10.1042/bj2620529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dano K, Andreasen PA, Grondahl-Hansen K, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 43.Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–36. [PubMed] [Google Scholar]
- 44.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J Cell Biol. 1996;271:10079–86. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 45.Kirchheimer JC, Wojta J, Christ G, Binder BR. Proliferation of a human epidermal cell line stimulated by urokinase. FASEB J. 1987;1:125–8. doi: 10.1096/fasebj.1.2.3038646. [DOI] [PubMed] [Google Scholar]
- 46.Plouet J, Moro F, Bertagnolli S, et al. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J Biol Chem. 1997;272:13390–6. doi: 10.1074/jbc.272.20.13390. [DOI] [PubMed] [Google Scholar]
- 47.Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143:949–58. [PMC free article] [PubMed] [Google Scholar]
- 48.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–7. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Rohan T. Role of insulin-like growth family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 50.Trusolino L, Comoglio PM. Scatter-factor and semaphoring receptors: cell signaling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 51.Devy L, Blacher S, Grignet-Debrus C, et al. The pro or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–54. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 52.Jo M, Thomas KS, Somloyo AV, Somlyo AP, Gonias SL. Cooperativity between the ras-ERK and Rho-Rho kinase pathway in urokinase-type plasminogen activator-stimulated cell migration. J Biol Chem. 2002;277:12479–85. doi: 10.1074/jbc.M111147200. [DOI] [PubMed] [Google Scholar]
- 53.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor- mediated cell adhesion and release? J Cell Biol. 1996;134:1563–71. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of 1196 Duffy: uPA and PAI-I in Breast Cancer the basal level activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–96. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 55.Pakneshan P, Szyf M, Farias-Eisner R, Rabbani SA. Reversal of the hypomethylation status of urokinase (uPA) promoter blocks breast cancer growth and metastasis. J Biol Chem. 2004;279(30):31735–44. doi: 10.1074/jbc.M401669200. [DOI] [PubMed] [Google Scholar]
- 56.Jovanovic J, Rønneberg JA, Tost J, Kristensen V. The epigenetics of breast cancer. Mol Oncol. 2010;4(3):242–54. doi: 10.1016/j.molonc.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephens RW, Brunner N, Janicke F, Schmitt M. The urokinase plasminogen activator system as a target for prognostic studies in breast cancer. Breast Cancer Res Treat. 1998;52:99–111. doi: 10.1023/a:1006115218786. [DOI] [PubMed] [Google Scholar]
- 58.Sternlicht MD, Dunning AM, Moore DH, et al. Prognostic value of PAI1 in invasive breast cancer: evidence that tumor-specific factors are more important than genetic variation in regulating PAI1 expression. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2107–14. doi: 10.1158/1055-9965.EPI-06-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janicke F, Prechtl A, Thomssen C, et al. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst. 2001;93:913–20. doi: 10.1093/jnci/93.12.913. [DOI] [PubMed] [Google Scholar]
- 60.Prechtl A, Harbeck N, Thomssen C, et al. Tumor-biological factors uPA and PAI-1 as stratification criteria of a multicenter adjuvant chemotherapy trial in node negative breast cancer. Int J Biol Markers. 2000;15:73–8. doi: 10.1177/172460080001500114. [DOI] [PubMed] [Google Scholar]
- 61.Harbeck N, Kates RE, Gauger K, et al. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thromb Haemost. 2004;91:450–6. doi: 10.1160/TH03-12-0798. [DOI] [PubMed] [Google Scholar]
- 62.Luporsi E, Bellocq JP, Barrière J, et al. uPA/PAI-1, Oncotype DX™, MammaPrint® Valeurs pronostique et prédictive pour une utilité clinique dans la prise en charge du cancer du sein. Oncologie. 2014;16(4):196–206. [Google Scholar]
- 63.Hery M, Delozier T, Ramaioli A, et al. Natural history of node-negative breast cancer: are conventional prognostic factors predictors of time to relapse? Breast. 2002;11:442–8. doi: 10.1054/brst.2002.0462. [DOI] [PubMed] [Google Scholar]
- 64.Luporsi É, André F, Bellocq J-P, et al. Rapport 2009 sur l’état des con-naissances relatives aux biomarqueurs tissulaires uPA/PAI-1, Oncotype DX™ et MammaPrint® dans la prise en charge du cancer du sein. Oncologie. 2010;12(2):158–63. [Google Scholar]
- 65.Ciccolini J. Editorial: targeted therapy, targeted dosing and targeted delivery in oncology: where do we stand? Curr Top Med Chem. 2012;12(15):1638. doi: 10.2174/156802612803531379. [DOI] [PubMed] [Google Scholar]
- 66.André F, Ciccolini J, Spano JP, et al. Personalized medicine in oncology: where have we come from and where are we going? Pharmacogenomics. 2013;14(8):931–9. doi: 10.2217/pgs.13.79. [DOI] [PubMed] [Google Scholar]
- 67.Hartung N, Mollard S, Barbolosi D, et al. Mathematical modeling of tumor growth and metastatic spreading: validation in tumor-bearing mice. Cancer Res. 2014;74(22):6397–407. doi: 10.1158/0008-5472.CAN-14-0721. [DOI] [PubMed] [Google Scholar]
- 68.Benzekry S, Tracz A, Mastri M, Corbelli R, Barbolosi D, Ebos JM. Modeling spontaneous metastasis following surgery: an in vivo-in silico approach. Cancer Res. 2016;76(3):535–47. doi: 10.1158/0008-5472.CAN-15-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Look MP, van Putten WLJ, Duffy MJ, et al. Pooled analysis of prognostic impact of uPA and PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst. 2002;94:116–28. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- 70.Harbeck N, Kates R, Schmitt M. Clinical relevance of invasion factors uPA and PAI-1 for individualized therapy decisions in primary breast cancer is greatest when used in combination. J Clin Oncol. 2002;20:1000–9. doi: 10.1200/JCO.2002.20.4.1000. [DOI] [PubMed] [Google Scholar]
- 71.Harbeck N, Alt U, Krüger A, et al. Prognostic impact of proteolytic factors (uPA, PAI-1, cathepsins B, D, L) in primary breast cancer reflects effects of adjuvant systemic therapy. Clin Cancer Res. 2001;7:2757–64. [PubMed] [Google Scholar]
- 72.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the treatment of primary breast cancer. J Clin Oncol. 2001;19:3817–27. doi: 10.1200/JCO.2001.19.18.3817. [DOI] [PubMed] [Google Scholar]
- 73.Jänicke F, Prechtl A, Thomssen C, et al. Randomized adjuvant therapy trial in high risk lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type I. J Natl Cancer Inst. 2001;93:913–20. doi: 10.1093/jnci/93.12.913. [DOI] [PubMed] [Google Scholar]
- 74.Harbeck N, Dettmar P, Thomssen C, et al. Risk group discrimination in node negative breast cancer using invasion and proliferation markers, six-year median follow-up. Br J Cancer. 1999;80:419–26. doi: 10.1038/sj.bjc.6690373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardoso F, Van’t Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ. Clinical application of the 70-gene profile: the MINDA CT trial. J Clin Oncol. 2008;26(5):729–35. doi: 10.1200/JCO.2007.14.3222. [DOI] [PubMed] [Google Scholar]
- 76.Schmitt M, Wilhelm OG, Reuning U, et al. The plasminogen activation system as a novel target for therapeutic strategies. Fibrinolysis. 2000;14:114–32. [Google Scholar]
- 77.Muehlenweg B, Sperl S, Magdolen V, et al. Interference with the urokinase plasminogen activator system: a promising therapy concept for solid tumors. Expert Opin Biol Ther. 2001;1(4):683–91. doi: 10.1517/14712598.1.4.683. [DOI] [PubMed] [Google Scholar]