Abstract
The angiotensin II type 1 receptor (AGTR1) plays an integral role in blood pressure control, and is implicated in the pathogenesis of hypertension. Polymorphisms within this gene have been extensively studied in association with hypertension; however, findings are conflicting. To clarify these data, we conducted a systematic review of association studies of AGTR1 polymorphisms and hypertension, and performed a meta-analysis of the rs5186 variant. Results show that the currently available literature is too heterogeneous to draw meaningful conclusions. The definition of hypertension and gender composition of individual studies helps to explain this heterogeneity. Although the structure and splicing pattern of AGTR1 would suggest a likely effect of polymorphisms within the promoter region on gene function, few studies have been conducted thus far. In conclusion, there is insufficient evidence that polymorphisms in the AGTR1 gene are risk factors for hypertension. However, most studies are inadequately powered, and larger well-designed studies of haplotypes are warranted.
BACKGROUND
Gene
The renin-angiotensin system (RAS) plays a fundamental role in blood pressure maintenance and is implicated as a likely etiologic factor in the development of hypertension.1 Angiotensin II is central to the pathway of the RAS, causing vasoconstriction, sodium, and water retention and is tightly intertwined with the cascade of inflammatory, thrombotic, and fibrotic factors.1,2 These effects are mediated directly and indirectly via two distinct receptors, angiotensin II receptor type 1 (AGTR1) and type 2 (AGTR2).2 The bulk of the literature supports AGTR1 as a likely culprit in pathologic states such as hypertension. In fact, angiotensin receptor blockers specific to AGTR1 are commonly prescribed antihypertensive medications which have also been shown to reduce the risk of other cardiovascular endpoints, independent of their antihypertensive effects.3,4 As such, the AGTR1 is an excellent candidate gene in the etiology of hypertension and other cardiovascular diseases (CVD).
The AGTR1 gene is located on chromosome 3q21–25 and is more than 55 Kb long.5,6 Four transcription initiation sites have been described.5,7–9 AGTR1 is composed of five exons, with the first four encoding the 5′ untranslated region (UTR) and the fifth being the coding region.5 Five transcript variants have been reported.5 The abundance of variant 5 is negligible and some have discounted the existence of exon 4.7 Exon 3 contains an ATG start site that is in-frame with the ATG start site of the open reading frame of exon 5. Hence, transcript variant 3 and 4 wherein exon 5 is juxtaposed to exon 5 produces a receptor isoform with an additional 32 amino acids at the N-terminus.7 Its presence has been substantiated in vivo and seems to result in decreased affinity for angiotensin II.10 The five AGTR1 receptor variants also vary with respect to their relative tissue expression, with the heart and kidney mostly expressing variants 1 and 2.10
The single promoter in the 5′ region contains multiple regulatory sequences including several potential TATA boxes, two CAAT boxes, two overlapping SP1 recognition sequences, a GC box, and a cyclic-AMP-induced responsive element.5,9,11 Located in the 3′ region are two polyadenylation signals (AATAAA) and six nucleotide motifs in the 3′ region which correspond to an AUUUA sequence which may influence mRNA stability during posttranscriptional regulation.8 The AGTR1 product is found predominantly in vascular smooth muscle cells and the heart, adrenal gland, and kidney.2 The predominant short isoform is composed of 359 amino acids and has a molecular mass of 41.1 kDa.8 The longer isoform is composed of 391 amino acids and is an additional 3.8 kDa larger.7 It is a G-protein coupled receptor spanning seven transmembrane domains.8 The protein sequence of the gene product is well conserved.7
Gene variants and frequency
Search of NCBI db single nucleotide polymorphisms (SNPs) build 127 for SNPs of the AGTR1 gene validated by both frequency and cluster data yielded 278 entries with 15 being insertion/deletion polymorphisms and 262 SNPs. Located 213–214 base pairs upstream from exon 1 are two adjacent SNPs listed in dbSNP as a single insertion/deletion polymorphism (rs422858).9 Review of dbSNP submissions and population data demonstrate them to be SNPs in linkage disequilibrium (LD) as no evidence of a deletion could be found. For this polymorphism, we refer to the major allele as AG and the minor allele as CC. Four SNPs lie within the coding region, two of which are synonymous (rs5182 and rs5183) and two nonsynonymous polymorphisms. One nonsynonymous SNP (rs12721226) results in a change from alanine to threonine at amino acid position 163 and another (rs12721225) a change from alanine to serine at amino acid position 244. The one polymorphism not documented in dbSNP is a CA dinucleotide repeat microsatellite polymorphism located in the 3′UTR, which has a polymerase chain reaction product ranging between 128 and 146 basepairs.12,13
Online-only Tables 1A and 1B display allele and genotype frequencies of polymorphisms genotyped in populations of at least 100 apparently healthy, unrelated individuals from similar ancestry. For inclusion, studies had to be published in the English language and genotype distribution in Hardy-Weinburg Equilibrium. Several studies refer to the same polymorphism by different names depending on the method of nomenclature, reference sequence, and choice of initiation site. Hence, for the purpose of clarity polymorphisms will be referred to by their rs numbers. As recommended by the Human Genome Variation Society the precise location of each SNP is listed according to the reference genomic sequence (AF24599), and that referenced by the literature.14
The most well-studied AGTR1 SNP is rs5186 (also termed the A1166C variant) located in the 3′ UTR. Although this SNP does not lie within a coding region or splice site, it has been hypothesized that it may affect mRNA stability and transcription, or alternatively be in LD with another polymorphism of regulatory significance. Frequencies of this variant range from 0.19–0.31 in populations of European descent,15–22 0.03–0.11 in populations of Asian descent,23–27 and 0.05–0.08 in studies of populations of African descent.28–33
Data on the function of AGTR1 polymorphisms is limited. One study utilized computational software to identify variations that have the potential to create and destroy transcription factor binding sites.34 The rs275652 SNP and the rs422858 polymorphisms may have such an effect; however, these need to be tested experimentally. Functional analysis of three nonsynonymous mutants have demonstrated altered binding affinities, cell surface expression and response to angiotensin II.35 Still, these mutants have yet to be confirmed by cluster or frequency data as they were induced experimentally. A haplotype within the promoter region, T(rs275651) T(rs275652) AG(rs422858) A(rs275653), may have increased promoter activity in adrenal cortical and vascular smooth muscle cells.36 It was proposed that this may be due to an increased binding of transcription factor C/EBP to the oligonucleotide containing the A(rs275653) variant. These data were presented in abstract form and complete publication including methods is awaited.
In vitro experiments of AUF1, an mRNA-binding protein, did not reveal any differences in binding capacity to the 3′ UTR between the A and C alleles of the rs5186 polymorphism.37 Some have found an increase in mRNA levels associated with the C allele of rs5186.38 On the contrary, Abdollahi et al.39 found rs5186 AGTR1 mRNA levels to be 0.8-fold lower in heterozygotes than major allele homozygotes, and 0.27-fold lower in minor allele homozygotes. Platelet AGTR1 binding sites do not vary by rs5186 genotype.40
Haplotypes
Data from the International HapMap Project reveal three recombination hotspots within the AGTR1 gene.41 Caucasian populations yield two haplotype blocks; combined Japanese and Chinese populations three blocks; and African populations four haplotype blocks. Block one is the largest, spanning 16 kb and encompassing exon 2 and flanking intronic sequences.41 Block two is restricted to intron 2 as is the third block for African populations. Haplotype block three in Asian populations encompasses exon 3 and flanking intronic sequences. African populations possess a fourth haplotype block that is isolated to intron 3.
Antonellis et al.6 established a SNP map of the AGTR1 gene using a new protocol of long-range PCR and fragment cloning. 6 LD was greatest within 30kb and D′ was at least 0.70 within 10 kb.6 Polymorphisms within the promoter region have exhibited extensive LD as have SNPs in exon five.39, 42–44 Additionally, LD has been demonstrated between rs5186 and SNPs within the coding region.39 Haplotype frequencies of the AGTR1 gene in populations >100 individuals of similar ancestry are illustrated in Table 1. For inclusion, variants had to be found within dbSNP build 127.
Table 1.
Allele frequencies for CA repeat polymorphism within the 3′ UTR of the AGTR1 gene between positions 60117 and 60256 of genomic reference sequence AF24599
Hypertension
According to the WHO, the current definition of hypertension is the persistent elevation of blood pressure above 140/90, while at rest, documented on at least three separate occasions. 45 In diabetics, blood pressure above 130/80 is defined as pathologic.45 There are multiple secondary causes of hypertension, including hyperaldosteronism, Cushing disease, and renal artery stenosis.46 Essential hypertension (EH) is diagnosed when there is no obvious etiology. It is a complex, multifactorial polygenic disease, and is likely not one disease unto itself. Physiologic processes that contribute to the development of hypertension and may account for phenotypic variation include salt sensitivity, arterial compliance, vasoreactivity, and activity of the renin-angiotensin and adrenergic systems.
Hypertension is extremely common, occurring in 24% of all Americans and 28% in the African American population.47 It was estimated in the year 2000 that more than one billion people have hypertension and this number may increase by an additional 4% by the year 2025.48 The highest prevalence of hypertension is in established market economies, former socialist economies, and Latin American and Caribbean nations. 48 Estimates in these regions range between 35 and 41% in men and 37–39% in women. The lowest prevalence of hypertension is in India and southeastern Pacific regions including Korea, Thailand, and Taiwan.48 These estimates are as low as 17–21% in men and 14–21% in women. In all regions, hypertension prevalence increases with age. Systolic blood pressure levels are higher, and hypertension more prevalent in men than women in younger age groups, whereas the reverse tends to be true in higher age groups across populations, suggesting a likely interaction between these two factors.48, 49 Hypertension is an insidious disease, rarely resulting in acute symptoms. When left uncontrolled, however, it can lead to a multitude of CVD such as ischemic heart disease (IHD), stroke, congestive heart failure (CHF), and kidney disease. There is a log-linear relationship between blood pressure and relative risk of cardiovascular endpoints.50 Worldwide, it is estimated that 49% of ischemic heart disease, 62% of stroke, 76% of a composite outcome of EH, congestive heart failure, and kidney disease and 14% of other CVD (retinopathy, peripheral vascular disease) are attributable to a mean systolic blood pressure (SBP) >115 mm Hg.51 In the year 2000, approximately 12.8% of all deaths (7.1 million) and 4.4% of all disability adjusted life-years (64.3 million) were due to suboptimally controlled blood pressure.51 In the United States, it is estimated that as many as 50% of hypertensives are uncontrolled even when under the care of a physician.45 In some populations, especially African Americans, hypertension is difficult to control even with multiple antihypertensive medicines.52 Blood pressure levels and estimates of hypertension prevalence are highly variable even within regions of similar ethnic ancestry.48,49 Historically, studies within the United States documented higher rates of hypertension in individuals of African descent than European descent, suggesting a genetic predisposition to hypertension.47 Recent data, however, demonstrate similar hypertension prevalence between African Americans and Caucasian individuals residing in European nations.53 It has been suggested that environmental factors play at least as great a role in blood pressure variation as does genetics.49
Obesity, insulin resistance/diabetes and obstructive sleep apnea are tightly intertwined; however, each poses an independent risk factor for hypertension.54–56 Obesity results in impaired natriuresis and glomerular hyperfiltration and resultant renal injury which then exacerbates sodium retention.54 Obstructive sleep apnea has been found to have similar physiologic effects.57 Both obesity and insulin resistance lead to endothelial dysfunction via shared and unshared pathways that impact hormone, cytokine, and redox balance.58 Hyperglycemia in addition to insulin resistance promotes vascular changes and resultant hypertension via formation of advanced glycation end products, increased reactive oxygen species, and altered growth factor and cytokine production.59
The effect of salt intake on blood pressure variation across populations is well established.60,61 An increase of 100mmol/day of salt intake has been estimated to account for a 3–6 mm Hg increase in systolic pressure and a 0–3 mm Hg increase in diastolic blood pressure.62 Epidemiologic data suggest that at least in some populations, individuals with active lifestyles are less prone to hypertension.63,64 Alcohol is also associated with hypertension, and the previous notion that moderate alcohol intake or specific forms of alcohol was cardioprotective has been dismissed.65 The negative effect of alcohol intake on blood pressure is dose-linear and seems to have a greater effect in men than women.66 Even when environmental factors are optimized, many continue to manifest with elevated blood pressures as a result of genetic predisposition. Hypertension has long been noted to exhibit familial clustering, with heritability estimates ranging between 30 and 50%.67–70 The genetic basis of hypertension and current methodologic/epidemiologic roadblocks to its unraveling has been extensively reviewed.71,72 Both linkage and association studies of hypertension-related phenotypes have largely been disappointing with discrepant results due to a combination of insufficient power, poor phenotyping, population stratification, epistasis, and insufficient attention to gene-environment interactions.71
Genome-wide linkage studies have yielded largely divergent results with the exception of a quantitative trait locus for hypertension and/or blood pressure on 2p.73–76 Evidence does exist in support of an association between the endothelial nitric oxide synthase gene3 27 base-pair variable number tandem repeat polymorphism in intron 4 (4a/b) and hypertension under a recessive model in Caucasian and Asian populations.77 Notably, findings were homogeneous even between studies of different ethnicity. Because of the major role of the RAS on blood pressure control, many of its components have been extensively studied with regard to hypertension. Results have been largely discrepant with respect to the angiotensionogen M235T, 78, 79 angiotensin converting enzyme (ACE) insertion/ deletion, 79 and aldosterone synthase (CYP11B2) C-344T polymorphisms.80
Objectives
Numerous studies have been published associating polymorphisms of AGTR1 with hypertension, however results have been inconsistent. The purpose of this review is (1) to systematically present data relating AGTR1 gene polymorphisms in association with hypertension; and (2) to perform a meta-analysis of association studies of the rs5186 SNP and hypertension to both understand the relationship between this genetic variant and hypertension across multiple populations, and the apparent heterogeneity of findings inherent in the current literature.
METHODS
We searched PubMed (The National Library of Medicine) using the search terms “AGTR1 polymorphism” including dates 1966–March 2007. Additionally, the bibliographies of retrieved articles were reviewed for additional papers. Abstracts from the annual meetings of the American Heart Association and American Society of Hypertension were also searched for the years 1994–2006. The ISI Web of Science database was also searched for abstracts using the terms “AGTR1 Polymorphism Hypertension.” Inclusion criteria were: publication in the English language, either a case-control or case cohort study design, outcome of EH only, allele and/or genotype frequencies reported, stratification by ethnic background when more than one ethnic group was included and study participants at least 18 years of age (as the etiology of hypertension in children may be distinct from that in adults). Minor allele frequencies and genotype distributions were extracted from publications which focused on haplotype analysis, when possible. We additionally attempted to obtain genotype distributions of hypertensive and control groups in publications that restricted their analysis to minor allele frequencies.
For the rs5186 meta-analysis, the Stata Version 9.0 (Stata-Corp: College Station, TX) user-written program METAN (Bradburn, Deeks, and Altman) was used for analysis. We began by assessing whether there was heterogeneity within populations of similar ancestral descent. Some populations were difficult to classify, and hence were placed a priori into the population with the closest minor allele frequency. The DerSimonian and Laird random-effects model was employed to assess heterogeneity.81 The cut point for the χ2 test of heterogeneity from the Mantel-Haenszel model was set at P<0.10; this value was chosen because tests of heterogeneity are often underpowered. 82 We further assessed heterogeneity using the χ2 statistic (the variance between studies) and the τ2 statistic (the proportion of total variance that is between studies); we interpreted at least 33% variance between studies as indicative of heterogeneity, precluding pooling of summary estimates across studies.83 Because there were only two studies of populations of African ancestry, we did not assess heterogeneity within this stratum.
Heterogeneity between studies may be due to variability in study design characteristics or populations of study. For example, studies with older populations may show weaker associations between genotype and hypertension because competing risk factors predominate in older adults. Therefore, we undertook a meta-regression of available and relevant study features including: hypertension definition (“SBP ≥ 160 mm Hg and/or on medication” vs. “SBP ≥ 140mmHg and no mention of medication”), mean age of cases, % male composition of cases, total cholesterol levels, and body mass index (BMI). Because of the limited number of studies conducted within Asian and African populations, meta-regression was conducted only within the European ancestry population. For predictors significant within the meta-regression (P < 0.10, due to poor power using a small number of studies), stratified meta-analysis was performed with testing of between-study heterogeneity within each stratum.84 Additionally, multiple publications reported gender-specific data, and hence we ran stratified meta-analyses for these studies.
Because multiple and varying models of genetic effects for the hypertension phenotype have been assessed in the literature, we calculated odds ratios using multiple genetic models. For all polymorphisms the minor allele (a) versus the major allele (A), is considered the risk marker for hypertension. First, we calculated pairwise contrasts of the aa versus AA genotype and the Aa versus AA genotype (2 df tests). Next, we employed an additive model, which assumes a monotonic increase in association with hypertension as one moves from zero to one and one to two copies of the minor allele. Finally, we calculated associations under an autosomal-dominant model (AD) that combines the aa and Aa genotypes in the numerator; and an autosomal-recessive model (AR) that combines the Aa and AA genotypes in the denominator. We attempted to infer the most likely model of inheritance by regressing the log-odds- ratio of hypertension of the homozygous aa-versus-AA contrast (x-axis) against the heterozygous Aa-versus-AA contrast (y-axis), using results of each study. The slope of the regression parameter (λ) will be zero under a perfectly AR model (only the aa genotype is associated with disease), one under a perfectly AD model (either one or two copies of the a allele confers risk of disease), and 0.5 under a codominant model.85
Publication bias was assessed using Begg rank correlation test, Egger weighted regression test, and visual inspection of funnel plots of standard error of the log-odds-ratio against the log-odds-ratio. Egger test is considered the most sensitive of these tests, although funnel plots may reveal publication bias not detected by underpowered formal statistical tests.86
RESULTS
Hypertension
There were 38 studies (one studied two distinct populations) which satisfied inclusion criteria and analyzed the rs5186 AGTR1 polymorphism (Table 2) and eight which analyzed other SNPs in relation to hypertension categorized as a dichotomous variable (Table 2). Two studies were excluded due to analysis of redundant populations,87, 88 one that did not genotype its own controls,89 and one because it did not clarify genotype categorization.90 Two studies analyzed haplotypes of the AGTR1 gene, and risks for individual SNPs could not be extracted so these findings will be reported separately. 36, 91
Table 2.
Association studies of angiotensin II Type 1 receptor polymorphisms and hypertention status
| rs# | Ethnicity | Genotyping method |
Definition of hypertension |
Source of cases | Source of controls | Total no: cases vs controls |
Mean age: cases vs controls (SD) |
% Male: cases vs controls |
Minor allele frequency |
AA cases vs controls |
Aa cases vs controls |
Aa cases vs controls |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs188018 | German | PCR-RFLP | NS | Community based volunteers | Same | 319 | 58 (NS) | 0.47 | 0.18 | 219 | 100 | Zhanga15 | |
| 304 | 208 | 96 | |||||||||||
| Tibetan | PCR-RFLP | >140/90 | Hospital | Hospital | 173 | 54 (13) | 0.51 | 0.30 | 131 | 41 | 1 | Sun130 | |
| 193 | 45 (11) | 0.53 | 138 | 53 | 2 | ||||||||
| Mongolian | PCR-RFLP | >140/90 or on Medication | Community-based volunteers | Same | 297 | 53 (11) | 0.38 | 0.13 | 233 | 62 | 2 | Wang131 | |
| 276 | 50 (10) | 0.28 | 210 | 60 | 6 | ||||||||
| rs1880765 | German | PCR-RFLP | NS | Community based volunteers | Same | 319 | 58 (NS) | 0.47 | 0.04 | 290 | 29 | Zhanga15 | |
| 304 | 289 | 15 | |||||||||||
| rs275650 | Japanese | PCR-RFLP | >160/96 or on medication | Cohort study of radiation effects | Same | 149 | 63 (9) | 0.28 | 0.09 | 118 | 31 | 0 | Takahashi133 |
| 156 | 62 (9) | 0.23 | 132 | 21 | 3 | ||||||||
| rs275651 | Japanese | PCR-RFLP | >160/96 or on medication | Cohort study of radiation effects | Same | 149 | 63 (9) | 0.28 | 0.09 | 118132 | 31 | 0 | Takahashi133 |
| 156 | 62 (9) | 0.23 | 21 | 3 | |||||||||
| Chinese Han | Sequencing | >140/90 | Hospital clinics | Same | 345 | 63 (10) | 0.57 | 0.11 | 253 | 77 | 15 | Jin135 | |
| 160 | 62 (9) | 0.61 | 130 | 26 | 4 | ||||||||
| rs275652 | Finnish | PCR-RFLP | on medication | Finnish twin cohort | Same or survey of heart disease | 50 | NS | NS | 0.08 | NS | NS | NS | Kainulainan137 |
| 122 | |||||||||||||
| Japanese | PCR-RFLP | >160/96 or on medication | Cohort study of radiation effects | Same | 149 | 63 (9) | 0.28 | 0.09 | 118 | 31 | 0 | Takahashi133 | |
| 156 | 62 (9) | 0.23 | 132 | 21 | 3 | ||||||||
| rs1492078 | African American | Taqman | Self report or on medication | DMV and HCFA master lists | Same | 666 | NS | 0.59 | 0.77 | 19 | 227 | 420 | Henderson et a139 |
| 586 | 41 | 192 | 353 | ||||||||||
| Hispanic American | 305 | NS | 0.52 | 0.27 | 162 | 117 | 26 | ||||||
| 737 | 387 | 301 | 49 | ||||||||||
| German | PCR-RFLP | NS | Community based volunteers | Same | 316 | 58 (NS) | 0.47 | NS | 128 | 159 | 29 | Zhang15 | |
| 305 | 112 | 145 | 48 | ||||||||||
| Japanese | PCR RFLP | >160/96 or on medication | Cohort study of atomic bomb survivors | Same | 149 | 63 (9) | 0.28 | 0.14 | 92 | 51 | 6 | Takahashi133 | |
| 156 | 62 (9) | 0.23 | 117 | 34 | 5 | ||||||||
| Chinese Han | sequencing | >140/90 | Hospital clinics | Same | 345 | 63 (10) | 0.57 | 0.18 | 213 | 105 | 27 | Jin135 | |
| 160 | 62 (9) | 0.61 | 110 | 41 | 9 | ||||||||
| Tibetan | PCR RFLP | >140/90 × 3 occasions | Hospital | Hospital | 173 | 53 (11) | 0.38 | 0.18 | 126 | 45 | 2 | Sun130 | |
| 193 | 50 (10) | 0.28 | 128 | 60 | 5 | ||||||||
| rs422858 | Japanese | PCR RFLP | >160/96 or on medication | Cohort study of radiation effects | Same | 149 | 63 (9) | 0.28 | 0.14 | 119 | 30 | 0 | Takahashi133 |
| 156 | 62 (9) | 0.23 | 133 | 22 | 1 | ||||||||
| rs275653 | Japanese | PCR-RFLP | >160/96 or on medication | Cohort study of radiation effects | Same | 149 | 63 (9) | 0.28 | 0.08 | 119 | 30 | 0 | Takahashi133 |
| 156 | 62 (9) | 0.23 | 133 | 22 | 1 | ||||||||
| Mongolian | PCR-RFLP | >140/90 or on medication | Community-based volunteers | Same | 297 | 53 (11) | 0.38 | 0.15 | 216 | 77 | 4 | Wang195 | |
| 275 | 50 (10) | 0.28 | 195 | 76 | 4 | ||||||||
| rs5182 | French | ASO | DBP >95 × 2 or on medication | Hypertension clinics | Transfusion center | 206 | 53 (10) | 0.49 | 0.54 | 34 | 113 | 59 | Bonnardeaux92 |
| 298 | 42 (7) | 0.48 | 61 | 151 | 86 | ||||||||
| Finnish | PCR-RFLP | on medication | Finnish twin cohort | Same or survey study of CHD | 50 | NS | NS | 0.35 | NS | NS | NS | Kanulainan137 | |
| 122 | |||||||||||||
| rs380400 | French | ASO | DBP >95 × 2 or on medication | Hypertension clinics | Transfusion clinics | 206 | 53 (10) | 0.49 | 0.14 | 34 | 113 | 59 | Bonnardeaux92 |
| 298 | 42 (7) | 0.48 | 61 | 151 | 86 | ||||||||
SD, standard deviation; PCR-RFLP, polymerase chain reaction - restriction fragment length polymorphism; NS, not stated; DMV, Department of Motor Vehicles; HCFA, health care financing administration; ASO, allele-specific oligonuclotide hybridization; DBP, diastolic blood pressure.
Case-cohort study.
Multiple methodological weaknesses were noted in the majority of studies listed in Tables 4 and 5. Sample sizes were small, definitions of hypertension were inconsistent between studies and sometimes inconsistent with currently accepted guidelines, classification of hypertension was based on a single point estimate, and often controls were sampled from different populations than cases. There was substantial variability in age, gender composition, cholesterol levels, and BMI both within and between studies.
Table 4.
Odds ratios and 95% confidence intervals for association of angiotensin II type 1 receptor single nucleotide polymorphisms and hypertension under dominant, recessive and additive genetic models
| rs# | Ethnicity | Reference | Autosomal dominant
|
Autosomal recessive
|
Additive
|
|||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| rs188018 | German | Zhang 2000 | 0.99 | (0.71–1.39) | 0.99 | (0.95–1.04) | ||
| Tibetan | Sun 2004 | 0.80 | (0.5–1.28) | 0.56 | (0.35–0.89) | 0.82 | (0.75–0.9) | |
| Mongolian | Wang 2006 | 0.87 | (0.59–1.29) | 0.31 | (0.21–0.45) | 0.83 | (0.78–0.89) | |
| Summary | 0.91a | 0.72–1.13 | 0.90b | 0.73–1.10 | ||||
| I2 = 0% | I2 = 0% | |||||||
| rs1880765 | German | Zhang 2000 | 1.93 | (1.01–3.67) | 1.88 | (1.53–2.31) | ||
| rs275650 | Japanese | Takahashi 2000 | 1.44 | (0.8–2.6) | 1.23 | (1.05–1.42) | ||
| rs275651 | Japanese | Takahashi 2000 | 1.44 | (0.8–2.6) | 1.23 | (1.05–1.42) | ||
| Chinese | Jin 2003 | 1.58 | (0.99–2.5) | 1.77 | (1.12–2.82) | 1.54 | (1.42–1.68) | |
| rs275652 | Japanese | Takahashi 2000 | 1.44 | (0.8–2.6) | 1.23 | (1.05–1.42) | ||
| rs1492078 | African American | Henderson 2004 | 2.56 | (1.47–4.47) | 1.13 | (0.65–1.96) | 1.23 | (1.21–1.25) |
| Hispanic American | Henderson 2004 | 0.98 | (0.75–1.28) | 1.31 | (1–1.71) | 1.03 | (1.01–1.06) | |
| German | Zhang 2000 | 0.85 | (0.62–1.18) | 0.54 | (0.39–0.75) | 0.8 | (0.78–0.82) | |
| Japanese | Takahashi 2000 | 1.86 | (1.14–3.04) | 1.27 | (0.78–2.07) | 1.63 | (1.49–1.79) | |
| Chinese Han | Jin 2003 | 1.36 | (0.92–2.03) | 1.42 | (0.96–2.12) | 0.32 | (1.25–1.4) | |
| Tibetan | Sun 2004 | 0.73 | (0.47–1.15) | 0.44 | (0.28–0.69) | 0.74 | (0.69–0.81) | |
| Summary | Heterogeneity P = 0.001 | Heterogeneity P = 0.08 | Heterogeneity P = 0.005 | |||||
| I2 = 76% | I2 = 49% | I2 = 70% | ||||||
| rs422858 | Japanese | Takahashi 2000 | 1.46 | (0.8–2.65) | 1.34 | (1.14–1.58) | ||
| rs275653 | Japanese | Takahashi 2000 | 1.46 | (0.8–2.65) | 1.34 | (1.14–1.58) | ||
| Mongolian | Wang 2006 | 0.9 | (0.63–1.3) | 0.93 | (0.64–1.34) | 0.92 | (0.87–0.97) | |
| rs5182 | French | Bonnardeaux 1994 | 1.3 | (0.82–2.07) | 0.99 | (0.62–1.57) | 1.08 | (1.04–1.11) |
| rs5186 | See Figures 1, A–C | |||||||
| rs380400 | French | Bonnardeaux 1994 | 0.92 | (0.61–1.39) | 0.57 | (0.38–0.87) | 0.91 | (0.84–0.97) |
OR, odds ratio; CI, confidence interval.
Heterogeneity P = 0.76; Egger test P = 0.11.
Heterogeneity P = 0.69; Egger test P = 0.14.
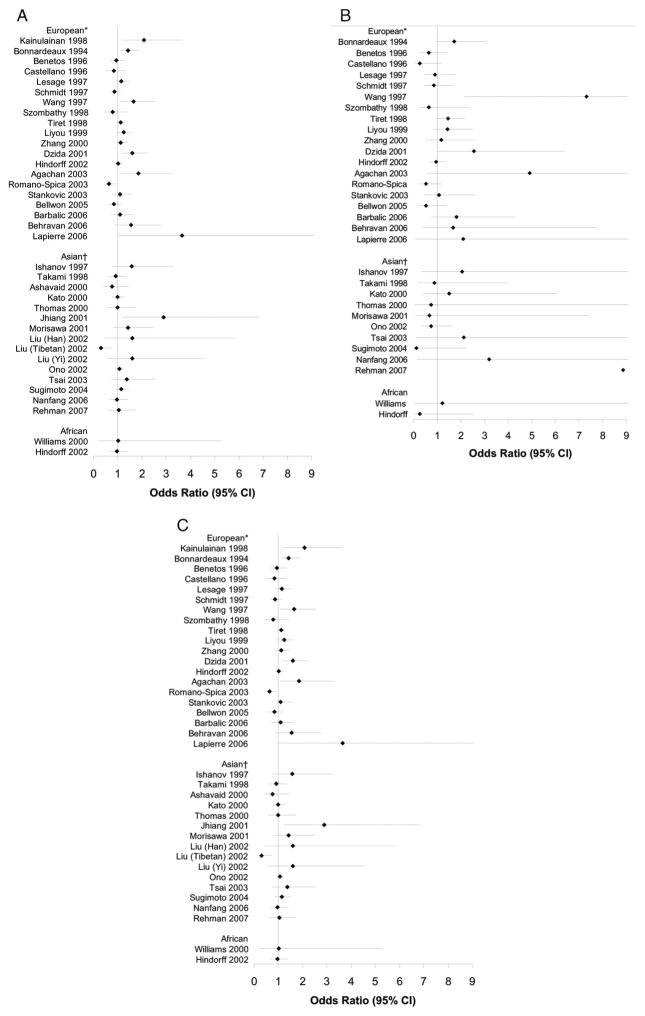
Forest plots for association studies of rs5186 and hypertension status under dominant, recessive and additive genetic models are shown in Figure 1. Table 3 reports the tests of heterogeneity (χ2, τ2, and I2), Egger publication bias test, and where appropriate, summary estimates (odds ratio (OR) and 95% confidence interval (CI)) of association studies of hypertension and rs5186 genotype under each genetic model. The heterogeneity χ2 and I2 tests led to identical conclusions. Pooled summary estimates are reported when studies may be regarded as nonheterogeneous (P<0.10 for the heterogeneity χ2 test, or I2 < 0.33). There was significant heterogeneity for both Asian and European populations under most genetic models. In searching for the most appropriate genetic model, results were difficult to interpret as we obtained slope values >1.0 for European populations and less than zero for Asian populations. These results may be related to the observed heterogeneity of studies.
Fig. 1.
A C, Forest plots for dominant, recessive, and additive genetic models of rs5186 polymorphism in association with hypertension, including all studies. (Dotted line represents null value.) Filled OR is the adjusted estimate using meta-bias to estimate results if publication bias were not present. (1A) Dominant Model: *heterogeneity χ2 P = 0.08; τ2 = 0.021; I2 = 34%; Egger’s publication bias P = 0.51.†Heterogeneity χ2 P = 0.09; τ2 = 0.028;I2 = 35%; Egger’s publication bias P = 0.75. (1B) Recessive Model:*heterogeneity χ2 P = 0.08; τ2 0.074; I2 = 34%; Egger’s publication bias P = 0.98.†Heterogeneity χ2 P = 0.68; τ2 = 0.000;I2 = 0%; Egger’s publication bias P = 0.09; Asian summary OR: 0.99 (95%CI: 0.59, 1.68). (1C) Additive Model: *heterogeneity χ2 P = 0.001; τ2 = 0.040;I2 =58%; Egger’s publication bias P = 0.47. †Heterogeneity χ2 P = 0.15; τ2 = 0.020;I2 =28%; Egger’s publication bias P = 0.93; Asian summary OR: 1.07 (0.93, 1.23).
Table 3.
Measures of heterogeneity of association (χ2 p-value, tau-squared, and I-squared), publication bias test, and summary estimates (odds ratio and 95% confidence intervals) of the angiotensin II Type 1 receptor gene A1166C(rs5186) single nucleotide polymorphisms and hypertension under dominant, recessive and additive genetic models for various subgroups
| Heterogeneity χ2 P | τ2 | I2 | Egger’s publication bias (%) | Summary estimate | OR (95% CI) |
|---|---|---|---|---|---|
| European | |||||
| AD | 0.08 | 0.021 | 34 | 0.51 | |
| AR | 0.08 | 0.074 | 34 | 0.98 | |
| Additive | 0.001 | 0.040 | 58 | 0.47 | |
| Asian | |||||
| AD | 0.09 | 0.028 | 35 | 0.75 | |
| AR | 0.68 | 0.000 | 0 | 0.09 | 0.99 (0.59–1.68) |
| Additive | 0.15 | 0.020 | 28 | 0.93 | 1.07 (0.93–1.23) |
| Hypertension defined by blood pressure ≥160/95 and/or use of antihypertensive medication | |||||
| AD | 0.17 | 0.015 | 30 | 0.01 | 1.23 (1.06–1.43) |
| AR | 0.13 | 0.066 | 35 | 0.46 | |
| Additive | 0.02 | 0.028 | 54 | 0.04 | |
| Hypertension defined by blood pressure ≥140/90, without mention of hypertensive medication | |||||
| AD | 0.33 | 0.008 | 13 | 0.62 | 0.90 (0.74–1.10) |
| AR | 0.01 | 0.399 | 68 | 0.28 | |
| Additive | 0.03 | 0.051 | 60 | 0.36 | |
| Cases mostly men | |||||
| AD | 0.85 | 0.000 | 0 | 0.48 | 1.02 (0.87–1.20) |
| AR | 0.08 | 0.186 | 47 | 0.10 | |
| Additive | 0.48 | 0.000 | 0 | 0.13 | 1.03 (0.91–1.16) |
| Cases mostly women | |||||
| AD | 0.28 | 0.009 | 18 | 0.01 | 1.28 (1.10–1.48) |
| AR | 0.04 | 0.164 | 51 | 0.05 | |
| Additive | 0.04 | 0.026 | 50 | 0.01 | |
| European men | |||||
| AD | 0.13 | 0.075 | 39 | 0.34 | |
| AR | 0.26 | 0.112 | 23 | 0.39 | 0.93 (0.53–1.63) |
| Additive | 0.09 | 0.063 | 45 | 0.82 | |
| European women | |||||
| AD | 0.24 | 0.053 | 25 | 0.93 | 1.36 (0.97–1.92) |
| AR | 0.62 | 0.000 | 0 | 0.35 | 1.65 (0.96–2.86) |
| Additive | 0.24 | 0.070 | 40 | 0.72 | |
| Asian men | |||||
| AD | 0.03 | 0.149 | 64 | 0.18 | |
| AR | 0.71 | 0.000 | 0 | 0.08 | 0.97 (0.35–2.66) |
| Additive | 0.04 | 0.120 | 61 | 0.31 | |
| Asian women | |||||
| AD | 0.04 | 0.150 | 61 | 0.47 | |
| AR | 0.99 | 0.000 | 0 | 0.34 | 0.70 (0.30–1.65) |
| Additive | 0.06 | 0.104 | 56 | 0.45 | |
None of the factors we examined explained the heterogeneity for the AR model using meta-regression. Under the AD model, the definition of hypertension and percent male composition of cases were significant predictors of heterogeneity (P < 0.10). For the studies that used “SBP ≥160 mm Hg and/or on medication,” odds ratios were 36% greater than studies with a definition of “SBP ≥ 140 mm Hg and no mention of medication” (OR 1.36, 95% CI 1.07–1.74). For the studies that were predominantly male, odds ratios were 60% lower than studies that were predominantly female (OR 0.40, 95% CI 0.20–0.82). Next, we repeated the meta-analysis within strata identified by the meta-regression results. Strata were: definition of hypertension “ 160 mm Hg and/or on medication” versus “ 140 mmHg and no mention of medication” and male gender composition of cases ≥ or <50% male. Results are given in Table 4. There was no evidence of heterogeneity within studies stratified by these variables (P > 0.10). The test of heterogeneity is least powerful when looking within strata, and given there were as few as five studies within any stratum these results should be interpreted cautiously. Association of rs5186 genotype and hypertension was found using an AD model within studies that used a definition of hypertension ≥160 mm Hg and/or on medication (D + L pooled OR 1.23, 95% CI 1.06–1.43) and studies that were predominantly female (D + L pooled OR 1.28, 95% CI 1.10–1.48). Publication bias was evident in both strata (Egger P < 0.001 and P=0.003, respectively). In assessing the most appropriate genetic model for analysis, results were difficult to interpret with a slope of >2.0 in the stratum using a definition of hypertension ≥160 mm Hg and/or on medication and no correlation between the aa-versus-AA and Aa versus-AA contrasts in all other strata.
Study-level odds ratios for association of SNPs other than rs5186 with hypertension under AD, AR, and additive genetic models are provided in Table 4. Heterogeneity was assessed for SNPs with at least three studies including rs1492078 and rs188018 with P-values of < 0.10 for all genetic models involving rs1492078 and the AR model for rs188018. Results were nonheterogeneous for the AD model of rs188018 (P=0.76), although there were a limited number of studies. The D + L pooled OR was 0.91 (95% CI 0.72–1.14), showing no association with hypertension. It should be noted that results for multiple SNPs were identical, given the presence of strong LD.
The AC repeat within the 3′UTR was analyzed in two studies. One found an increased risk with the 140 bp fragment in comparison with any other allele (OR 3.21, 95% CI 1.28–8.04).13 The other examined allele frequencies between the case and control groups and found no difference, and did not report genotype frequencies to allow for additional analyses.92
Zhu et al.91 demonstrated a haplotype block containing the C allele from rs5182 and the G allele from rs5183 was possibly associated with hypertension in a case-control analysis of African Americans (P<0.05). In this same study, using a haplotype transmission disequilibrium test in African American families, the CG haplotype was overtransmitted to hypertensive offspring (P =0.0002). No association was found in Caucasian Americans in this study.91 Another study reported association of a haplotype block within the promoter region consisting of: T(rs275651) T(rs275652) AG(rs422858) A(rs275653), to be associated with hypertension but only inwomen.36 This haplotype reportedly also has functional significance as discussed earlier. Finally, although Gu et al.93 did not report genotype frequencies, it is worth mentioning due to its innovative approach which incorporated both additive and up to a four-way interactive effect. There were 503 stage two hypertensives and 490 age/gender-matched controls genotyped for 33 SNPs located in 11 genes physiologically relevant to hypertension. AGTR1 SNPs rs275650, rs1492078, and rs5186 were not found to predict hypertension in a single locus analysis.
Interactions
Epistasis
Interaction between AGT M235T and AGTR1 rs5186 on both hypertension and blood pressure was investigated but no effect was demonstrated.92,94,95 In studying interaction of the AGTR1 rs5186 with the angiotensin converting enzyme I/D polymorphism and hypertension, Ashavaid et al.97 found the C allele to be more frequent in hypertensive individuals with the DD genotype. Others have found no interaction between these two polymorphisms with regard to hypertension or the normal variation of blood pressure.96–98 These studies were all underpowered to investigate interaction.
One analysis examined gene-gene interaction between rs275650, rs1492078, and rs5186 with polymorphisms from 11 other candidate genes on hypertension. There was epistasis between the CYP11B2 conversion polymorphism and AGTR1 rs275650 with an OR of 2.10 (95% CI 1.26–3.51) for A allele homozygotes versus T allele carriers.93
Age of onset of hypertension
Interaction with age of onset of hypertension and rs5186 genotype was examined in one small study, which reported null results.99
Diabetes
Interaction between the AGTR1 rs5186 genotype and diabetes was explored in a stratified analysis of individuals with diabetes versus those with a normal glucose tolerance test.100 No associations were noted.
Cholesterol
One paper investigated the interaction between the AGTR1 rs5186 polymorphism and cholesterol level on the pathogenesis of hypertension.101 Given that cholesterol levels varied between groups in much of the literature, this possibility is intriguing. Among those with total cholesterol levels >220 mg/dL, there was a dramatic increase in theORfrom1.6 (0.9–3.0) to 6.7 (1.8–24.7). Although CIs are wide, and other confounders such as BMI, triglyceride levels, and fasting serum glucose were not accounted for, the evidence for biologic plausibility of this interaction is strong. In vitro studies have shown that low density lipoprotein (LDL) upregulates AGTR1 expression.102 Other evidence exists that the vascular response to angiotensin II is modified by LDL levels.103
Gender
As mentioned above, the effect of AGTR1 variants on hypertension risk may vary by gender. In fact, the response to angiotensin II may be altered by gender104 via regulation of the AGTR1.105 One article that examined the effect of gender on blood pressure as a continuous variable, did find a numeric increase in systolic and diastolic blood pressures in males but not females with the C allele.104
Odds ratios for hypertension in association with rs5186, stratified by gender, are presented in Table 4. Under the AD and additive models, Asian male and female strata demonstrated significant heterogeneity; however, we were able to pool results for the Asian AR models. Results for European populations were nonheterogeneous and summary results are displayed. As illustrated in the figures, results are imprecise, but there is suggestion of an increased risk that is specific to European females.
DISCUSSION
In summary, the literature regarding the AGTR1 rs5186 polymorphismis heterogeneous, and hence use of an overall summary estimate is inappropriate. Variation among studies with regard to the definition of hypertension and gender composition helps explain the heterogeneity of findings, and the role of these covariates should be explored further. The variability between studies with regard to age, cholesterol levels, and BMI also could explain the heterogeneity of the prevalent literature given their biologic plausibility. Although we didn’t find evidence of their influence on heterogeneity, meta regression with this relatively limited number of studies is lacking in power.
Gender-stratified analyses merely suggest that the rs5186 C allele may pose an increased risk for hypertension in Caucasian women, as the presence of publication bias is concerning and the results were not statistically significant. Nonetheless, supportive of our findings is the report of interaction between rs5186 genotype and gender on the renal hemodynamic response to angiotensin II.104 Further testing with larger studies or meta-analysis using patient-level data are necessary before forming any definitive conclusions.
We must mention two limitations inherent in conducting numerous subgroup analyses, including 30 homogeneity χ2 P-value tests and the estimation of 12 CIs (Table 4). First, the application of the homogeneity χ2 test to many subgroups (and using a P-value cut point of 0.10) will sometimes lead to rejection of the null hypothesis by chance alone, and analysts generally prefer to be conservative in rejecting the null. Here, the null hypothesis is homogeneity, and the alternative hypothesis is heterogeneity. In the context of deciding whether or not to pool study results, the multiple-testing approach should err on the side of being too conservative (i.e., not pooling results when they should be pooled). Our choice of whether to pool or not was supported by the I2 statistic, which was even more conservative than the homogeneity P-value in rejecting pooling. We therefore do not feel multiple testing for homogeneity is problematic. A second, more vexing problem is that two CIs lie above the null value (Table 4), which could have been due to chance. We caution against using the CI as a test of statistical significance, noting that these subgroups show relatively precise estimates, and precision is a more robust standard of judging estimates than hypothesis testing.106 In judging the validity of the subgroup analyses, other considerations must be incorporated (including biological plausibility), and the evidence is weak in this regard.
Another explanation for the inconsistency in results is that binary phenotypes are underpowered. It might be that study of blood pressure as a continuous variable would yield more consistent findings. The few studies conducted thus far, have found no association between rs5186 genotype and blood pressure variation whether studied in population-based cohorts, 107–109 hypertensives off antihypertensive medication, 110–112 or nonhypertensive populations.94, 95, 98, 113, 114 The lack of a functional role of the rs5186 polymorphism makes this SNP somewhat unappealing as a “true” influence of hypertension susceptibility. The available evidence for functionality of AGTR1 polymorphisms is mostly confined to those within the promoter region. Thus far, studies of promoter region polymorphisms have yielded encouraging results especially given many of these SNPs are in LD with one another. Given the dedication of the AGTR1 gene to the promoter region and the role of splicing variants, this region warrants further investigation. Identification of genetic susceptibilities to hypertension has important public health implications given its high prevalence and large impact on cardiovascular morbidity and mortality. It is premature to conclude any relationship between AGTR1 polymorphic variants and hypertension, and hence there is no role for genetic screening at this time. The a priori probability for an influential role of the AGTR1 gene on hypertension is quite strong given its biologic relevance. Many physiologic mechanisms contribute to hypertension such as activity of the noradrenergic and RASs, arterial stiffness, and salt sensitivity. It may be that hypertension represents multiple diseases with distinct etiologies. Investigation of these processes as a function of genotypic variation might yield more consistent results, as population stratification could be minimized.
A handful of studies of the rs5186 SNP in association with hypertension-related physiologic mechanisms have been conducted thus far. Studies of the sympathetic postural response114, 115 and vascular reactivity to alpha-adrenergic stimulation116–118 have been conflicting. Similarly, there have been a variety of contradictory findings in the blood pressure109, 119–123 and renal response109, 120, 121 to angiotensin II. There is no variation in the aldosterone response to angiotensin II with relation to rs5186 or rs188018 genotypes.109, 119–121,124 Neither salt sensitivity125, 126 nor renal sodium handling, 127 seems to vary according to rs5186 genotype. Studies of arterial stiffness as indicated by pulse wave velocity110, 128 or pulse pressure129 have been conflicting.
Further study of gene-environment interactions are indicated, particularly with powerful population determinants of hypertension such as obesity and salt intake. Study of single SNPs in isolation is likely to be fruitless given the complexity of the hypertensive phenotype and the need to incorporate multiple variants from multiple genes. Haplotype analysis helps to alleviate some of the burden of genotyping and should likely be the rule for future association studies rather than the exception. Larger studies with a robust definition of hypertension, investigation of processes physiologically relevant to hypertension, appropriate sampling of controls, and consideration of epistasis and gene-environment interaction are necessary to make a true determination of whether AGTR1 polymorphisms have a role in the pathogenesis of hypertension.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Laragh J, Pickering TG. Essential hypertension. In: Brenner B, editor. Brenner & Rector’s the kidney. Philadelphia: W.B. Saunders Company; 1991. pp. 1913–1967. [Google Scholar]
- 2.Gasparo M, Bullock G. The AT1 and AT2 angiotensin receptors. In: Oparil S, Webber M, editors. Hypertension: a companion to Brenner & Rector’s the kidney. Philadelphia: W.B. Saunders Company; 2000. pp. 101–110. [Google Scholar]
- 3.Brenner B, Cooper M, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 5.Guo D, Furuta H, Mizukoshi M, Inagami T. The genomic organization of human angiotensin II type 1 receptor. Biochem Biophys Res Commun. 1994;200:313–319. doi: 10.1006/bbrc.1994.1450. [DOI] [PubMed] [Google Scholar]
- 6.Antonellis A, Rogus JJ, Canani LH, et al. A method for developing high-density SNP maps and its application at the type 1 angiotensin II receptor (AGTR1) locus. Genomics. 2002;79:326–332. doi: 10.1006/geno.2002.6713. [DOI] [PubMed] [Google Scholar]
- 7.Curnow KM, Pascoe L, White PC. Genetic analysis of the human type-1 angiotensin II receptor. Mol Endocrinol. 1992;6:1113–1118. doi: 10.1210/mend.6.7.1508224. [DOI] [PubMed] [Google Scholar]
- 8.Furuta H, Guo D, Inagami T. Molecular cloning and sequencing of the gene encoding human angiotensin II type 1 receptor. Biochem Biophys Res Commun. 1992;183:8–13. doi: 10.1016/0006-291x(92)91600-u. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi R, Ohnaka K, Sakai Y, Ikuyama S, Nawata H. Molecular cloning and characterization of the promoter for the human type-1 angiotensin II receptor gene. Biochem Biophys Res Commun. 1994;200:1264–1270. doi: 10.1006/bbrc.1994.1587. [DOI] [PubMed] [Google Scholar]
- 10.Martin MM, Willardson BM, Burton GF, et al. Human angiotensin II type 1 receptor isorforms encoded by messenger RNA splice variants are functionally distinct. Mol Endocrinol. 2001;15:281–293. doi: 10.1210/mend.15.2.0598. [DOI] [PubMed] [Google Scholar]
- 11.Su B, Martin MM, Beason KB, Miller PJ, Elton TS. The genomic organization and functional analysis of the promoter for the human angiotensin II type 1 receptor. Biochem Biophys Res Commun. 1994;204:1039–1046. doi: 10.1006/bbrc.1994.2567. [DOI] [PubMed] [Google Scholar]
- 12.Davies E, Bonnardeaux A, Lathrop GM, Corvol P, Clauser E, Soubrier F. Angiotensin II (type-1) receptor locus: CA repeat polymorphism and genetic mapping. Hum Mol Genet. 1994;3:838. doi: 10.1093/hmg/3.5.838. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Qiu C, Zhou W, Zheng Y, Hou S, Cao J. Gene polymorphisms of the renin angiotensin system in essential hypertension. Chin Med J (Engl) 1999;112:115–120. [PubMed] [Google Scholar]
- 14.den Dunnen J, Antonarakis S. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Erdmann J, Regitz-Zagrosek V, Kurzinger S, Hense HW, Schunkert H. Evaluation of three polymorphisms in the promoter region of the angiotensin II type I receptor gene. J Hypertens. 2000;18:267–272. doi: 10.1097/00004872-200018030-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kee F, Morrison C, Poirier O, et al. Angiotensin II type-I receptor and ACE polymorphisms and risk of myocardial infarction in men and women. Eur J Clin Invest. 2000;30:1076–1082. doi: 10.1046/j.1365-2362.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 17.Hindorff LA, Heckbert SR, Tracy R, et al. Angiotensin II type 1 receptor polymorphisms in the cardiovascular health study: relation to blood pressure, ethnicity and cardiovascular events. Am J Hypertens. 2002;15:1050–1056. doi: 10.1016/s0895-7061(02)03063-7. [DOI] [PubMed] [Google Scholar]
- 18.Ermis C, Tsai MY, Hanson NQ, Akar N, Aras O. Angiotensin I converting enzyme, angiotensin II type 1 receptor and angiotensinogen polymorphisms and early myocardial infarction in Turkish population. Thromb Haemost. 2002;88:693–694. [PubMed] [Google Scholar]
- 19.Olsson M, Annerbrink K, Westberg L, et al. Angiotensin-related genes in patients with panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2004;127:81–84. doi: 10.1002/ajmg.b.20164. [DOI] [PubMed] [Google Scholar]
- 20.Asselbergs FW, Williams SM, Hebert PR, et al. The gender-specific role of polymorphisms from the fibrinolytic, renin-angiotensin, and bradykinin systems in determining plasma t-PA and PAI-1 levels. Thromb Haemost. 2006;96:471–477. [PubMed] [Google Scholar]
- 21.Andrikopoulos GK, Tzeis SM, Needham EW, et al. Lack of association between common polymorphisms in genes of the renin-angiotensin system and mortality after myocardial infarction. Cardiology. 2005;103:185–188. doi: 10.1159/000084592. [DOI] [PubMed] [Google Scholar]
- 22.Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A. Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant. 2006;21:979–983. doi: 10.1093/ndt/gfk012. [DOI] [PubMed] [Google Scholar]
- 23.Kato N, Sugiyama T, Morita H, et al. Comprehensive analysis of the renin-angiotensin gene polymorphisms with relation to hypertension in the Japanese. J Hypertens. 2000;18:1025–1032. doi: 10.1097/00004872-200018080-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hirakawa M, Tanaka T, Hashimoto Y, Kuroda M, Takagi T, Nakamura Y. JSNP: a database of common gene variations in the Japanese population. Nucleic Acids Res. 2002;30:159–162. doi: 10.1093/nar/30.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabara Y, Kohara K, Miki T. Polymorphisms of genes encoding components of the sympathetic nervous system but not the renin-angiotensin system as risk factors for orthostatic hypotension. J Hypertens. 2002;20:651–656. doi: 10.1097/00004872-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Mannami T, Baba S, Yasui N, Ogihara T, Iwai N. Lack of association between angiotensin II type 1 receptor gene polymorphism and hypertension in Japanese. Hypertens Res. 2003;26:131–134. doi: 10.1291/hypres.26.131. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto K, Katsuya T, Ohkubo T, et al. Association between angiotensin II type 1 receptor gene polymorphism and essential hypertension: the Ohasama Study. Hypertens Res. 2004;27:551–556. doi: 10.1291/hypres.27.551. [DOI] [PubMed] [Google Scholar]
- 28.Hindorff LA, Heckbert SR, Tracy R, et al. Angiotensin II type 1 receptor polymorphisms in the cardiovascular health study: relation to blood pressure, ethnicity, and cardiovascular events. Am J Hypertens. 2002;15:1050–1056. doi: 10.1016/s0895-7061(02)03063-7. [DOI] [PubMed] [Google Scholar]
- 29.Hsu CC, Bray MS, Kao WH, Pankow JS, Boerwinkle E, Coresh J. Genetic variation of the renin-angiotensin system and chronic kidney disease progression in black individuals in the atherosclerosis risk in communities study. J Am Soc Nephrol. 2006;17:504–512. doi: 10.1681/ASN.2005050468. [DOI] [PubMed] [Google Scholar]
- 30.Hooper WC, Dowling NF, Wenger NK, Dilley A, Ellingsen D, Evatt BL. Relationship of venous thromboembolism and myocardial infarction with the renin-angiotensin system in African-Americans. Am J Hematol. 2002;70:1–8. doi: 10.1002/ajh.10078. [DOI] [PubMed] [Google Scholar]
- 31.Ranjith N, Pegoraro RJ, Rom L, Lanning PA, Naidoo DP. Renin-angiotensin system and associated gene polymorphisms in myocardial infarction in young South African Indians. Cardiovasc J S Afr. 2004;15:22–26. [PubMed] [Google Scholar]
- 32.Jiang Z, Zhao W, Yu F, Xu G. Association of angiotensin II type 1 receptor gene polymorphism with essential hypertension. Chin Med J (Engl) 2001;114:1249–1251. [PubMed] [Google Scholar]
- 33.Takami S, Katsuya T, Rakugi H, et al. Angiotensin II type 1 receptor gene polymorphism is associated with increase of left ventricular mass but not with hypertension. Am J Hypertens. 1998;11(3 Pt 1):316–321. doi: 10.1016/s0895-7061(97)00457-3. [DOI] [PubMed] [Google Scholar]
- 34.Jin W, Liu Y, Sheng HH, et al. Single nucleotide polymorphisms in promoter of angiotensin II type 1 receptor gene associated with essential hypertension and coronary heart disease in Chinese population. Acta Pharmacol Sin. 2003;24:1083–1088. [PubMed] [Google Scholar]
- 35.Hansen JL, Haunso S, Brann MR, Sheikh SP, Weiner DM. Loss-of-function polymorphic variants of the human angiotensin II type 1 receptor. Mol Pharmacol. 2004;65:770–777. doi: 10.1124/mol.65.3.770. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Prater A, Li Y, et al. A haplotype of the angiotensin-II receptor subtype is associated with hypertension in caucasian women. Circulation. 2005;11:E52. [Google Scholar]
- 37.Pende A, Giacche M, Castigliola L, et al. Characterization of the binding of the RNA-binding protein AUF1 to the human AT(1) receptor mRNA. Biochem Biophys Res Commun. 1999;266:609–614. doi: 10.1006/bbrc.1999.1862. [DOI] [PubMed] [Google Scholar]
- 38.Lehtonen J, Paukku K, Daviet L, et al. Angiotensin II type 1 receptor 1166 polymorphism A to C Increases mRNA stability and steady-state levels. Circulation. 2006;114(18 Suppl S):190. [Google Scholar]
- 39.Abdollahi MR, Lewis RM, Gaunt TR, et al. Quantitated transcript haplotypes (QTH) of AGTR1, reduced abundance of mRNA haplotypes containing 1166C (rs5186:A>C), and relevance to metabolic syndrome traits. Hum Mutat. 2007;28:365–373. doi: 10.1002/humu.20454. [DOI] [PubMed] [Google Scholar]
- 40.Paillard F, Chansel D, Brand E, et al. Genotype-phenotype relationships for the renin-angiotensin-aldosterone system in a normal population. Hypertension. 1999;34:423–429. doi: 10.1161/01.hyp.34.3.423. [DOI] [PubMed] [Google Scholar]
- 41.The international HapMap consortium. The international HapMap project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Yan D, Cooper RS, et al. Linkage disequilibrium and haplotype diversity in the genes of the renin-angiotensin system: findings from the family blood pressure program. Genome Res. 2003;13:173–181. doi: 10.1101/gr.302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erdmann J, Riedel K, Rohde K, et al. Characterization of polymorphisms in the promoter of the human angiotensin II subtype 1 (AT1) receptor gene. Ann Hum Genet. 1999;63(Pt 4):369–374. doi: 10.1046/j.1469-1809.1999.6340369.x. [DOI] [PubMed] [Google Scholar]
- 44.Su S, Chen J, Zhao J, et al. Angiotensin II type I receptor gene and myocardial, infarction: tagging SNPs and haplotype based association study. The Beijing atherosclerosis study. Pharmacogenetics. 2004;14:673–681. doi: 10.1097/00008571-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 46.Laragh JH, Brenner BM. Pathophysiology, diagnosis and management. 2. New York: Raven Press; 1995. Hypertension. [Google Scholar]
- 47.Burt V, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: results from the third NHANES survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 48.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 49.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part 1: estimates of blood pressure levels. J Hypertens. 2006;24:413–422. doi: 10.1097/01.hjh.0000199801.72563.6f. [DOI] [PubMed] [Google Scholar]
- 50.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 51.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24:423–430. doi: 10.1097/01.hjh.0000209973.67746.f0. [DOI] [PubMed] [Google Scholar]
- 52.Wright JJ, Jr, Agodoa L, Contreras G, et al. Successful blood pressure control in the African American study of kidney disease and hypertension. Arch Intern Med. 2002;162:1636–1643. doi: 10.1001/archinte.162.14.1636. [DOI] [PubMed] [Google Scholar]
- 53.Cooper RS, Wolf-Maier K, Luke A, et al. An international comparative study of blood pressure in populations of European vs. African descent. BMC Med. 2005;3:2. doi: 10.1186/1741-7015-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3 Pt 2):625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 55.Diabetes Prevention Program Research Group. Hypertension, insulin, and proinsulin in participants with impaired glucose tolerance. Hypertension. 2002;40:679–686. doi: 10.1161/01.hyp.0000035706.28494.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep heart health study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 57.Wolk R, Shamsuzzaman A, Somers V. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 58.Caballero A. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 59.Sheetz M, King G. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 60.Bragulat E, de la Sierra A. Salt intake, endothelial dysfunction, and salt-sensitive hypertension. J Clin Hypertens (Greenwich) 2002;4:41–46. doi: 10.1111/j.1524-6175.2002.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 62.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65(Suppl 2):626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 63.Pereira MA, Folsom AR, McGovern PG, et al. Physical activity and incident hypertension in black and white adults: the atherosclerosis risk in communities study. Prev Med. 1999;28:304–312. doi: 10.1006/pmed.1998.0431. [DOI] [PubMed] [Google Scholar]
- 64.Hayashi T, Tsumura K, Suematsu C, Okada K, Fujii S, Endo G. Walking to work and the risk for hypertension in men: the Osaka Health Survey. Ann Intern Med. 1999;131:21–26. doi: 10.7326/0003-4819-131-1-199907060-00005. [DOI] [PubMed] [Google Scholar]
- 65.Beilin LJ, Puddey IB. Alcohol and hypertension: an update. Hypertension. 2006;47:1035–1038. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- 66.Moreira LB, Fuchs FD, Moraes RS, Bredemeier M, Duncan BB. Alcohol intake and blood pressure: the importance of time elapsed since last drink. J Hypertens. 1998;16:175–180. doi: 10.1097/00004872-199816020-00007. [DOI] [PubMed] [Google Scholar]
- 67.Hunt SC, Hasstedt SJ, Kuida H, Stults BM, Hopkins PN, Williams RR. Genetic heritability and common environmental components of resting and stressed blood pressures, lipids, and body mass index in Utah pedigrees and twins. AmJ Epidemiol. 1989;129:625–638. doi: 10.1093/oxfordjournals.aje.a115175. [DOI] [PubMed] [Google Scholar]
- 68.Longini IM, Jr, Higgins MW, Hinton PC, Moll PP, Keller JB. Environmental and genetic sources of familial aggregation of blood pressure in Tecumseh, Michigan. Am J Epidemiol. 1984;120:131–144. doi: 10.1093/oxfordjournals.aje.a113862. [DOI] [PubMed] [Google Scholar]
- 69.Rice T, Vogler GP, Perusse L, Bouchard C, Rao DC. Cardiovascular risk factors in a French Canadian population: resolution of genetic and familial environmental effects on blood pressure using twins, adoptees, and extensive information on environmental correlates. Genet Epidemiol. 1989;6:571–588. doi: 10.1002/gepi.1370060503. [DOI] [PubMed] [Google Scholar]
- 70.Snieder H, Harshfield G, Treiber F. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 71.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 72.Gong M, Hubner N. Molecular genetics of human hypertension. Clin Sci. 2006;110:315–326. doi: 10.1042/CS20050208. [DOI] [PubMed] [Google Scholar]
- 73.Liu W, Zhao W, Chase GA. Genome scan meta-analysis for hypertension. Am J Hypertens. 2004;17(12 Pt 1):1100–1106. doi: 10.1016/j.amjhyper.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 74.Koivukoski L, Fisher SA, Kanninen T, et al. Meta-analysis of genome-wide scans for hypertension and blood pressure in Caucasians shows evidence of susceptibility regions on chromosomes 2 and 3. Hum Mol Genet. 2004;13:2325–2332. doi: 10.1093/hmg/ddh237. [DOI] [PubMed] [Google Scholar]
- 75.Rice T, Cooper RS, Wu X, et al. Meta-analysis of genome-wide scans for blood pressure in African American and Nigerian samples. The National Heart, Lung, and Blood Institute GeneLink Project. Am J Hypertens. 2006;19:270–274. doi: 10.1016/j.amjhyper.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Wu X, Kan D, Province M, et al. An updated meta-analysis of genome scans for hypertension and blood pressure in the NHLBI Family Blood Pressure Program (FBPP) Am J Hypertens. 2006;19:122–127. doi: 10.1016/j.amjhyper.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Zintzaras E, Kitsios G, Stefanidis I. Endothelial NO synthase gene polymorphisms and hypertension: a meta-analysis. Hypertension. 2006;48:700–710. doi: 10.1161/01.HYP.0000238124.91161.02. [DOI] [PubMed] [Google Scholar]
- 78.Sethi AA, Nordestgaard BG, Tybjaerg-Hansen A. Angiotensinogen gene polymorphism, plasma angiotensinogen, and risk of hypertension and ischemic heart disease: a meta-analysis. Arterioscler Thromb Vasc Biol. 2003;23:1269–1275. doi: 10.1161/01.ATV.0000079007.40884.5C. [DOI] [PubMed] [Google Scholar]
- 79.Mondry A, Loh M, Liu P, Zhu AL, Nagel M. Polymorphisms of the insertion / deletion ACE and M235T AGT genes and hypertension: surprising new findings and meta-analysis of data. BMC Nephrol. 2005;6:1. doi: 10.1186/1471-2369-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staessen JA, Wang JG, Ginocchio G, et al. The deletion/insertion polymorphism of the angiotensin converting enzyme gene and cardiovascular-renal risk. J Hypertens. 1997;15(12 Pt 2):1579–1592. doi: 10.1097/00004872-199715120-00059. [DOI] [PubMed] [Google Scholar]
- 81.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 82.Greenland S. Meta-analysis. In: Rothman K, Greenland S, editors. Modern epidemiology. Philadelphia: Lippincott Williams and Wilkins; pp. 643–673. [Google Scholar]
- 83.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: metaanalysis in context. London: BMJ Publishing Group; 2001. pp. 285–312. [Google Scholar]
- 85.Minelli C, Thompson JR, Abrams KR, Thakkinstian A, Attia J. The choice of a genetic model in the meta-analysis of molecular association studies. Int J Epidemiol. 2005;34:1319–1328. doi: 10.1093/ije/dyi169. [DOI] [PubMed] [Google Scholar]
- 86.Sterne JA, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis D, Liyou N, Johnson A. The ACE gene I/D polymorphism, but not the angiotensin II type I receptor gene A1166C polymorphism is associated with isolated systolic hypertension. J Hum Hypertens. 2001;15:653–654. doi: 10.1038/sj.jhh.1001221. [DOI] [PubMed] [Google Scholar]
- 88.Mettimano M, Romano-Spica V, Ianni A, Specchia M, Migneco A, Savi L. AGT and AT1R gene polymorphism in hypertensive heart disease. Int J Clin Pract. 2002;56:574–577. [PubMed] [Google Scholar]
- 89.Miyama N, Hasegawa Y, Suzuki M, et al. Investigation of major genetic polymorphisms in the renin-angiotensin-aldosterone system in subjects with young-onset hypertension selected by a targeted-screening system at university. Clin Exp Hypertens. 2007;29:61–67. doi: 10.1080/10641960601096968. [DOI] [PubMed] [Google Scholar]
- 90.Rolfs A, Weber-Rolfs I, Regitz-Zagrosek V, Kallisch H, Riedel K, Fleck E. Genetic polymorphisms of the angiotensin II type 1 receptor gene. Eur Heart J. 1994;15(Suppl D):108–112. doi: 10.1093/eurheartj/15.suppl_d.108. [DOI] [PubMed] [Google Scholar]
- 91.Zhu X, Chang YP, Yan D, et al. Associations between hypertension and genes in the renin-angiotensin system. Hypertension. 2003;41:1027–1034. doi: 10.1161/01.HYP.0000068681.69874.CB. [DOI] [PubMed] [Google Scholar]
- 92.Bonnardeaux A, Davies E, Jeunemaitre X, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 93.Gu D, Su S, Ge D, et al. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 94.Berge KE, Berg K. Polymorphisms at the angiotensinogen (AGT) and angiotensin II type 1 receptor (AT1R) loci and normal blood pressure. Clin Genet. 1998;53:214–219. doi: 10.1111/j.1399-0004.1998.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Zhu H, Dong Y, Treiber FA, Snieder H. Effects of angiotensinogen and angiotensin II type I receptor genes on blood pressure and left ventricular mass trajectories in multiethnic youth. Twin Res Hum Genet. 2006;9:393–402. doi: 10.1375/183242706777591335. [DOI] [PubMed] [Google Scholar]
- 96.Dzida G, Sobstyl J, Puzniak A, Golon P, Mosiewicz J, Hanzlik J. Polymorphisms of angiotensin-converting enzyme and angiotensin II receptor type 1 genes in essential hypertension in a Polish population. Med Sci Monit. 2001;7:1236–1241. [PubMed] [Google Scholar]
- 97.Ashavaid TF, Shalia KK, Nair KG, Dalal JJ. ACE and AT1R gene polymorphisms and hypertension in Indian population. J Clin Lab Anal. 2000;14:230–237. doi: 10.1002/1098-2825(2000)14:5<230::AID-JCLA6>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henskens LH, Spiering W, Stoffers HE, et al. Effects of ACE I/D and AT1R-A1166C polymorphisms on blood pressure in a healthy normotensive primary care population: first results of the hippocates study. J Hypertens. 2003;21:81–86. doi: 10.1097/00004872-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt S, Beige J, Walla-Friedel M, Michel MC, Sharma AM, Ritz E. A polymorphism in the gene for the angiotensin II type 1 receptor is not associated with hypertension. J Hypertens. 1997;15(12 Pt 1):1385–1388. doi: 10.1097/00004872-199715120-00003. [DOI] [PubMed] [Google Scholar]
- 100.Lesage S, Velho G, Vionnet N, et al. Genetic studies of the renin-angiotensin system in arterial hypertension associated with non-insulin-dependent diabetes mellitus. J Hypertens. 1997;15:601–606. doi: 10.1097/00004872-199715060-00005. [DOI] [PubMed] [Google Scholar]
- 101.Morisawa T, Kishimoto Y, Kitano M, Kawasaki H, Hasegawa J. Influence of angiotensin II type 1 receptor polymorphism on hypertension in patients with hypercholesterolemia. Clin Chim Acta. 2001;304:91–97. doi: 10.1016/s0009-8981(00)00402-2. [DOI] [PubMed] [Google Scholar]
- 102.Nickenig G, Wassmann S, Bohm M. Regulation of the angiotensin AT1 receptor by hypercholesterolaemia. Diabetes Obes Metab. 2000;2:223–228. doi: 10.1046/j.1463-1326.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 103.John S, Delles C, Klingbeil AU, Jacobi J, Schlaich MP, Schmieder RE. Low-density lipoprotein-cholesterol determines vascular responsiveness to angiotensin II in normocholesterolaemic humans. J Hypertens. 1999;17(12 Pt 2):1933–1939. doi: 10.1097/00004872-199917121-00024. [DOI] [PubMed] [Google Scholar]
- 104.Reich H, Duncan JA, Weinstein J, Cattran DC, Scholey JW, Miller JA. Interactions between gender and the angiotensin type 1 receptor gene polymorphism. Kidney Int. 2003;63:1443–1449. doi: 10.1046/j.1523-1755.2003.00867.x. [DOI] [PubMed] [Google Scholar]
- 105.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part II: AT(1) receptor regulation. Circulation. 2002;105:530–536. doi: 10.1161/hc0402.102619. [DOI] [PubMed] [Google Scholar]
- 106.Poole C. Low P-values or narrow confidence intervals: which are more durable? Epidemiology. 2001;12:291–294. doi: 10.1097/00001648-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Castellano M, Muiesan ML, Beschi M, et al. Angiotensin II type 1 receptor A/C1166 polymorphism. Relationships with blood pressure and cardiovascular structure. Hypertension. 1996;28:1076–1080. doi: 10.1161/01.hyp.28.6.1076. [DOI] [PubMed] [Google Scholar]
- 108.Kikuya M, Sugimoto K, Katsuya T, et al. A/C1166 gene polymorphism of the angiotensin II type 1 receptor (AT1) and ambulatory blood pressure: the Ohasama Study. Hypertens Res. 2003;26:141–145. doi: 10.1291/hypres.26.141. [DOI] [PubMed] [Google Scholar]
- 109.Hilgers KF, Langenfeld MR, Schlaich M, Veelken R, Schmieder RE. 1166 A/C polymorphism of the angiotensin II type 1 receptor gene and the response to short-term infusion of angiotensin II. Circulation. 1999;100:1394–1399. doi: 10.1161/01.cir.100.13.1394. [DOI] [PubMed] [Google Scholar]
- 110.Lajemi M, Labat C, Gautier S, et al. Angiotensin II type 1 receptor-153A/G and 1166A/C gene polymorphisms and increase in aortic stiffness with age in hypertensive subjects. J Hypertens. 2001;19:407–413. doi: 10.1097/00004872-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Rudnichi A, Safar ME, Lajemi M, Benetos A. Gene polymorphisms of the renin angiotensin system and age-related changes in systolic and diastolic blood pressure in subjects with hypertension. Am J Hypertens. 2004;17:321–327. doi: 10.1016/j.amjhyper.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Spiering W, Zwaan IM, Kroon AA, de Leeuw PW. Genetic influences on 24 h blood pressure profiles in a hypertensive population: role of the angiotensin-converting enzyme insertion/deletion and angiotensin II type 1 receptor A1166C gene polymorphisms. Blood Press Monit. 2005;10:135–141. doi: 10.1097/00126097-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Barbeau P, Kulharya A, Harshfield G, Snieder H, Davis H, Treiber F. Association between angiotensin II type I receptor polymorphism and resting hemodynamics in black and white youth. Ethn Dis. 2002;12:S168–71. [PubMed] [Google Scholar]
- 114.Nishikino M, Matsunaga T, Yasuda K, et al. Genetic variation in the renin-angiotensin system and autonomic nervous system function in young healthy Japanese subjects. J Clin Endocrinol Metab. 2006;91:4676–4681. doi: 10.1210/jc.2006-0700. [DOI] [PubMed] [Google Scholar]
- 115.Stolarz K, Staessen JA, Kawecka-Jaszcz K, et al. Genetic variation in CYP11B2 and AT1R influences heart rate variability conditional on sodium excretion. Hypertension. 2004;44:156–162. doi: 10.1161/01.HYP.0000135846.91124.a5. [DOI] [PubMed] [Google Scholar]
- 116.Henrion D, Amant C, Benessiano J, et al. Angiotensin II type 1 receptor gene polymorphism is associated with an increased vascular reactivity in the human mammary artery in vitro. J Vasc Res. 1998;35:356–362. doi: 10.1159/000025605. [DOI] [PubMed] [Google Scholar]
- 117.Steeds RP, Toole LO, Channer KS, Morice AH. Human vascular reactivity and polymorphisms of the angiotensin-converting enzyme and the angiotensin type 1 receptor genes. J Vasc Res. 1999;36:445–455. doi: 10.1159/000025687. discussion 535–8. [DOI] [PubMed] [Google Scholar]
- 118.Amant C, Hamon M, Bauters C, et al. The angiotensin II type 1 receptor gene polymorphism is associated with coronary artery vasoconstriction. J Am Coll Cardiol. 1997;29:486–490. doi: 10.1016/s0735-1097(96)00535-9. [DOI] [PubMed] [Google Scholar]
- 119.Delles C, Erdmann J, Jacobi J, et al. Lack of association between polymorphisms of angiotensin II receptor genes and response to short-term angiotensin II infusion. J Hypertens. 2000;18:1573–1578. doi: 10.1097/00004872-200018110-00007. [DOI] [PubMed] [Google Scholar]
- 120.Miller JA, Thai K, Scholey JW. Angiotensin II type 1 receptor gene polymorphism predicts response to losartan and angiotensin II. Kidney Int. 1999;56:2173–2180. doi: 10.1046/j.1523-1755.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 121.Spiering W, Kroon AA, Fuss-Lejeune MM, Daemen MJ, de Leeuw PW. Angiotensin II sensitivity is associated with the angiotensin II type 1 receptor A (1166)C polymorphism in essential hypertensives on a high sodium diet. Hypertension. 2000;36:411–416. doi: 10.1161/01.hyp.36.3.411. [DOI] [PubMed] [Google Scholar]
- 122.Vuagnat A, Giacche M, Hopkins PN, et al. Blood pressure response to angiotensin II, low-density lipoprotein cholesterol and polymorphisms of the angiotensin II type 1 receptor gene in hypertensive sibling pairs. J Mol Med. 2001;79:175–183. doi: 10.1007/s001090100205. [DOI] [PubMed] [Google Scholar]
- 123.Lim HS, Cho JY, Oh DS, et al. Angiotensin II type 1 receptor 1166A/C polymorphism in association with blood pressure response to exogenous angiotensin II. Eur J Clin Pharmacol. 2007;63:17–26. doi: 10.1007/s00228-006-0228-6. [DOI] [PubMed] [Google Scholar]
- 124.Kosachunhanun N, Hunt SC, Hopkins PN, et al. Genetic determinants of nonmodulating hypertension. Hypertension. 2003;42:901–908. doi: 10.1161/01.HYP.0000095615.83724.82. [DOI] [PubMed] [Google Scholar]
- 125.Giner V, Poch E, Bragulat E, et al. Renin-angiotensin system genetic polymorphism and salt sensitivity in essential hypertension. Hypertension. 2000;35(1 Pt 2):512–517. doi: 10.1161/01.hyp.35.1.512. [DOI] [PubMed] [Google Scholar]
- 126.Pamies-Andreu E, Ramirez-Lorca R, Garcia-Junco A, et al. Renin-angiotensin aldosterone system and G-protein beta-3 subunit gene polymorhpisms in salt sensitive essential hypertension. J Hum Hypertens. 2003;17:187–191. doi: 10.1038/sj.jhh.1001534. [DOI] [PubMed] [Google Scholar]
- 127.Siani A, Russo P, Paolo Cappuccio F, et al. Combination of renin-angiotensin system polymorphisms is associated with altered renal sodium handling and hypertension. Hypertension. 2004;43:598–602. doi: 10.1161/01.HYP.0000117985.57001.b3. [DOI] [PubMed] [Google Scholar]
- 128.Gardier S, Vincent M, Lantelme P, Rial MO, Bricca G, Milon H. A1166C polymorphism of angiotensin II type 1 receptor, blood pressure and arterial stiffness in hypertension. J Hypertens. 2004;22:2135–2142. doi: 10.1097/00004872-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 129.Mourad JJ, Ducailar G, Rudnicki A, Lajemi M, Mimran A, Safar ME. Age-related increase of pulse pressure and gene polymorphisms in essential hypertension: a preliminary study. J Renin Angiotensin Aldosterone Syst. 2002;3:109–115. doi: 10.3317/jraas.2002.011. [DOI] [PubMed] [Google Scholar]
- 130.Sun B, Dronma T, Qin WJ, et al. Polymorphisms of renin-angiotensin system in essential hypertension in Chinese Tibetans. Biomed Environ Sci. 2004;17:209–216. [PubMed] [Google Scholar]
- 131.Gui-yan W, Yan-Hua W, Qun X, et al. Associations between RAS Gene polymorphisms, environmental factors and hypertension in Mongolian people. Eur J Epidemiol. 2006;21:287–292. doi: 10.1007/s10654-005-6006-4. [DOI] [PubMed] [Google Scholar]
- 132.Poirier O, Georges JL, Ricard S, et al. New polymorphisms of the angiotensin II type 1 receptor gene and their associations with myocardial infarction and blood pressure: the ECTIM study. Etude Cas-Temoin de l’Infarctus du Myocarde. J Hypertens. 1998;16:1443–1447. doi: 10.1097/00004872-199816100-00007. [DOI] [PubMed] [Google Scholar]
- 133.Takahashi N, Murakami H, Kodama K, et al. Association of a polymorphism at the 5′-region of the angiotensin II type 1 receptor with hypertension. Ann Hum Genet. 2000;64(Pt 3):197–205. doi: 10.1017/S0003480000008083. [DOI] [PubMed] [Google Scholar]
- 134.Liu KP, Lin CY, Chen HJ, Wei CF, Lee-Chen GJ. Renin-angiotensin system polymorphisms in Taiwanese primary vesicoureteral reflux. Pediatr Nephrol. 2004;19:594–601. doi: 10.1007/s00467-003-1379-7. [DOI] [PubMed] [Google Scholar]
- 135.Jin W, Liu Y, Sheng HH, et al. Single nucleotide polymorphisms in promoter of angiotensin II type 1 receptor gene associated with essential hypertension and coronary heart disease in Chinese population. Acta Pharmacol Sin. 2003;24:1083–1088. [PubMed] [Google Scholar]
- 136.Koh WP, Yuan JM, Van Den Berg D, Lee HP, Yu MC. Polymorphisms in angiotensin II type 1 receptor and angiotensin I-converting enzyme genes and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:459–464. doi: 10.1093/carcin/bgh309. [DOI] [PubMed] [Google Scholar]
- 137.Kainulainen K, Perola M, Terwilliger J, et al. Evidence for involvement of the type 1 angiotensin II receptor locus in essential hypertension. Hypertension. 1999;33:844–849. doi: 10.1161/01.hyp.33.3.844. [DOI] [PubMed] [Google Scholar]
- 138.Hashizume K, Mashima Y, Fumayama T, et al. Genetic polymorphisms in the angiotensin II receptor gene and their association with open-angle glaucoma in a Japanese population. Invest Ophthalmol Vis Sci. 2005;46:1993–2001. doi: 10.1167/iovs.04-1100. [DOI] [PubMed] [Google Scholar]
- 139.Henderson SO, Haiman CA, Mack W. Multiple polymorphisms in the renin- angiotensin- aldosterone system (ACE, CYP11B2, AGTR1) and their contribution to hypertension in African Americans and Latinos in the multiethnic cohort. Am J Med Sci. 2004;328:266–273. doi: 10.1097/00000441-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 140.Tiret L, Mallet C, Poirier O, et al. Lack of association between polymorphisms of eight candidate genes and idiopathic dilated cardiomyopathy: the CARDIGENE study. J Am Coll Cardiol. 2000;35:29–35. doi: 10.1016/s0735-1097(99)00522-7. [DOI] [PubMed] [Google Scholar]
- 141.Haga H, Yamada R, Ohnishi Y, Nakamura Y, Tanaka T. Gene-based SNP discovery as part of the Japanese millennium genome project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet. 2002;47:605–610. doi: 10.1007/s100380200092. [DOI] [PubMed] [Google Scholar]
- 142.Chaves FJ, Corella D, Sorli JV, Marin-Garcia P, Guillen M, Redon J. Polymorphisms of the renin-angiotensin system influence height in normotensive women in a Spanish population. J Clin Endocrinol Metab. 2004;89:2301–2305. doi: 10.1210/jc.2003-031058. [DOI] [PubMed] [Google Scholar]
- 143.van Rijn MJ, Schut AF, Aulchenko YS, et al. Heritability of blood pressure traits and the genetic contribution to blood pressure variance explained by four blood-pressure- related genes. J Hypertens. 2007;25:565–570. doi: 10.1097/HJH.0b013e32801449fb. [DOI] [PubMed] [Google Scholar]
- 144.Benetos A, Gautier S, Ricard S, et al. Influence of angiotensin-converting enzyme and angiotensin II type 1 receptor gene polymorphisms on aortic stiffness in normotensive and hypertensive patients. Circulation. 1996;94:698–703. doi: 10.1161/01.cir.94.4.698. [DOI] [PubMed] [Google Scholar]
- 145.Gainer JV, Hunley TE, Kon V, Nadeau JH, Muldowney JA, III, Brown NJ. Angiotensin II type I receptor polymorphism in African Americans lower frequency of the C1166 variant. Biochem Mol Biol Int. 1997;43:227–231. doi: 10.1080/15216549700204001. [DOI] [PubMed] [Google Scholar]
- 146.Tiret L, Blanc H, Ruidavets JB, et al. Gene polymorphisms of the renin-angiotensin system in relation to hypertension and parental history of myocardial infarction and stroke: the PEGASE study. Projet d’Etude des Genes de l’Hypertension Arterielle Severe a moderee Essentielle. J Hypertens. 1998;16:37–44. doi: 10.1097/00004872-199816010-00007. [DOI] [PubMed] [Google Scholar]
- 147.Liyou N, Davis D, James K, et al. The A1166C mutation in the angiotensin II type I receptor and hypertension in the elderly. Clin Exp Pharmacol Physiol. 1999;26:525–526. doi: 10.1046/j.1440-1681.1999.03066.x. [DOI] [PubMed] [Google Scholar]
- 148.Rice GI, Foy CA, Grant PJ. Angiotensin converting enzyme and angiotensin II type 1-receptor gene polymorphisms and risk of ischaemic heart disease. Cardiovasc Res. 1999;41:746–753. doi: 10.1016/s0008-6363(98)00246-6. [DOI] [PubMed] [Google Scholar]
- 149.Nalogowska-Glosnicka K, Lacka BI, Zychma MJ, et al. Angiotensin II type 1 receptor gene A1166C polymorphism is associated with the increased risk of pregnancy induced hypertension. Med Sci Monit. 2000;6:523–529. [PubMed] [Google Scholar]
- 150.Canavy I, Henry M, Morange PE, et al. Genetic polymorphisms and coronary artery disease in the south of France. Thromb Haemost. 2000;83:212–216. [PubMed] [Google Scholar]
- 151.Fatini C, Abbate R, Pepe G, et al. Searching for a better assessment of the individual coronary risk profile. The role of angiotensin-converting enzyme, angiotensin II type 1 receptor and angiotensinogen gene polymorphisms. Eur Heart J. 2000;21:633–638. doi: 10.1053/euhj.1999.1738. [DOI] [PubMed] [Google Scholar]
- 152.Steeds RP, Wardle A, Smith PD, Martin D, Channer KS, Samani NJ. Analysis of the postulated interaction between the angiotensin II sub-type 1 receptor gene A1166C polymorphism and the insertion/deletion polymorphism of the angiotensin converting enzyme gene on risk of myocardial infarction. Atherosclerosis. 2001;154:123–128. doi: 10.1016/s0021-9150(00)00438-x. [DOI] [PubMed] [Google Scholar]
- 153.Coto E, Rodrigo L, Alvarez R, et al. Variation at the angiotensin-converting enzyme and endothelial nitric oxide synthase genes is associated with the risk of esophageal varices among patients with alcoholic cirrhosis. J Cardiovasc Pharmacol. 2001;38:833–839. doi: 10.1097/00005344-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 154.Fernandez-Arcas N, Dieguez-Lucena JL, Munoz-Moran E, et al. Both alleles of the M235T polymorphism of the angiotensinogen gene can be a risk factor for myocardial infarction. Clin Genet. 2001;60:52–57. doi: 10.1034/j.1399-0004.2001.600108.x. [DOI] [PubMed] [Google Scholar]
- 155.Mettimano M, Lanni A, Migneco A, Specchia ML, Romano-Spica V, Savi L. Angiotensin- related genes involved in essential hypertension: allelic distribution in an Italian population sample. Ital Heart J. 2001;2:589–593. [PubMed] [Google Scholar]
- 156.Sunder-Plassmann G, Kittler H, Eberle C, et al. Angiotensin converting enzyme DD genotype is associated with hypertensive crisis. Crit Care Med. 2002;30:2236–2241. doi: 10.1097/00003246-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 157.Strazzullo P, Iacone R, Iacoviello L, et al. Genetic variation in the renin-angiotensin system and abdominal adiposity in men: the Olivetti prospective heart study. Ann Intern Med. 2003;138:17–23. doi: 10.7326/0003-4819-138-1-200301070-00007. [DOI] [PubMed] [Google Scholar]
- 158.Jones A, Dhamrait SS, Payne JR, et al. Genetic variants of angiotensin II receptors and cardiovascular risk in hypertension. Hypertension. 2003;42:500–506. doi: 10.1161/01.HYP.0000088853.27673.D0. [DOI] [PubMed] [Google Scholar]
- 159.Luther Y, Bantis C, Ivens K, Fehsel K, Kolb-Bachhofen V, Heering P. Effects of the genetic polymorphisms of the renin-angiotensin system on focal segmental glomerulosclerosis. Kidney Blood Press Res. 2003;26:333–337. doi: 10.1159/000073939. [DOI] [PubMed] [Google Scholar]
- 160.Papp F, Friedman A, Bereczki C, et al. Renin-angiotensin gene polymorphism in children with uremia and essential hypertension. Pediatr Nephrol. 2003;18:150–154. doi: 10.1007/s00467-002-1032-x. [DOI] [PubMed] [Google Scholar]
- 161.Stankovic A, Zivkovic M, Glisic S, Alavantic D. Angiotensin II type 1 receptor gene polymorphism and essential hypertension in Serbian population. Clin Chim Acta. 2003;327:181–185. doi: 10.1016/s0009-8981(02)00340-6. [DOI] [PubMed] [Google Scholar]
- 162.Bouba I, Makrydimas G, Kalaitzidis R, Lolis DE, Siamopoulos KC, Georgiou I. Interaction between the polymorphisms of the renin-angiotensin system in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;110:8–11. doi: 10.1016/s0301-2115(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 163.Kostic M, Stankovic A, Zivkovic M, et al. ACE and AT1 receptor gene polymorphisms and renal scarring in urinary bladder dysfunction. Pediatr Nephrol. 2004;19:853–857. doi: 10.1007/s00467-004-1511-3. [DOI] [PubMed] [Google Scholar]
- 164.Andrikopoulos GK, Richter DJ, Needham EW, et al. The paradoxical association of common polymorphisms of the renin-angiotensin system genes with risk of myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2004;11:477–483. doi: 10.1097/00149831-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 165.Bellwon J, Sobiczewski W, Gruchala M, et al. An A1166C polymorphism of the angiotensin II AT1 receptor gene does not influence the diagnosis of arterial hypertension during 7 years follow up. Am J Hypertens. 2005;18(5 part 2):100A. [Google Scholar]
- 166.Tabel Y, Berdeli A, Mir S, Serdaroglu E, Yilmaz E. Effects of genetic polymorphisms of the renin-angiotensin system in children with nephrotic syndrome. J Renin Angiotensin Aldosterone Syst. 2005;6:138–144. doi: 10.3317/jraas.2005.020. [DOI] [PubMed] [Google Scholar]
- 167.Sekuri C, Cam FS, Ercan E, et al. Renin-angiotensin system gene polymorphisms and premature coronary heart disease. J Renin Angiotensin Aldosterone Syst. 2005;6:38–42. doi: 10.3317/jraas.2005.005. [DOI] [PubMed] [Google Scholar]
- 168.Ece A, Tekes S, Gurkan F, Bilici M, Budak T. Polymorphisms of the angiotensin converting enzyme and angiotensin II type 1 receptor genes and renal scarring in non-uropathic children with recurrent urinary tract infection. Nephrology (Carlton) 2005;10:377–381. doi: 10.1111/j.1440-1797.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 169.Ozkaya O, Soylemezoglu O, Gonen S, et al. Renin-angiotensin system gene polymorphisms: association with susceptibility to Henoch-Schonlein purpura and renal involvement. Clin Rheumatol. 2006;25:861–865. doi: 10.1007/s10067-006-0207-4. [DOI] [PubMed] [Google Scholar]
- 170.Seremak-Mrozikiewicz A, Dubiel M, Drews K, Breborowicz GH, Mrozikiewicz PM. 1166C mutation of angiotensin II type 1 receptor gene is correlated with umbilical blood flow velocimetry in women with preeclampsia. J Matern Fetal Neonatal Med. 2005;17:117–121. doi: 10.1080/14767050500043400. [DOI] [PubMed] [Google Scholar]
- 171.Buraczynska M, Ksiazek P, Zaluska W, Spasiewicz D, Nowicka T, Ksiazek A. Angiotensin II type 1 receptor gene polymorphism in end-stage renal disease. Nephron. 2002;92:51–55. doi: 10.1159/000064455. [DOI] [PubMed] [Google Scholar]
- 172.Nakauchi Y, Suehiro T, Yamamoto M, et al. Significance of angiotensin I-converting enzyme and angiotensin II type 1 receptor gene polymorphisms as risk factors for coronary heart disease. Atherosclerosis. 1996;125:161–169. doi: 10.1016/0021-9150(96)05866-2. [DOI] [PubMed] [Google Scholar]
- 173.Ishanov A, Okamoto H, Watanabe M, et al. Angiotensin II type 1 receptor gene polymorphisms in patients with cardiac hypertrophy. Jpn Heart J. 1998;39:87–96. doi: 10.1536/ihj.39.87. [DOI] [PubMed] [Google Scholar]
- 174.Takemoto Y, Sakatani M, Takami S, et al. Association between angiotensin II receptor gene polymorphism and Serum Angiotensin Converting Enzyme (SACE) activity in patients with sarcoidosis. Thorax. 1998;53:459–462. doi: 10.1136/thx.53.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thomas GN, Young RP, Tomlinson B, Woo KS, Sanderson JE, Critchley JA. Renin angiotensin-aldosterone system gene polymorphisms and hypertension in Hong Kong Chinese. Clin Exp Hypertens. 2000;22:87–97. doi: 10.1081/ceh-100100064. [DOI] [PubMed] [Google Scholar]
- 176.Aoki S, Mukae S, Itoh S, et al. Genetic background in patients with acute myocardial infarction. Jpn Heart J. 2001;42:15–28. doi: 10.1536/jhj.42.15. [DOI] [PubMed] [Google Scholar]
- 177.Liu Y, Zhuoma C, Shan G, et al. A1166C polymorphism of the angiotensin II type 1 receptor gene and essential hypertension in Han, Tibetan and Yi populations. Hypertens Res. 2002;25:515–521. doi: 10.1291/hypres.25.515. [DOI] [PubMed] [Google Scholar]
- 178.Lee KB, Kim UK. Angiotensinogen and angiotensin II type 1 receptor gene polymorphism in patients with autosomal dominant polycystic kidney disease: effect on hypertension and ESRD. Yonsei Med J. 2003;44:641–647. doi: 10.3349/ymj.2003.44.4.641. [DOI] [PubMed] [Google Scholar]
- 179.Fukazawa R, Sonobe T, Hamamoto K, et al. Possible synergic effect of angiotensin- I converting enzyme gene insertion/deletion polymorphism and angiotensin-II type-1 receptor 1166A/C gene polymorphism on ischemic heart disease in patients with Kawasaki disease. Pediatr Res. 2004;56:597–601. doi: 10.1203/01.PDR.0000139426.16381.C8. [DOI] [PubMed] [Google Scholar]
- 180.Kobashi G, Hata A, Ohta K, et al. A1166C variant of angiotensin II type 1 receptor gene is associated with severe hypertension in pregnancy independently of T235 variant of angiotensinogen gene. J Hum Genet. 2004;49:182–186. doi: 10.1007/s10038-004-0129-4. [DOI] [PubMed] [Google Scholar]
- 181.Yan C, Zhan J, Feng W. Gene polymorphisms of angiotensin II type 1 receptor and angiotensin-converting enzyme in two ethnic groups living in Zhejiang Province, China. J Renin Angiotensin Aldosterone Syst. 2005;6:132–137. doi: 10.3317/jraas.2005.019. [DOI] [PubMed] [Google Scholar]
- 182.Nanfang L, Tao L, Ling Z. The study for relationship between the angiotensin II type 1 receptor gene polymorphism and essential hypertension in Kazakans of Xinjiang. J Hypertens. 2004;24:240. [Google Scholar]
- 183.Kim Y, Kim JH, Nam YJ, et al. Sequence variants of ACE, AGT, AT1R, and PAI-1 as genetic risk factors for vascular dementia. Neurosci Lett. 2006;401:276–279. doi: 10.1016/j.neulet.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 184.Rehman A, Rasool AH, Naing L, Roshan TM, Rahman AR. Influence of the angiotensin II type I receptor gene 1166A > C polymorphism on BP and aortic pulse wave velocity among Malays. Ann Hum Genet. 2007;71(Pt 1):86–95. doi: 10.1111/j.1469-1809.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 185.Williams SM, Addy JH, Phillips JA, III, et al. Combinations of variations in multiple genes are associated with hypertension. Hypertension. 2000;36:2–6. doi: 10.1161/01.hyp.36.1.2. [DOI] [PubMed] [Google Scholar]
- 186.Szombathy T, Szalai C, Katalin B, Palicz T, Romics L, Csaszar A. Association of angiotensin II type 1 receptor polymorphism with resistant essential hypertension. Clin Chim Acta. 1998;269:91–100. doi: 10.1016/s0009-8981(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 187.Wang W, Zee R, Morris B. Association of angiotensin II type 1 receptor genepoly morphism with essential hypertension. Clin Genet. 1997;51:31–34. doi: 10.1111/j.1399-0004.1997.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 188.Romano-Spica V, Mettimano M, Ianni A, Specchia ML, Ricciardi G, Savi L. Epidemiology of essential hypertension: the role of genetic polymorphism. Eur J Epidemiol. 2003;18:211–219. doi: 10.1023/A:1023360410810. [DOI] [PubMed] [Google Scholar]
- 189.Agachan B, Isbir T, Yilmaz H, Akoglu E. Angiotensin converting enzyme I/D, angiotensinogen T174M-M235T and angiotensin II type 1 receptor A1166Cgene polymorphisms in Turkish hypertensive patients. Exp Mol Med. 2003;35:545–549. doi: 10.1038/emm.2003.71. [DOI] [PubMed] [Google Scholar]
- 190.Barbalic M, Skaric-Juric T, Cambien F, et al. Gene polymorphisms of the renin angiotensin system and early development of hypertension. Am J Hypertens. 2006;19:837–842. doi: 10.1016/j.amjhyper.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 191.Behravan J, Naghibi M, Mazloomi MA, et al. Polymorphism of angiotensin II type 1 receptor gene in essential hypertension in Iranian population. DARU. 2006;14:82–86. [Google Scholar]
- 192.Lapierre AV, Arce ME, Lopez JR, Ciuffo GM. Angiotensin II type 1 receptorA1166C gene polymorphism and essential hypertension in San Luis. Biocell. 2006;30:447–455. [PubMed] [Google Scholar]
- 193.Wu K, Lin C, Lin M, et al. The relationships between angiotensin II type 1 receptor gene polymorphism and essential hypertension and its complications (abstract) J Hypertens. 2000;18(Supp 4):S176. [Google Scholar]
- 194.Tsai CT, Fallin D, Chiang FT, et al. Angiotensinogen gene haplotype and hypertension: interaction with ACE gene I allele. Hypertension. 2003;41:9–15. doi: 10.1161/01.hyp.0000045080.28739.12. [DOI] [PubMed] [Google Scholar]
- 195.Wang JG, Staessen JA. Genetic polymorphisms in the renin-angiotensin system: relevance for susceptibility to cardiovascular disease. Eur J Pharmacol. 2000;410:289–302. doi: 10.1016/s0014-2999(00)00822-0. [DOI] [PubMed] [Google Scholar]