Abstract
The Occupational Medicine Forum is prepared by the ACOEM Occupational and Environmental Medical Practice Committee and does not necessarily represent an official ACOEM position. The Forum is intended for health professionals and is not intended to provide medical or legal advice, including illness prevention, diagnosis or treatment, or regulatory compliance. Such advice should be obtained directly from a physician and/or attorney.
RESULTS
Six patients were evaluated including one women patient. The average age was 56.5 years with the age range between 43 and 66 years. There were four welders, one laboratory worker from a steel company, and one manganese ore separator worker. Occupational exposure was between 11 and 35 years. Each presented with neurological symptoms within 2 years of cession of work. Three patients met criteria for parkinsonism. Four patients had elevated urine manganese, three patients had elevated blood manganese (Table 1 and 2), and one had an abnormal magnetic resonance imaging (MRI) with an increased pallidal index Fig. 1. One of them had a significant improvement from levodopa but four of them had tried levodopa and had equivocal responses. Two patients had equivocal responses from calcium EDTA. These clinical characteristics are presented in (Table 3).
TABLE 1.
Manganese Urine Levels
| Case No | Date | Manganese Urine (μg/L)* |
|---|---|---|
| 1 | October 27,1995 | 0.004 |
| October 27,1995 | 0.010 | |
| October 27,1995 | 0.0064 | |
| December 15, 1995 | 0.0062 | |
| December 15, 1995 | 0.0064 | |
| December 15, 1995 | 0.0072 | |
| 2 | April 4, 1990 | 0.04† |
| April 10, 1990 | <0.01 | |
| June 4, 1990 | <0.01 | |
| 3 | August 18, 1994 | 2.1† |
| 4 | September 8, 2004 | 3.24† |
| September 8, 2004 | 3.24‡ | |
| September 12, 2004 | 20.52† | |
| September 13, 2004 | 17.94 | |
| 5 | July 11, 2004 | 1.96 |
| August 6, 2004 | 3.23 | |
| August 18, 2004 | 11.12 | |
| August 27, 2004 | 7.64 | |
| 6 | October 26, 1998 | 15.4‡ |
| October 26, 1998 | 20.6 | |
| October 26, 1998 | 21.1 | |
| October 26, 1998 | 28.8 |
Reference range is <10μg/L.
Based on 24-hour analysis.
24-hour manganese urine levels Blank
TABLE 2.
Manganese Blood Levels
| Case No | Date | Manganese Blood Levels (μg/L)* |
|---|---|---|
| 1 | October 9, 1995 | 35.1 |
| 5 | July 2004 | 0.041 |
| 6 | October 26, 1998 | 35.5 |
Normal manganese blood levels ranges from 7.1 to 10.5 μg/L.
FIGURE 1.
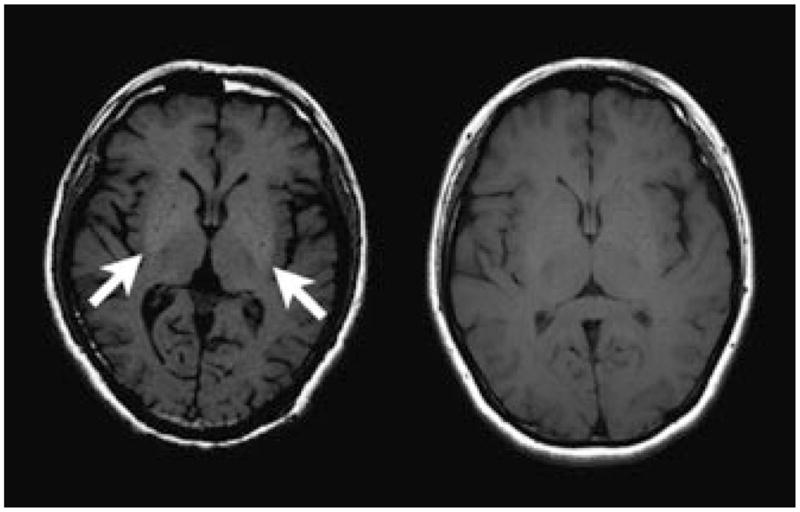
Axial T1-weighted images at the level of the basal ganglia in a patient with manganese toxicity (left) and a normal volunteer (right). Note symmetric high-T1 signal intensity within the globi pallidi compared with the surrounding white matter, a finding that has been described in manganese toxicity.
TABLE 3.
Clinical Snapshot of 6 Patients Occupationally Exposed With Manganese With Varying Neurological Findings
| No. | Age, Sex | Exposure Years, Occupation | First Symptom | Age of Onset | Clinical Characteristics | Chelation, or LDA | Abnormal MRI, MnB, MnU | Clinical Diagnosis | Further Testing? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66, Female | 35, laboratory assistant analyzing metals in ferroalloy plant | Stiffness | 54 | Resting Leg and arm tremor, hyperreflexia | Reduced tremors from DMSA, Brief response to LDA and amantadine | MnB, (2 years postexposure). No. increased MnU post–DMSA. | Unclear. Rule out cervical myelopathy, psychogenic tremor. | Cervical and Brain MRI |
| 2 | 60, Male | 32, metallurgy mine company. Stick welder, casting worker | Difficulty walking | 45 | Hypomimia, bilateral increased tone and postural tremor, retropulsion | EDTA and LDA Inconsistent | None. No increase of. MnU post–EDTA. | Early onset Atypical (postsynaptic) PD, manganese induced | Brain MRI |
| 3 | 62, Male | 19, Diesel engine factory worker. Welder | Pain | 46 | Mild bilateral postural tremor | None attempted | None | Mild Bilateral tremor. Rule out liver dysfunction. | MRI brain. Repeat LFT. |
| 4 | 50, Male | 20, Architecture and machinery factory. Stick and wire feed welder | Hand and leg tremors | 49 | Hypomimia, right resting tremor, action and postural tremor and no rigidity | Responsive, dose unknown. | Mn U, post–EDTA chelation, months after exposure | Early onset (typical/presynaptic) possibly manganese induced | MRI brain |
| 5 | 58, Male | 25, Director of Mn mining company | Bilateral hand tremors | 48 | Bilateral postural tremors, rigidity | EDTA and LDA, Not responsive | MRI: Increased signal in globus pallidus. MnU elevated post–EDTA, MnB. | Atypical parkinsonism, manganese induced | |
| 6 | 43, Male | 11, Stick welder for iron tower factory, indoor. | Bilateral hand tremors | 35 | Mild action tremor | EDTA: Brief improvement. LDA: Not attempted | MnU elevated post–EDTA, MnB | Unclear | MRI brain |
DMSA, dimercaptosuccinic acid; EDTA, ethylenediaminetetraacetic acid; LDA, lithium diisopropylamide; MnB, manganese blood levels; MnU, manganese urine levels; MRI, magnetic resonance imaging; PD, Parkinson’s disease.
Of the three patients that had clinical evidence of Parkinsonism, one of them (No. 4) presented with asymmetrical tremor and rigidity. This patient, being a welder, developed symptoms beginning at the age of 49 years. Blood and urine chemistry as well as imaging studies were not performed for this patient. He is presently taking levodopa and no longer welding. He carried the diagnosis of Parkinson’s disease (PD). One of the other patients (No. 5) whose examination met the clinical criteria for parkinsonism worked in a factory with manganese ore, and presently is the director of that factory. Urine and blood manganese concentrations were elevated in the past and MRI did in fact reveal abnormalities in the globus pallidus bilaterally. The examination revealed that he had bilateral and symmetrical action and postural tremor along with rigidity. He also had a mild resting tremor and head titubation. The third patient (No. 2) in this group developed symptoms at the age of 45 years and was a welder for 20 years before presenting. His examination revealed bilateral and symmetrical action and postural tremor with evidence of rigidity bilaterally. His urine manganese level was elevated upon first evaluation. He has tried levodopa but experienced no improvement. Two other patients (Nos. 3 and 6) were both welders and had mild bilateral postural tremor that was symmetrical without resting tremor, significant rigidity, or postural instability. One had an unusual tongue tremor. Both of these patients had abnormal urine manganese levels; one had an abnormal blood manganese level. Neither had an MRI testing. One (No. 6) did not respond significantly to calcium EDTA and the other (No. 3) did not get treatment. The patient receiving treatment presented at the age of 36 years. The other welder presented at the age of 47 years with clear neurological symptoms.
The last patient (No. 1) was a laboratory worker in a steel company. She presented with a number of complaints and was found to have elevated urine manganese and elevated blood manganese. Her examination was significant for bilateral resting upper extremity tremors as well as bilateral postural and action tremors. These were of large amplitude and changed when she stood compared to when she sat in a chair or was laid down. Reflexes were significantly increased while she sat but when she laid down the reflexes became somewhat symmetrical and normal. Magnetic resonance imaging of the brain was not accomplished. Levodopa as well as calcium EDTA, led to no significant improvement. She also was tried on the dopamine agonist. A number of these patients continued to take levodopa and reported some positive affect but could not admit to either hallucinations or in on or off phenomena.
DISCUSSION & LIMITATIONS
Occupational exposure to manganese among Chinese welders and steelworkers is evident.1–6 The evaluation of six Chinese workers in Guangxi Province with occupational exposure to manganese led to the discovery of three with evidence of parkinsonism based on the Unified Parkinson’s Disease Rating Scale. One welder presented with a classic presynaptic syndrome that responded to levodopa, but developed these symptoms at the age of 49 years, after 20 years of occupational exposure, suggesting that manganese may facilitate the development of this syndrome, clinically similar to PD. The second of four welders, who developed symptoms at the age of 45 years, had bilateral action and postural tremor with rigidity and has not responded to medication. He also had 20 years of welding experience. Two other welders were noted to have tremor but did not meet the Unified Parkinson’s Disease Rating Scale criteria. Elevated urine manganese was noted in three welders; elevated blood manganese in two. A 25-year occupational exposure to manganese in a factory worker led to a syndrome of parkinsonism that presented with bilateral action, postural tremor, mild head and resting tremor, as well as significant rigidity at the age of 48 years. In this patient, MRI results revealed abnormalities bilaterally in the globus pallidus (Fig. 1). The sixth, a steel plant laboratory worker, presented also with tremor without satisfying PD criteria. These patients reflect the varying presentation of occupational exposure to manganese. On the basis of these limited cases, the authors conclude that the clinical presentation of manganese-induced movement disorder varies considerably and some may overlap with PD among manganese-exposed patients.
This study was limited by several factors. Although data gathering was vigorous, the issues, such as language barrier, the lack of full and complete personal, medical, as well as incomplete occupational and family medical histories, are of concern. It is possible that these confounding factors may impact the ultimate diagnosis. Further limitations include the lack of clarification about the criteria for chronic manganism used in the Chinese criteria document mentioned above. It is our hope that this international collaboration will prompt further evaluation of these patients with both neuroimaging and MRI, and possibly PET along with more complete blood and urine manganese levels.
CONCLUSIONS
This preliminary clinical study reveals that occupational exposure to manganese leads to clinical manifestations of Parkinsonian syndromes with considerable variations. While the response to levodopa has been used to differentiate a manganese-induced movement disorder from PD, one patient who had a classic presynaptic syndrome and responded to levodopa is clearly manganese-intoxicated, Moreover, a case with 25-year manganese exposure shows a syndrome of parkinsonism at an early age with MRI abnormalities bilaterally in the globus pallidus. Thus, these observations support an overlap in syndromes between manganese-induced movement disorder and PD. Since occupational exposure to manganese appears to be more prevalent in China than in the United States, this continuing international collaboration, aiming at diagnosis of a large body of patients with occupational exposure and clear movement disorders, should further our understanding of the clinical presentation of manganese-related health effects from occupational exposure.
Footnotes
Part II, will present a synopsis of the evaluation including blood and urine manganese and results from one magnetic resonance image and discuss and analyze the results. Limitations and conclusions will be presented. Part I, from an earlier JOEM issue, focused on the historical background and literature supporting parkinsonism and manganese exposure, both from mining and welding. Differential diagnosis, including radiological assessment and methods were discussed.
Contributor Information
Dr. Jonathan S. Rutchik, Assistant Clinical Professor in the Department of Occupational Medicine, Division of Medicine, UCSF and practices occupational and environmental neurology with Environmental and Occupational Medicine Associates, Mill Valley, Ca.
Dr. Wei Zheng, Professor and Head, School of Health Sciences, Purdue University, West Lafayette, Ind.
Dr. Yueming Jiang, Professor and Chair, Department of Occupational Health and Toxicology, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China.
Dr. Xuean Mo, Professor and Chair, Department of Neurology, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China.
References
- 1.Cowan DM, Fan QY, Zou Y, et al. Manganese exposure among smelting workers: blood manganese-iron ratio as a novel tool for manganese exposure assessment. Biomarke rs. 2009;14:3–16. doi: 10.1080/13547500902730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan DM, Zheng W, Zou Y, et al. Manganese exposure among smelting workers: Relationship between blood manganese-iron ratio and early onset neurobehavioral alternations. Neurotoxicology. 2009;30:1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y, Zheng W, Long L, et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li GJ, Zhang L, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46:241–248. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, Zhang L-L, Li GJ, Guo W, Liang W, Zheng W. Serum concentrations of manganese and iron as the potential biomarkers for manganese exposure in welders. Neurotoxicology. 2005;26:257–265. doi: 10.1016/j.neuro.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang DX, Du XQ, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176:40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


