Abstract
We used an ultraviolet-ozone (UVO) cleaner to create substrates for atom-transfer radical polymerization (ATRP) with varying surface initiator coverage. We collected complementary time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron spectroscopy (XPS) measurements to investigate the precise chemical origin of the variation in grafting density. At short exposure times, the atomic composition underwent minor changes except for the relative amount of bromine. At longer UVO exposure times, there is clear evidence of exposure-dependent surface initiator oxidation. We interpret these data as evidence of a bromine ablation process within the UVO cleaner, with additional oxidative modification of the rest of the surface. We then used these substrates to create a series of poly(methyl methacrylate) (PMMA) brushes varying in grafting density, demonstrating the utility of this tool for the control of polymer brush density. The measured brush grafting densities were correlated with the bromine concentration measured by both ToF-SIMS and XPS. XPS and brush thicknesses correlate strongly, following an exponential decay with a half-life of (18 ± 1) s.
Graphical Abstract
Introduction
Grafting density, the number of tethered chains per unit area, is a key parameter in determining the configuration and behavior of an end-tethered polymer film.1 In the specific case of solvated films, interaction with the solvent and surface dominate the segment density profile at low grafting densities,2 while at high grafting densities, inter-chain interactions work together with the solvation effects to determine the overall configuration.3,4 It is not straightforward to target low and high grafting density regimes with a single polymerization strategy. Therefore, we seek a one-step, controllable method to vary grafting density between these regimes.
Surface-initiated atom transfer radical polymerization (SI-ATRP) is a widely used technique for the synthesis of high grafting density, end-tethered polymer films – “polymer brushes”5 – due to the flexibility, simplicity, and ease-of-access of the technique.6,7 In SI-ATRP, chains are initiated from sites bearing an appropriate halogen-bearing moiety.7 In an idealized synthesis, the grafting density of a brush would be equal to the surface density of halogen atoms in an initiator layer. In real syntheses, however, the grafting density is actually less and is considered to be proportional to the surface density of halogen atoms, with the main factors, such as Cu(II)Br2 or reducing agent concentration, contributing to the proportionality being lumped in to the “initiation efficiency.”8 In some applications, one may wish to control the grafting density of a brush by controlling the density of initiation sites. It is possible to reduce the density of initiation sites by simply depositing less initiator on the surface, but the methods of doing so present some challenges.
One method to reduce grafting density is to exert kinetic control of the deposition process, essentially halting the synthesis early. This is conceptually simple; however, we may expect deviations from a linear dependence of grafting density on deposition time, and a resulting film that may not be homogenous. Furthermore, if the film is a sub-monolayer or has gaps, the substrate chemistry remains exposed, which may be undesirable in some situations.
A similar idea is to deposit a complete initiator film and then chemically deactivate the initiating moiety. In the case of bromine, nucleophilic elimination or substitution are plausible options.9 Historically, researchers have been interested in replacing halogens with active moieties like azide,10,11 but relatively inert options like hydroxide exist as well.12 This method carries many of the disadvantages of the kinetic control method.
Another method to reduce grafting density is to “dilute” the initiator film with an inert molecule. This has been used widely, according to Barbey et al.13 (See section 2.2.8 and references therein). As an example, consider the deposition of an equal parts mixture of aminopropyltriethoxysilane and propyltriethoxysilane. If the inert molecule is similar enough to the initiator that one can assume equal reactivity, solubility, and surface coverage per molecule, we should expect a film with half the surface density of amino groups of a pure amino silane deposition. However, these assumptions are not generally valid14,15 so the dependence of grafting density on solution composition may be complicated.
Wu et al. have varied and studied grafting density by circumventing the notion of grafting density control and instead created grafting density gradients,16,17 where the grafting density (or molecular mass) of the brush is some smooth function of position on the substrate that depends on experimental conditions, e.g., the diffusion of chlorosilane vapor across a reaction chamber. Measurements performed after the polymerization from the gradient can then reveal the grafting density profile.
Ultraviolet-ozone (UVO) oxidation of surfaces can be used to make a gradient of surface properties. Roberson et al. used UVO to change the surface energy of polycaprolactone and measured the correlation of surface energy with time-of-flight secondary ion mass spectrometry (ToF-SIMS) data and cell binding.18,19 Gallant et al. involved the creation of a universal clickable gradient of alkyne groups by chemically modifying the new oxygen bearing moieties created during UVO treatment.20 These UVO processes have the interesting characteristic that they offer precise, programmable spatial control, unlike the other gradient production strategies mentioned above.
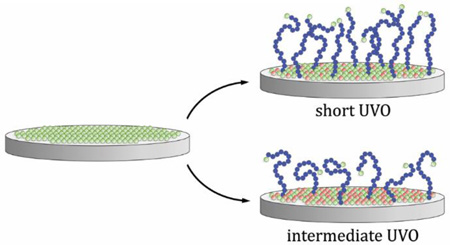
In this work, we describe and evaluate a new method of controlling the grafting density of polymer brushes grown via SI-ATRP using timed UVO treatment of the substrate prior to polymerization. We show that this treatment has the effect of eliminating or rendering inert the active halogen initiator sites, as shown schematically in Figure 1.
Figure 1.
Visualization of the reduction in polymer brush (violet chains) grafting density via the elimination of active surface initiator sites (gray circles: active initiator, red circles: deactivated initiator.) The top wafer represents an idealized SI-ATRP brush synthesis, with no exposure to UVO. The subsequent wafers represent SI-ATRP reactions performed on initiator layers subjected to increasing levels of UVO deactivation treatment.
Although this method is inspired by the aforementioned gradient studies, we use it here in a batch mode for simplicity, and for the purpose of creating larger surfaces for measurement methods that require bigger sampling volumes. UVO exposure has been use to eliminate surface initiators from regions in a pattern using a photomask,15,21 but not with limited exposure to modify grafting density in a controlled fashion. After exposing the samples to UVO over controlled time intervals, we investigated the ATRP surface initiator (Br-SI) films with ToF-SIMS and X-ray photoelectron spectroscopy (XPS) in order to gain insight into the nature of the UVO promoted chemical transformation, and studied the correlation between those measurements and the properties of brushes grown from comparable functionalized substrates.
Experimental Section
SI-ATRP
The surface initiator 11-(trichlorosilyl)undecyl 2-bromo-2-methylpropanoate (Br-SI) was synthesized and deposited on fresh, UVO-cleaned, N-doped (100) Si wafers from University Wafer as reported elsewhere.7,22 Br-SI-coated wafers were stored in the dark submerged in toluene to protect of the film from light and contamination until use. The SI-ATRP polymerization was also conducted as reported earlier,22 except the duration of the reaction was 2 h and no solution phase ATRP initiator (ethyl α-bromoisobutyrate) was used.
UVO Treatment
Oxidation treatments were performed by placing the film-coated silicon wafers in a Jelight UVO Cleaner model 342, with the sample at a nominal 3 mm sample distance from the grid lamp, which was operating at nominally 32 mW/cm2 at 253.7 nm. Wafers were placed in the chamber on top of a 3 mm glass shim, with the platform at the highest setting. The cleaner was activated for at least 10 min before use to warm the lamp. Samples were removed or inserted as quickly as possible (~ 2 s to 3 s) to minimize cooling of the lamp between treatments. Some transient effect over short (e.g., 10 s) treatments is anticipated and unavoidable.
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) was performed on a Kratos AXIS Ultra DLD spectrometer with a monochromated Al Kα source operating at 1486.6 eV and 140 W. The base pressure of the sample analysis chamber was ~ 2.0 × 10−9 Pa, and spectra were collected from a nominal spot size of 300 µm × 700 µm. The 1487 eV X-rays used in XPS cause continual bromine ablation over a wide, ill-characterized region of the sample surface during measurement (See Figure S1 in the supporting information.) Therefore, the sample was exposed only at two spots on the sample for a total count time of only 4 min each. Atomic composition was determined from survey scans over a binding energy range of (0 to 600) eV, pass energy of 160 eV, step size of 0.5 eV and dwell time of 0.1 s. Peak fitting was performed on high resolution scans of the C 1s and Br 3d regions collected using a pass energy of 20 eV, step size of 0.1 eV and sweep time of 60 s. All XPS data analysis was performed using the CasaXPS software package.
Time-of-flight secondary ion mass spectrometry
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) measurements were performed using an Iontof IV instrument equipped with a 15 keV Bi3+ analysis source oriented at an angle of 45°. The ion source was operated at a current of 0.04 pA, pulsed at 10 kHz, and rastered within a 500 µm × 500 µm area to acquire mass spectra in both positive and negative polarities. Spectra were mass calibrated to CH3+, C3H4+, C3H7O+, and C7H3+ peaks for positive polarity, and CH−, C2H−, C3H−, and C4H− peaks for negative polarity. The average ion dose density at 0.04 pA was 8.2 × 1010 ions/cm2, well below the static limit of 1012 ions/cm2 where just 1 % of the sample is consumed during analysis. SurfaceLab and MATLAB software were used to analyze the collected data. (See Figure S7 and Figure S8 in the supporting information).
Variable angle spectroscopic ellipsometry
Film and brush thicknesses were measured by variable angle spectroscopic ellipsometry (VASE). VASE was conducted on a J.A. Woolam spectroscopic ellipsometer M2000 with spectral scans from 200 nm to 1000 nm and at 65°, 70°, and 75°. Library refractive index data were used to fit the thickness of PMMA layers. The initiator layer was assumed to behave as a Cauchy material with parameters A = 1.45, B = 0.01, C = 0. Br-SI initial film thickness was (5.5 ± 0.1) nm by ellipsometry. The initiator layer thickness at each oxidation time was calculated using the C 1s signal from X-ray photoelectron spectroscopy. The C 1s signal was normalized to the Br-SI initial film thickness, multiplied by the difference between the initial film thickness and the final “brush” thickness (1.9 ± 0.3) nm. This thickness for the degraded initiator was then subtracted from the ellipsometric thickness of the entire film to yield just the thickness of the PMMA brush layer.
Contact angle
Advancing water contact angles were collected using image analysis on backlit images of 2 µL drops of deionized water taken with a JAI BM500GE camera with Computar lens (working distance 90 mm).
Results and Discussion
XPS
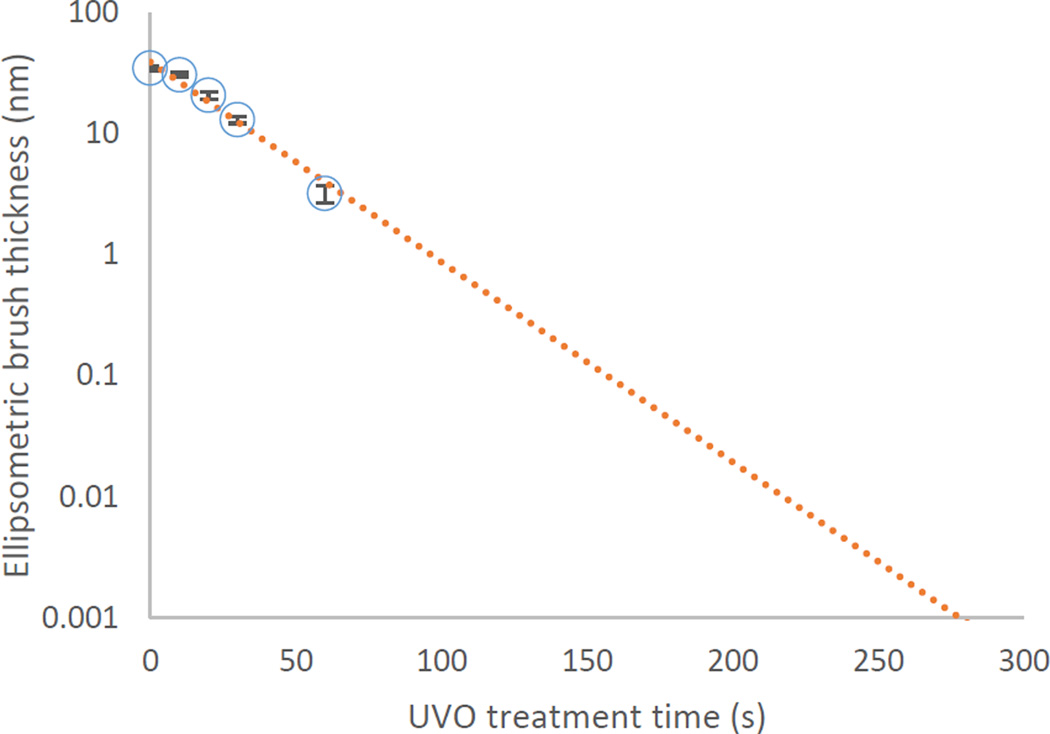
XPS is a powerful tool for the inspection of the atomic and chemical composition of the first ~ 8 nm of surfaces. However, we are predominantly interested in the surface density of bromine, which poses some challenges for quantitative measurement by XPS, namely that the hard X-rays cause bromine ablation during measurement (see Figure S1 in the supporting information). Therefore, we minimized scan time, resulting in a consistent loss of about 12 % of the bromine signal. As an unfortunate side-effect, this substantially limits the signal to noise ratio of the data, making precise component fitting challenging. Fortunately, the Br 3d peak appears strongly at about 71 eV, well separated from other signals (see Figure S5 in the supporting information). We can use this peak to obtain a relative surface density of bromine across one UVO treatment batch. A representative UVO treatment series is shown in Figure 2.
Figure 2.
Representative graph of the XPS signal Br 3d area at ≈ 71 eV (circles) and C 1s area at 286 eV (squares) from high resolution scans of Br-SI films as a function of UVO treatment time. The high resolution spectra are shown in Figure S5. An exponential fit through the Br 3d data is plotted (dotted line). Error bars represent a standard deviation of the integrated area as calculated by CasaXPS’s Monte Carlo method, which is taken as the uncertainty of the measurement.
The peak area always shows a decreasing trend with respect to UVO treatment time that appears to be well approximated by an exponential decay, which would be consistent with a first-order bromine ablation reaction during UVO treatment. We estimate from this the overall bromine half-life of (20.9 ± 1.1) s from the series in Figure 2. We reproduced this treatment across three other Br-SI films, resulting in half-lives of (19.1 ± 0.9), (15.8 ± 0.4), and (18.8 ± 1.0) s, indicating the level of consistency and reproducibility of bromine removal via UVO treatment. (See Figure S6 in the supporting information.) We performed all the rest of the data analysis and collection only on the Br-SI batch displayed in Figure 2 with a half-life of 21 s.
The C 1s signal (also shown in Figure 2) does not track the Br 3d curve exactly. It reduces only slowly at first, and then faster with longer oxidation time. We speculate that multiple oxidation reactions are necessary to transform the CH3 and CH2 groups of Br-SI into oxidized species that may escape, such as CO2. We expected C-Br bonds, being the most labile in our system (bond-dissociation energy ≈ 272 kJ/mol vs 340 to 370 kJ/mol for C-C),12 to be cleaved first, and these data seem to bear that hypothesis out to an extent. Since the ability to initiate ATRP solely depends on the presence of the alkyl halide, this was also treated as a closer approximation of initiating power of a substrate after a given exposure time.
ToF-SIMS
ToF-SIMS provides molecular information from the analyzed surface with an information depth of roughly 3 nm to 5 nm,23 making it another powerful technique to study the Br ablation with UVO exposure time. This technique provides semi-quantitative information for systems with consistent surface chemistries,24,25 such as the one being studied here. As can be seen in Figure 3, the 79Br− signal shows an exponential decay as with the XPS data, but with a measured bromine half-life of (34.6 ± 0.4) s. This discrepancy with the XPS reported half-life is possibly due to the interaction of the fitting weights from measurement error and the slightly faster-than-exponential drop over the first 20 s.
Figure 3.
ToF-SIMS 79Br− peak intensity (open circles) as a function of UVO treatment time. An exponential fit through the data is plotted (dotted line). Error bars represent a standard deviation of three scans on the same chip, which is taken as the uncertainty of the measurement.
In addition to observing the loss of bromine from the surface, the high mass resolution allows monitoring of the loss of molecules from the existing structure or the addition of oxygen, offering an insight into how UVO treatment affects the chemistry of the polymers on the surface. This is shown in Figure 4, where peaks were selected for having high loadings in the first and second principal components. (See Figure S8 in the supporting information.). The first four peaks represent signals present in the original film and absent (or reduced) from the ablated surface like 79Br−. In addition, fragments such as C279Br−/C281Br− and C2H4O79Br−/C2H4O81Br− decrease with UVO exposure (See Figure S7 in the supporting information), suggesting that bromine loss does not solely occur at the C-Br bond, but to some extent through various cleavage mechanisms. The next six peaks represent signals showing the relative concentration of O− and OH− and other oxidized species that are increasing with UVO exposure time. The last three peaks represent species that are generated during the UVO treatment but then ablate away. The species C2HO− and CHO2− in Figure 4 are not present in either the virgin or ablated films, but seem to be generated during the UVO process.
Figure 4.
ToF-SIMS signals normalized by total ion counts for several negative ions after different UVO treatment times from 0 s (left bars, black) through (10, 20, 30, 60, 100, 180) s to 300 s (right bars, white). The oxygen and hydroxide signals increase slowly as the bromine signal drops rapidly.
Contact angle
Complimentary advancing water contact angle data for the UVO treatment time series demonstrated a decreasing contact angle with increasing UVO treatment (Figure S9). While increasing hydrophilicity with exposure to UVO is expected, the correlation between these measurements and ToF-SIMS/XPS was limited. Figure S Because the measurement of contact angle represents a composite property of many factors that determine hydrophilicity, quantitative correlation of this value to prevalence of a specific chemical species would be inappropriate. Contact angle was used as a qualitative check on the accumulation of other products of the surface oxidation.
SI-ATRP
We now investigate the effect of UVO treatment on the properties of brushes grown from Br-SI films. PMMA brushes grown in the same reaction solution from one batch of Br-SI films vary in thickness depending on the extent of UVO treatment. As seen in Figure 5, the trend is apparently exponential, and with a similar half-life to the one seen in the bromine signals of XPS and ToF-SIMS, (18 ± 1) s. By 100 s, the thickness of the brush has fallen below the experimental noise level and in fact are calculated to be slightly negative via the spectroscopic ellipsometry fit. It may be possible to detect the presence of some grafted chains after 100 s by another technique, but at this point we cannot demonstrate control of thickness below 3 nm (11 % of the thickness of the brush grown from the untreated film).
Figure 5.
PMMA brush thickness as measured by VASE (circles) as a function of UVO treatment time for ATRP reactions. An exponential fit through the data is plotted (dotted line). Error bars represent a standard deviation of three scans on the same chip, which is taken as the uncertainty of the measurement. UVO treatments of 100 s or longer had a nominal thickness of less than zero, and so cannot be plotted.
If we assume that grafting density plays little or no role in determining the final degree of polymerization, or the average density, of the PMMA brush chains, then this trend in thickness can be ascribed directly to variation in grafting density via dimensional analysis:26
| (1) |
Here, σ is the grafting density in chains per nm2, h is the thickness of the dry brush, ρ is the density of the brush, usually taken to be equal to the bulk density of the free polymer, NA is Avogadro’s number, and Mn is the number average molar mass of the end-tethered polymer chains. After linking thickness and grafting density in this way, Figure 5 serves as a demonstration that UVO treatment of Br-SI films can be used to vary the grafting density by reducing the amount of active bromine sites. Since the polymers are tethered to the surface, we have not measured Mn for this brush, so although Equation 1 would hold for all our results, we are unable to directly calculate a number for grafting density. We can, however, compare the brush grafting densities to the XPS and SIMS bromine density results by a judicious normalization of the data.
The integrated Br 3d area measured in XPS and the intensity of the 79Br− bromine peak signal measured in ToF-SIMS are proportional to the bromine density on the surface, and therefore also proportional to grafting density assuming equal reactivity and initiation rates of each bromine on the surface. Likewise, the thickness of the dry brush is proportional to grafting density according to Equation 1. Let us collectively term these measurements y. Then:
| (2) |
We have argued that each measurement is well described by an exponential decay with fitted prefactor y0 and half-life t½:
| (3) |
We now define a normalized grafting density that should overlap for each measurement:
| (4) |
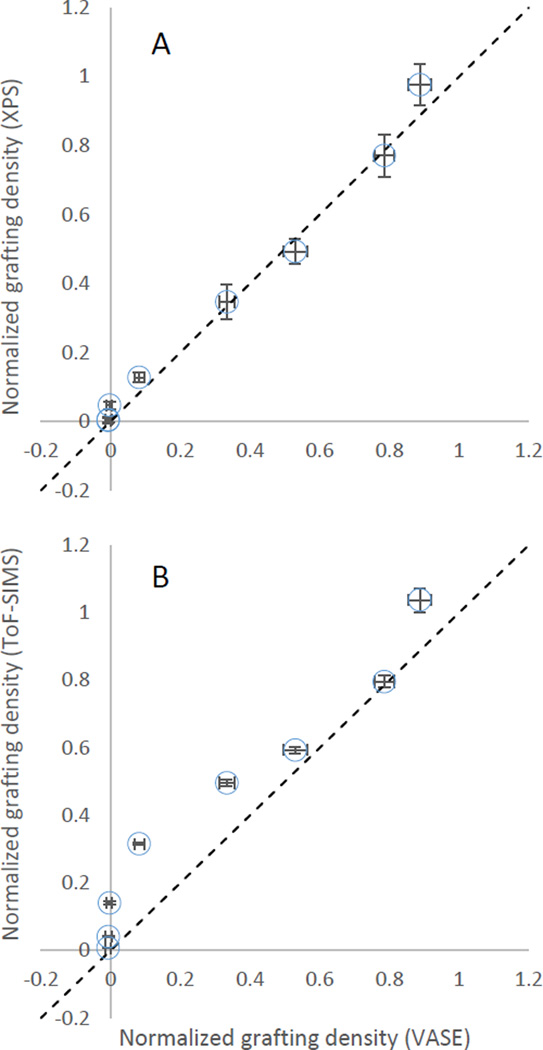
bringing the data from VASE, ToF-SIMS, and XPS into the same scale without giving too much weight to the initial point. This treatment results in Figure 6 and shows the degree to which bromine removal measurements correlate with final brush thickness.
Figure 6.
Comparison of the normalized grafting densities calculated from ATRP brush thickness measured via ellispsometry and the normalized estimates of bromine density measured via (A) XPS and (B) ToF-SIMS. The dashed line is the identity line y = x.
It would appear from the correlation plots that the XPS bromine signal has a stronger correlation with the final brush thickness than the ToF-SIMS bromine signal. We do not know the origin of the difference in performance between these measurement techniques, although we may speculate. The ToF-SIMS data could be biased due to gas-phase secondary ion reactions or some other non-linear effect on surface concentration versus signal intensity, which would not occur in XPS. Alternatively, ToF-SIMS is more surface sensitive than XPS (3 nm to 5 nm vs 8 nm penetration depth, respectively) and so may be sensitive to bromine gradients developing in the film which do not affect final brush thickness. Nevertheless, it is clear that the best balance between instrument availability and predictive strength is found in XPS.
Summary and Conclusions
In this work we created bromine-bearing Br-SI films and treated them with UVO to reduce the bromine surface density and ultimately achieve a reduction of the grafting density of the PMMA brushes grown from these substrates. We have measured the effect of this UVO treatment on Br-SI films with two surface sensitive techniques: XPS and ToF-SIMS. Comparison of those measurements to the thickness of PMMA brushes shows that XPS measurements of bromine have the strongest correlation to grafting density. With the information we have presented here, researchers interested in performing experiments on varying-grafting-density ATRP brushes have a new method of creating their desired films. Grafting densities can be controlled over an order of magnitude, and the relative grafting density can be predicted before polymerization if desired by measuring the initiator concentration. If selective ablation of the initiation site is desired, oxidation time can be limited to 30 s where the rate of bromine removal is much larger than the rate of carbon removal. The only side-product of the process is the hydrophilic change in surface energy from the new oxygen bearing moieties, which we have identified (see Figure 4 for reference.) Using this method to reduce grafting density relative to the “native” maximum is effective, controllable, and repeatable for flat, open surfaces like the wafers used in this study. We have used UVO to ablate bromine in this study; however, we suspect that it should be broadly effective for other organic surface initiation films, such as those bearing nitroxide or trithiocarbonate groups. We have already used this process to control grafting density for an upcoming manuscript,27 and we hope other investigators will find it useful to study the effects of grafting density in their own systems.
Supplementary Material
Acknowledgments
Funding Sources
R. Sheridan wishes to acknowledge postdoctoral research support from the National Research Council Research Associateship Program.
Abbreviations
- UVO
ultraviolet/ozone
- ATRP
atom transfer radical polymerization
- ToF-SIMS
time of flight secondary ion mass spectrometry
- XPS
X-ray photoelectron spectroscopy
- PMMA
poly(methylmethacrylate)
- SI-ATRP
surface initiated atom transfer radical polymerization
- Br-SI
bromine surface initiator (11-(trichlorosilyl)undecyl 2-bromo-2-methylpropianoate)
- VASE
variable angle spectroscopic ellipsometry.
Footnotes
Associated Content
Supporting information. XPS (Figure S1 – Figure S5) and ToF-SIMS (Figure S7 and Figure S8) data and analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose. Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.de Gennes PG. Conformations of Polymers Attached to an Interface. Macromolecules. 1980;13(5):1069–1075. [Google Scholar]
- 2.Adamuţi-Trache M, McMullen WE, Douglas JF. Segmental Concentration Profiles of End-Tethered Polymers with Excluded-Volume and Surface Interactions. J. Chem. Phys. 1996;105(11):4798. [Google Scholar]
- 3.Milner ST, Witten TA, Cates ME. A Parabolic Density Profile for Grafted Polymers. Europhys. Lett. 1988;5(5):413–418. [Google Scholar]
- 4.Zhulina EB, Borisov OV, Pryamitsyn VA, Birshtein TM. Coil-Globule Type Transitions in Polymers. 1. Collapse of Layers of Grafted Polymer Chains. Macromolecules. 1991;24(1):140–149. [Google Scholar]
- 5.Edmondson S, Osborne VL, Huck WTS. Polymer Brushes via Surface-Initiated Polymerizations. Chem. Soc. Rev. 2004;33(1):14–22. doi: 10.1039/b210143m. [DOI] [PubMed] [Google Scholar]
- 6.Matyjaszewski K, Dong H, Jakubowski W, Pietrasik J, Kusumo A. Grafting from Surfaces For “everyone”: ARGET ATRP in the Presence of Air. Langmuir. 2007;23(8):4528–4531. doi: 10.1021/la063402e. [DOI] [PubMed] [Google Scholar]
- 7.Matyjaszewski K, Miller PJ, Shukla N, Immaraporn B, Gelman A, Luokala BB, Siclovan TM, Kickelbick G, Vallant T, Hoffmann H, et al. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules. 1999;32(26):8716–8724. [Google Scholar]
- 8.Sumerlin BS, Neugebauer D, Matyjaszewski K. Initiation Efficiency in the Synthesis of Molecular Brushes by Grafting from via Atom Transfer Radical Polymerization. Macromolecules. 2005;38(3):702–708. [Google Scholar]
- 9.Hänsch C. Surface Chemistry on Self-Assembled Monolayers. Eindhoven University of Technology. 2009 [Google Scholar]
- 10.Balachander N, Sukenik CN. Functionalized Siloxy-Anchored Monolayers with Exposed Amino, Azido, Bromo, or Cyano Groups. Tetrahedron Lett. 1988;29(44):5593–5594. [Google Scholar]
- 11.Balachander N, Sukenik CN. Monolayer Transformation by Nucleophilic Substitution: Applications to the Creation of New Monolayer Assemblies. Langmuir. 1990;6(11):1621–1627. [Google Scholar]
- 12.Wade LG. Organic Chemistry. Upper Saddle River, NJ: Prentice Hall; 2003. The Study of Chemical Reactions; p. 134. [Google Scholar]
- 13.Barbey R, Lavanant L, Paripovic D, Schüwer N, Sugnaux C, Tugulu S, Klok H-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009;109(11):5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 14.Chechik V, Stirling CJM. Reactivity in Self-Assembled Monolayers: Effect of the Distance from the Reaction Center to the Monolayer–Solution Interface. Langmuir. 1998;14(1):99–105. [Google Scholar]
- 15.Tugulu S, Barbey R, Harms M, Fricke M, Volkmer D, Rossi A, Klok H-A. Synthesis of Poly(methacrylic Acid) Brushes via Surface-Initiated Atom Transfer Radical Polymerization of Sodium Methacrylate and Their Use as Substrates for the Mineralization of Calcium Carbonate. Macromolecules. 2007;40(2):168–177. [Google Scholar]
- 16.Wu T, Efimenko K, Genzer J. Combinatorial Study of the Mushroom-to-Brush Crossover in Surface Anchored Polyacrylamide. J. Am. Chem. Soc. 2002;124(32):9394–9395. doi: 10.1021/ja027412n. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Efimenko K, Vlček P, Šubr V, Genzer J. Formation and Properties of Anchored Polymers with a Gradual Variation of Grafting Densities on Flat Substrates. Macromolecules. 2003;36(7):2448–2453. [Google Scholar]
- 18.Roberson SV, Fahey AJ, Sehgal A, Karim A. Multifunctional ToF-SIMS: Combinatorial Mapping of Gradient Energy Substrates. Appl. Surf. Sci. 2002;200(1–4):150–164. [Google Scholar]
- 19.Roberson SV, Sehgal A, Fahey AJ, Karim A. Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) for High-Throughput Characterization of Biosurfaces. Appl. Surf. Sci. 2003;203–204:855–858. [Google Scholar]
- 20.Gallant ND, Lavery KA, Amis EJ, Becker ML. Universal Gradient Substrates for “Click” Biofunctionalization. Adv. Mater. 2007;19(7):965–969. [Google Scholar]
- 21.Chen T, Amin I, Jordan R. Patterned Polymer Brushes. Chem. Soc. Rev. 2012;41(8):3280. doi: 10.1039/c2cs15225h. [DOI] [PubMed] [Google Scholar]
- 22.Orski SV, Sheridan RJ, Chan EP, Beers KL. Utilizing Vapor Swelling of Surface-Initiated Polymer Brushes to Develop Quantitative Measurements of Brush Thermodynamics and Grafting Density. Polymer. 2015;72(0):471–478. [Google Scholar]
- 23.Muramoto S, Brison J, Castner DG. Exploring the Surface Sensitivity of TOF-Secondary Ion Mass Spectrometry by Measuring the Implantation and Sampling Depths of Bi N and C 60 Ions in Organic Films. Anal. Chem. 2012;84(1):365–372. doi: 10.1021/ac202713k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner MS, Castner DG. Characterization of Adsorbed Protein Films by Time-of-Flight Secondary Ion Mass Spectrometry with Principal Component Analysis. Langmuir. 2001;17(15):4649–4660. doi: 10.1002/1097-4636(20011205)57:3<432::aid-jbm1186>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Wagner MS, McArthur SL, Shen M, Horbett TA, Castner DG. Limits of Detection for Time of Flight Secondary Ion Mass Spectrometry (ToF-SIMS) and X-Ray Photoelectron Spectroscopy (XPS): Detection of Low Amounts of Adsorbed Protein. J. Biomater. Sci. Polym. Ed. 2002;13(4):407–428. doi: 10.1163/156856202320253938. [DOI] [PubMed] [Google Scholar]
- 26.Brittain WJ, Minko S. A Structural Definition of Polymer Brushes. J. Polym. Sci. Part A Polym. Chem. 2007;45(16):3505–3512. [Google Scholar]
- 27.Orski SV, Chan EP, Sheridan RJ, Beers KL. Contributions of Chain Conformation to Solvent Quality within Polymer Thin Films: Brushes versus Gels. (in prep) [Google Scholar]
- 28.Muramoto S, Graham DJ, Wagner MS, Lee TG, Moon DW, Castner DG. ToF-SIMS Analysis of Adsorbed Proteins: Principal Component Analysis of the Primary Ion Species Effect on the Protein Fragmentation Patterns. J. Phys. Chem. C. 2011;115(49):24247–24255. doi: 10.1021/jp208035x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belu AM, Graham DJ, Castner DG. Time-of-Flight Secondary Ion Mass Spectrometry: Techniques and Applications for the Characterization of Biomaterial Surfaces. Biomaterials. 2003;24(21):3635–3653. doi: 10.1016/s0142-9612(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 30.Wagner MS, Shen M, Horbett TA, Castner DG. Quantitative Analysis of Binary Adsorbed Protein Films by Time of Flight Secondary Ion Mass Spectrometry. J. Biomed. Mater. Res. 2003;64A(1):1–11. doi: 10.1002/jbm.a.10263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.