Abstract
Harmane, one of the heterocyclic amines (HCAs), is a potent neurotoxin linked to human diseases. Dietary exposure, especially in cooked meats, is the major source of exogenous exposure for humans. However, knowledge of harmane concentrations in cooked meat samples is limited. Our goals were to (1) quantify the concentration of harmane in different types of cooked meat samples, (2) compare its concentration to that of other more well-understood HCAs, and (3) examine the relationship between harmane concentration and level of doneness. Thirty barbecued/grilled meat samples (8 beef steak, 12 hamburger, 10 chicken) were analyzed for harmane and four other HCAs (2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine [PhIP], amino-3,8-dimethylimidazo[4,5-f]quinoxaline [MeIQx], 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline [DiMeIQx], and 2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine [IFP]). Mean (± SD) harmane concentration was 5.63 (± 6.63) ng/g; harmane concentration was highest in chicken (8.48 ± 9.86 ng/g) and lowest in beef steak (3.80 ± 3.6 ng/g). Harmane concentration was higher than that of the other HCAs and significantly correlated with PhIP concentration. Harmane concentration was associated with meat doneness in samples of cooked beef steak and hamburger, although the correlation between meat doneness and concentration was greater for PhIP than for harmane. Evidence indicates that harmane was detectable in nanograms per gram quantities in cooked meat (especially chicken) and, moreover, was more abundant than other HCAs. There was some correlation between meat doneness and harmane concentration, although this correlation was less robust than that observed for PhIP. Data such as these may be used to improve estimation of human dietary exposure to this neurotoxin.
The heterocyclic amines (HCAs), which have been identified in cooked foods (especially meats), exert a variety of adverse health effects and have been linked with human cancer risks (Skog & Solyakov, 2002). More than 20 HCAs have been identified in cooked foods (Felton et al., 2000; Skog, 2002). Among these is harmane (1-methyl-9H-pyrido[3,4-b]indole), which is both a comutagen that enhances mutagenic activity of other compounds and a potent neurotoxin, producing tremors and psychiatric manifestations (Zetler et al., 1972; Sakai, 1995). Although harmane is less well understood than many of the other HCAs, recent studies have demonstrated an association between blood concentrations of harmane and odds of essential tremor development (Louis et al., 2002, 2005), the most common tremor disorder in humans (Louis et al., 1998). Other studies demonstrated elevated concentrations of harmane in cerebrospinal fluid in patients with Parkinson’s disease (Kuhn et al., 1996). Given the neurotoxic effects of harmane, it is important to assess human exposure to/intake of this HCA.
In general, HCA concentrations increase with certain meat cooking practices (e.g., concentrations are correlated with duration of cooking and meat doneness) (Sinha et al., 1998). Cooking under intense heat, frying, and especially grilling on an open flame have resulted in high levels of harmane and other HCAs (Pfau & Skog, 2004; Herraiz, 2000). There are a number of studies examining harmane concentrations in cooked meat samples (Johansson et al., 1995; Chiu et al., 1998; Gross & Gruter, 1992; Gross et al., 1993; Skog et al., 1997, 1998; Solyakov et al., 1999; Totsuka et al., 1999; Pias et al., 1999; Herraiz, 2000; Solyakov & Skog, 2002; Olsson et al., 2002), although the number of meat samples in these studies was generally small and there are few data on the relationship between type of meat, meat doneness, and harmane concentrations. Hence, assessing dietary exposure to harmane is difficult. To address these issues, a study of HCA concentrations in meats prepared in residential settings was conducted. Our specific aims were to (1) quantify the concentration of harmane in different types (beef steak, hamburger, chicken) of barbecued/grilled meat samples, (2) compare its concentration to that of other more well-understood HCAs (PhIP, MeIQx, DiMeIQx, and IFP), and (3) examine the relationship between harmane concentration and the level of doneness. Our overarching goal was to improve estimation of dietary exposure to this neurotoxin.
METHODS
Meat Sample Collection
The Agricultural Health Study (AHS) cohort is a prospective health study of pesticide applicators and their families (Alavanja et al., 1996). Participants responded that, more than once per month, they consumed barbecued/grilled meats that were either well done or very well done. Our interest in barbecued/grilled meats related to the observations that cooking under intense heat and especially grilling on an open flame can produce very high levels of harmane and other HCAs (Pfau & Skog, 2004; Herraiz, 2000). Participants were sent a meat sampling kit, as described previously (Keating et al., 2000). The sampling kit consisted of instructions on how to obtain the sample of barbecued/grilled meat, Zip-Loc plastic bags to store and ship the meat sample, and a disposable Kodak flash camera. Participants were also provided a questionnaire requesting information about how the meat was prepared and cooked as well as instructions to take photographs of the meat before cooking, after cooking, and after being cut in half after cooking. Meat samples were initially stored frozen by participants and then shipped on dry ice, with the corresponding camera, to Lawrence Livermore National Laboratory, where the samples were stored frozen until analysis (Keating et al., 2000).
Meat Sample Analysis for HCA Concentrations
For the current study, 30 frozen meat samples (8 beef steak, 12 hamburger, and 10 chicken [6 skinless breasts, 2 legs [1 with skin], 1 wing with skin, 1 hindquarter with skin]) were homogenized at Lawrence Livermore National Laboratory and analyzed for concentrations (ng/g of cooked meat) of 4 HCAs: (1) 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), (2) amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), (3) 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx), and (4) 2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine (IFP). The analyses and high-performance liquid chromatography (HPLC) system were described previously (Keating et al., 2000). Aliquots (4.8–20.3 g) of the homogenized frozen meat samples were shipped to Purdue University, where they were further analyzed for concentrations (ng/g) of harmane. The method to quantify harmane in biological samples was described previously (Louis et al., 2002, 2005; Zheng et al., 2000) with some modifications for meat sample analysis. Briefly, triplicate samples of each frozen meat sample were prepared individually as follows: The removed samples were homogenized in a buffer (1:2–4, g:ml) containing 20 mM Tris, pH 7.5, 5 mM EGTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], and protease inhibitor cocktail (Calbiochem, San Diego, CA) on ice. After digestion with NaOH, the samples were centrifuged at 800 × g, followed by removal of top-layer fat tissues using a filter paper. The samples were then extracted with ethyl acetate and methyl t-butyl ether according to the established protocol (Zheng et al., 2000). The method has the detection limit to measure 1 ng harmane per gram of meat tissue. The recovery from chicken samples relative to harmane in the buffer, which underwent the same extraction procedure, was 102.6%. A typical HPLC chromatogram is presented (Figure 1).
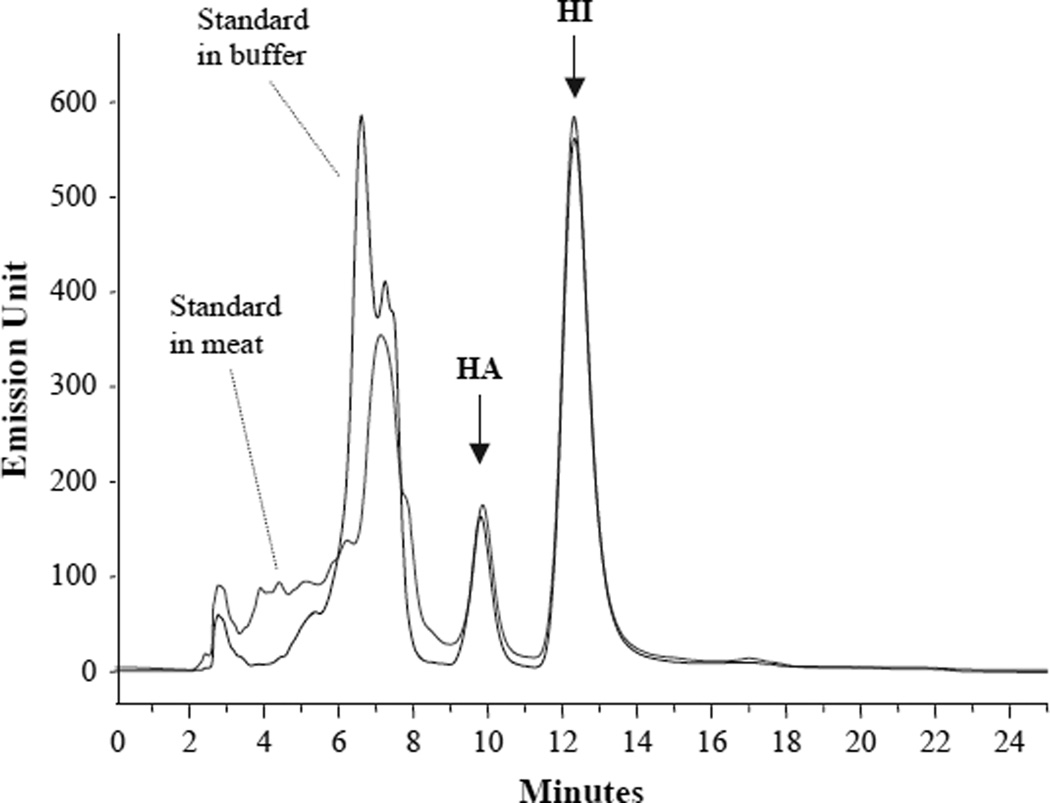
FIG. 1.
A typical HPLC chromatogram of harmane (HA) and harmine (HI) in chicken sample as well as in buffer. Standard harmane and harmine (100 ng each) were mixed with either 2 ml buffer or 1 g chicken meat in 2 ml buffer. Both samples underwent the same extraction procedure prior to HPLC. Arrows indicate harmane eluted at 9.864 min and harmine at 12.32 min. The curve showing the maximum emission between 6 and 7 min represents the standards in the buffer.
Assignment of Level of Doneness
Meat doneness is commonly used as an indicator of HCA content in grilled/barbequed meats. Although AHS participants indicated the level of doneness to which the meat samples were cooked, doneness assessment can be a subjective; hence, a more objective measure of meat doneness was developed to classify samples. For each of the 30 meat samples, doneness was assessed using the participant’s Kodak color photographs of the sample (Keating et al., 2000). As previously described, six reviewers were separately shown four photographs taken by the participant of each meat sample and asked questions about the interior and exterior colors of the cooked meat. A value was assigned to each sample based on the responses of the reviewers (1 [medium], 2 [well done], 3 [very well done]) and the six values were averaged to provide a doneness level for each meat sample (Keating et al., 2000).
Statistical Analyses
All analyses were performed in SPSS (Version 13.0). The criterion for significance was set at p < .05. Concentrations of HCAs were not normally distributed and therefore, values were log-transformed. Paired t-tests were used to compare log harmane concentration with log concentrations of PhIP, MeIQx, DiMeIQx, or IFP. In one analysis, the concentration of harmane was compared to the combined concentration of PhIP, MeIQx, DiMeIQx, and IFP (the sum of these four was referred to as the “total HCA concentration”). Pearson’s correlation coefficients (R) were used to compare log harmane concentrations with log concentrations of other HCAs. For some analyses, meat doneness was treated as a continuous variable and in others, meat doneness (range = 1–3) was stratified into tertiles (lowest meat doneness tertile = 1.0–1.78 [mean meat doneness = 1.4], middle = 1.79–2.25 [mean = 2.0], and highest tertile = 2.26–3.0 [mean = 2.8]), and the association between tertile (independent variable) and HCA concentration (dependent variable) was assessed in linear regression analyses.
A sample size of 30 meat samples was chosen to allow us to assess approximately 10 samples (with a range of doneness) from each of the 3 meat types; this sample size of 30 was larger than that of most other previous studies of meat concentration of harmane (Gross & Gruter, 1992; Gross et al., 1993; Skog et al., 1997, 1998; Solyakov et al., 1999; Totsuka et al., 1999; Herraiz, 2000), which generally used 10 or fewer meat samples.
RESULTS
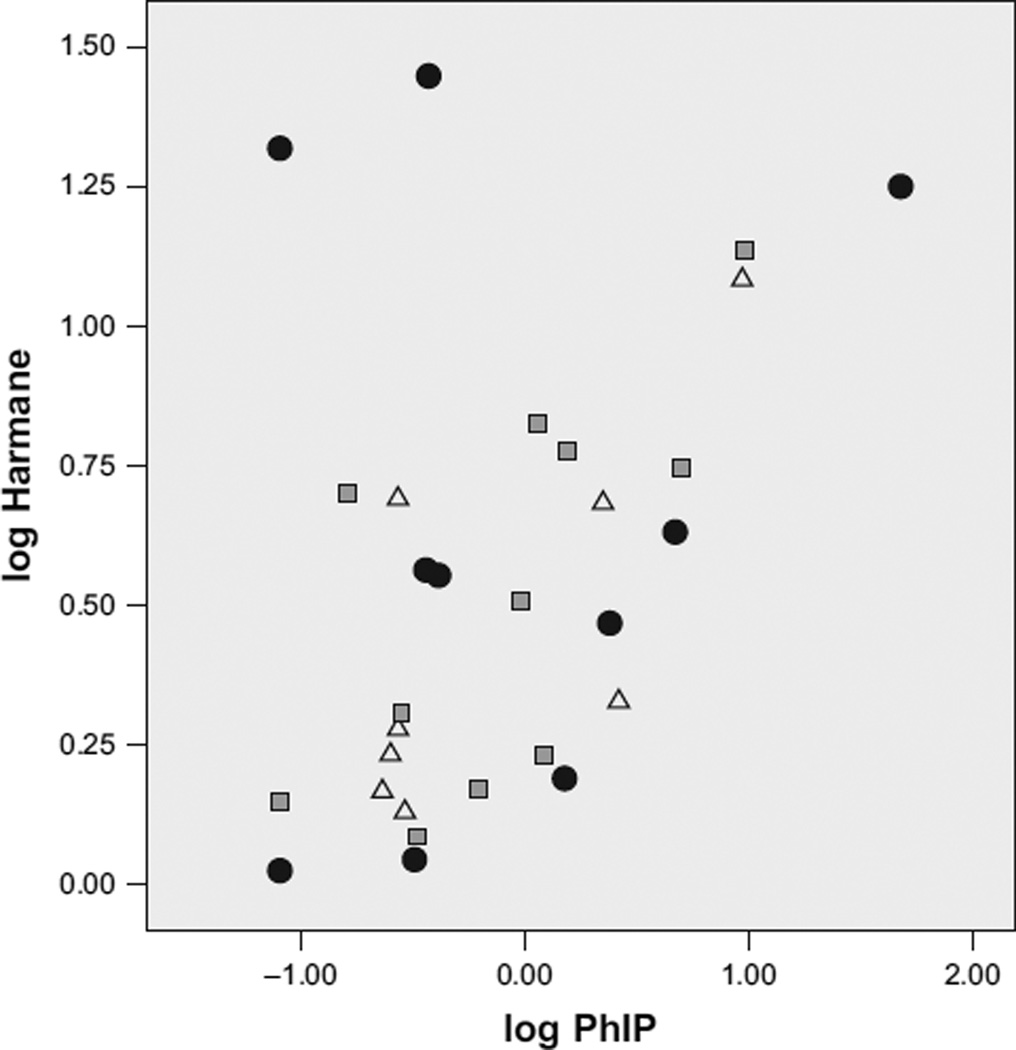
The mean (± SD) harmane concentration (n = 30, all meats) was 5.63 (± 6.63) ng/g in cooked meat (range = 1.06–28.05 ng/g) (Table 1). Harmane concentration was highest in chicken (8.48 ± 9.86 ng/g) and lowest in beef steak (3.8 ± 3.66 ng/g) (Table 1). The concentration of harmane was higher than that of the other four HCAs; this was followed by the concentration of PhIP (e.g., in Table 1, in four paired t-tests, log harmane concentration was higher than that of each of the other four HCAs in the “All Meats” category). Log harmane concentration was significantly correlated with log total HCA concentration (i.e., PhIP + MeIQx + DiMeIQx + IFP) in beef steak (r = .73) and hamburger (r = .63) but not in chicken (r = .20) (not shown in Table 2). Log harmane concentration was most markedly correlated with log PhIP concentration (for All Meats, r = .44, Table 2 and Figure 2) and, in terms of meat types, most significantly correlated with the concentrations of other HCAs in beef and hamburger (Table 2).
TABLE 1.
Concentrations (ng/g) and Log Concentrations of Five HCAs in Three Meat Types
| Beef (n = 8) | Hamburger(n = 12) | Chickena(n = 10) | All meats(n = 30) | |
|---|---|---|---|---|
| Harmane | 3.80 ± 3.66 (1.35–12.11) | 4.36 ± 3.71 (1.22–13.68) | 8.48 ± 9.86 (1.06–28.05) | 5.63 ± 6.63 (1.06–28.05) |
| Log harmane | 0.45 ± 0.34 | 0.51 ± 0.35 | 0.65 ± 0.52 | 0.54 ± 0.41 |
| PhIP | 1.94 ± 3.15 (0.23–9.35) | 3.23 ± 5.36 (0.08–17.80) | 5.80 ± 14.83 (0.08–47.79) | 3.74 ± 9.16 (0.08–47.79) |
| Log PhIP | −0.15 ± 0.63 | 0.01 ± 0.71 | −0.10 ± 0.85 | −0.07 ± 0.72 |
| MeIQx | 0.92 ± 1.83 (0.03–5.41) | 1.04 ± 1.04 (0.03–3.11) | 0.28 ± 0.28 (0.03–0.82) | 0.75 ± 1.17 (0.03–5.41) |
| Log MeIQx | −0.59 ± 0.72 | −0.30 ± 0.63 | −0.81 ± 0.55 | −0.55 ± 0.65 |
| DeMeIQx | 0.17 ± 0.36 (0.02–1.06) | 0.21 ± 0.24 (0.02–0.74) | 0.08 ± 0.07 (0.02–0.22) | 0.15 ± 0.24 (0.02–1.06) |
| Log DeMeIQx |
−1.32 ± 0.62 | −1.06 ± 0.64 | −1.29 ± 0.41 | −1.21 ± 0.56 |
| IFP | 0.88 ± 2.33 (0.01–6.64) | 0.05 ± 0.14 (0.01–0.51) | 0.15 ± 0.30 (0.01–0.87) | 0.30 ± 1.21 (0.01–6.64) |
| Log IFP | −1.46 ± 1.06 | −1.86 ± 0.49 | −1.63 ± 0.77 | −1.68 ± 0.76 |
Note. Values are mean ± SD (range). Log harmane concentration was compared by paired t tests with log PhIP, log MeIQx, log DeMeIQx, and log IFP concentrations (in ‘All Meats’ category), and all p ≤ .001.
For chicken, mean harmane concentration (ng/g) in 6 skinless breasts = 9.8 ± 11.6, 2 legs (1 with skin) = 2.4 ± 1.7, 1 wing (with skin) = 17.8 and 1 hindquarter (with skin) = 3.7.
TABLE 2.
Correlation Between Log Harmane Concentration and Log Concentrations of Four Other HCA in Three Meat Types
| Beef | Hamburger | Chicken | All meats | |
|---|---|---|---|---|
| Log PhIP | R = .74 p = .037 | R = .68 p = .021 | R = .21 p = .567 | R = .44 p = .018 |
| Log MeIQx | R = .67 p = .068 | R = .57 p = .069 | R = .01 p = .985 | R = .28 p = .135 |
| Log DeMeIQx | R = .53 p = .176 | R = .37 p = .266 | R = .03 p = .925 | R = .25 p = .201 |
| Log IFP | R = .59 p = .122 | R = −.01 p = .987 | R = −.11 p = .774 | R = .17 p = .548 |
Note. Pearson’s correlation coefficients (R) were used to compare log harmane concentration with log concentrations of PhIP, MeIQx, DiMeIQx, and IFP.
FIG. 2.
Log harmane by log PhIP concentrations in chicken (closed circles), hamburger (gray squares), and beef (triangles).
Harmane concentration was associated with meat doneness in samples of cooked beef steak and hamburger, although the correlation between meat doneness and concentration was greater for PhIP than for harmane (Table 3). The correlation (R) between doneness and log harmane concentration was not significant; after excluding the samples of chicken, there was significance (r = .48) (not shown in Table 3).
TABLE 3.
Mean ± SD Concentration (ng/g) of Harmane and PhIP by Doneness Tertile
| Doneness tertile | Harmane | Log harmane | PhIP | LogPhIP |
|---|---|---|---|---|
| Including all meat types | ||||
| Lowest tertile | 4.18 ± 6.00 | 0.40 ± 0.40 | 0.86 ± 1.42 | −0.46 ± 0.59 |
| Middle tertile | 5.47 ± 8.13 | 0.51 ± 0.41 | 0.91 ± 0.92 | −0.24 ± 0.44 |
| Highest tertile | 7.41 ± 5.74 p = .30 |
0.74 ± 0.38 p = .076 |
9.46 ± 14.60 p = .033 |
0.49 ± 0.75 p = .002 |
| Including beef steak and hamburger (excluding chicken) | ||||
| Lowest tertile | 2.36 ± 1.45 | 0.31 ± 0.24 | 0.37 ± 0.30 | −0.54 ± 0.34 |
| Middle tertile | 3.15 ± 2.17 | 0.42 ± 0.27 | 0.94 ± 0.95 | −0.24 ± 0.49 |
| Highest tertile | 6.47 ± 4.75 p = .035 |
0.69 ± 0.38 p = .035 |
5.80 ± 6.10 p = .017 |
0.45 ± 0.64 p = .002 |
Note. Meat doneness score (range = 1–3) was stratified into tertiles: lowest meat doneness tertile = 1.0–1.78 (mean doneness = 1.4), middle = 1.79–2.25 (mean = 2.0), and highest tertile = 2.26–3.0 (mean = 2.8), and the association between tertile (independent variable) and HCA concentration (dependent variable) was assessed in linear regression analyses.
DISCUSSION
Harmane is a potent neurotoxin, linked in several studies with essential tremor and Parkinson’s disease (Kuhn et al, 1996, Louis et al., 2002, 2005). As with other HCAs, a major dietary source of harmane is cooked protein-rich foods, with the quantity of these HCAs varying by meat type and meat cooking practices. Once ingested, harmane is distributed among bodily tissues, with the oral bioavailability in rats estimated to be 19% (Guan et al., 2001) and an increase in serum levels 5 min after the start of a meal (Pfau & Skog, 2004). Estimates of exogenous intake (as high as 1 µg/kg/d) greatly exceed estimates of endogenous harmane formation (20 ng/d), suggesting that exogenous intake is the more important of the two sources (Pfau & Skog, 2004). Hence, it is of great importance to quantify the amounts of harmane in different cooked meats.
Harmane was detectable in our residentially-prepared meat samples in nanograms per gram quantities (range = 1.06–28.05 ng/g). Such nanograms per gram concentrations of harmane in various meats were also reported by previous investigators (Johansson et al., 1995; Herraiz, 2000; Olsson et al., 2002). In our study, the harmane concentration was on average highest in chicken and lowest in beef steak; one other investigator suggested that the concentration of harmane might be higher in chicken than beef (Pais et al., 1999), although further studies are needed to assess this finding.
One of our goals was compare the concentration of harmane with that of other more well-understood HCAs in order to gain an appreciation of their relative burden in cooked meats. Although the other HCAs studied (PhIP, MeIQx, DiMeIQx, IFP) are known carcinogens rather than neurotoxins (i.e., their adverse health effects differ), it is nevertheless important to establish their relative concentrations so as to obtain a broad understanding of the quantities of the various HCA toxin/carcinogens in cooked meats. Data showed that the concentration of harmane was higher than that of the other measured HCAs, including PhIP. These data may be compared to those from several previous studies. Skog et al. (1997) reported that harmane was detected in the largest number of cooked meat samples (14 of 16 samples) and in concentrations that were higher than those of other HCAs. In a second study (Skog et al., 1998), harmane and norharmane were detected at levels up to 200 ng/g in all analyzed meat samples; concentrations were uniformly higher than those of other HCAs. In a study of process flavors made of meat products (Solyakov et al., 1999), harmane and norharmane were the most abundant HCA found in the samples. Solyakov and Skog (2002) demonstrated that the concentrations of harmane and norharmane were higher than those of PhIP and other HCAs in cooked chicken products, and Olsson et al. (2002) demonstrated that harmane concentrations exceeded those of PhIP in fried pork samples. In sum, these data and our own suggest that humans are exposed in daily life to harmane to a larger extent than to many other HCAs, making harmane a particularly abundant HCA. This abundance, along with its activities as both a comutagen and potent neurotoxin, make harmane an important HCA.
Harmane was detectable in our residentially-prepared meat samples in nanograms per gram quantities. Some investigators have estimated total dietary intake of harmane from all sources to be on the order of 1 µg/kg per day (Pfau & Skog, 2004). While 22.2 mg/kg of subcutaneously administered harmane is needed to acutely produce tremor in mice (Zetler et al., 1972), the tremor-producing potential of chronic, daily lower levels of exposure (as in the human diet) is not known.
Harmane concentrations were correlated with those of another HCA, PhIP. Because of its role as a mutagenic HCA, a substantially larger literature is available for PhIP than for harmane. Several other investigators have found such correlations, with correlation coefficients (R) ≥.90 (Gross & Gruter, 1992; Gross et al., 1993; Chiu et al., 1998). These data suggest that estimates of dietary PhIP intake, which may be more widely available than those of harmane, may be used to infer information on dietary harmane intake.
Harmane concentration was correlated with meat doneness in samples of cooked beef steak and hamburger but not in samples of chicken. The correlation between meat doneness and concentration was greater for PhIP than harmane. Previous studies suggested that PhIP concentrations are particularly dependent on cooking temperatures (Skog & Solyakov, 2002).
In the current study, harmane concentrations were assessed in cooked meats. While cooked meats are an important source of dietary harmane, they are not the only source and there are other important sources as well. For example, harmane is present in high concentrations (e.g., 5.06–814 ng/g) in coffee and cigarette smoke (Herraiz, 2004), and very high amounts of harmane are found in vinegar, soy sauce, cocoa, cheese and tomato ketchup (Pfau & Skog, 2004).
This study was not without imitations. First, the sample size, while allowing us to detect harmane and its correlations with other HCAs in these meat samples, was not optimal for fully assessing the associations between meat doneness and harmane concentrations. Despite this limitation, the number of meat samples analyzed in the current study was significantly larger than that analyzed in numerous other studies (Gross & Gruter, 1992; Gross et al., 1993; Skog et al., 1997, 1998; Solyakov et al., 1999; Totsuka et al., 1999; Herraiz, 2000). Second, we did not analyze norharmane concentrations; while norhamane is present in food in higher concentrations than harmane (Pfau & Skog, 2004), its tremor-producing potential is less well documented than that of harmane (Zetler et al., 1972). Third, while a worthwhile goal would be to provide data on the concentration of harmane in the meats prior to cooking, the goal of this research was to study dietary (i.e., postcooking) sources of harmane. A strength of the study was the analysis of several different meat types and the presentation of doneness data.
Given the adverse health effects of harmane, it is important to assess human intake of this HCA. Few studies have quantified harmane concentrations in cooked meat samples and assessed its relationship with meat doneness. Harmane was present in nanograms per gram quantities in cooked meat (especially chicken) and was more abundant than other HCAs, including PhIP. There was some correlation between meat doneness and harmane concentration, although this correlation was less robust than that observed for PhIP. Better understanding of the meat properties and cooking conditions that lead to the formation of HCAs is needed to evaluate the occurrence of these compounds in the diet. It is hoped that these types of data, along with others, will help to improve our ability to estimate dietary exposure to harmane.
Acknowledgments
This work was supported by R01 NS39422 and P30 ES09089 (National Institutes of Health, Bethesda, MD). We are grateful to Cynthia P. Salmon at Lawrence Livermore National Laboratory for high-performance liquid chromatography analysis of heterocyclic amine content.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Elan D. Louis, Gertrude H. Sergievsky Center, College of Physicians and Surgeons, Columbia University, New York, New York, USA Department of Neurology, College of Physicians and Surgeons, Columbia University, New York, New York, USA.
Wei Zheng, School of Health Sciences, Purdue University, West Lafayette, Indiana, USA.
Wendy Jiang, School of Health Sciences, Purdue University, West Lafayette, Indiana, USA.
Kenneth T. Bogen, Energy and Environmental Directorate, Lawrence Livermore National Laboratory, Livermore, California, USA
Garrett A. Keating, Energy and Environmental Directorate, Lawrence Livermore National Laboratory, Livermore, California, USA
REFERENCES
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A. The Agricultural Health Study. Environ. Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CP, Yang DY, Chen BH. Formation of heterocyclic amines in cooked chicken legs. J. Food. Prot. 1998;61:712–719. doi: 10.4315/0362-028x-61.6.712. [DOI] [PubMed] [Google Scholar]
- Felton JS, Jagerstad M, Knize MG, Skog K, Wakabayashi K. Contents in food, beverages and tobacco. In: Nagao M, Sugimura T, editors. Food borne carcinogens: Heterocyclic amines. John Wiley & Sons; West Sussex; 2000. pp. 31–72. [Google Scholar]
- Gross GA, Gruter A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatog. A. 1992;592:271–278. doi: 10.1016/0021-9673(92)85095-b. [DOI] [PubMed] [Google Scholar]
- Gross GA, Turesky RJ, Fay LB, Stillwell WG, Skipper PL, Tannenbaum SR. Heterocyclic aromatic amine formation in grilled bacon, beef and fish and in grill scrapings. Carcinogenesis. 1993;14:2313–2318. doi: 10.1093/carcin/14.11.2313. [DOI] [PubMed] [Google Scholar]
- Guan Y, Louis ED, Zheng W. Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. J. Toxicol. Environ. Health A. 2001;64:101–116. doi: 10.1080/152873901753246241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MAE, Fredholm L, Bjerne I, Jagerstad M. Influence of frying fat on the formation of heterocyclic amines in fried beef-burgers and pan residues. Food Chem. Toxicol. 1995;33:993–1004. doi: 10.1016/0278-6915(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Herraiz T. Tetrahydro-β-carboline-3-carboxylic acid compounds in fish and meat: Possible precursors of co-mutagenic β-carbolines norharman and harman in cooked foods. Food Addit. Contam. 2000;17:859–866. doi: 10.1080/026520300420439. [DOI] [PubMed] [Google Scholar]
- Herraiz T. Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit. Contam. 2004;21:1041–1050. doi: 10.1080/02652030400019844. [DOI] [PubMed] [Google Scholar]
- Keating GA, Sinha R, Layton D, Salmon P, Knize MG, Bogen KP, Lynch CF, Alavanj M. Comparison of heterocyclic amine levels in home-cooked meats with exposure indicators (United States) Cancer Causes Control. 2000;11:731–739. doi: 10.1023/a:1008935407971. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Muller T, Grosse H, Rommelspacher H. Elevated levels of harmane and norharmane in cerebrospinal fluid of parkinsonian patients. J. Neural Trans. 1996;103:1435–1440. doi: 10.1007/BF01271257. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder?: Estimates of the prevalence of essential tremor throughout the world. Move. Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, Parides M. Elevation of blood β-carboline alkaloids in essential tremor. Neurology. 2002;59:1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson V, Solyakov A, Skog K, Lundstrom K, Jagerstad M. Natural variations of precursors in pig meat affect the yield of heterocyclic amines—Effects of RN genotype, feeding regime, and sex. J. Agric. Food Chem. 2002;50:2962–2969. doi: 10.1021/jf011239h. [DOI] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to β-carbolines norharman and Harman. J. Chromatogr. B. 2004;802:115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Pias P, Salmon CP, Knize MG, Felton JS. Formation of mutagenic/carcinogenic heterocyclic amines in dry-heated model systems, meats, and meat drippings. J. Agric. Food Chem. 1999;47:1098–1108. doi: 10.1021/jf980644e. [DOI] [PubMed] [Google Scholar]
- Sakai S. Chemical studies of indole alkaloids. Yakugaku Zasshi. 1995;115:351–369. doi: 10.1248/yakushi1947.115.5_351. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, Swanson CA, Rhodes D, Rossi S, Felton JS, Levander OA. Heterocyclic amine content of beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem. Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- Skog K. Problems associated with the determination of heterocyclic amines in cooked foods and human exposure. Food Chem. Toxicol. 2002;40:1197–1203. doi: 10.1016/s0278-6915(02)00052-2. [DOI] [PubMed] [Google Scholar]
- Skog K, Augustsson K, Steineck G, Steinberg M, Jagerstad M. Polar and non-polar heterocyclic amines in cooked fish and meat products and their corresponding pan residues. Food Chem. Toxicol. 1997;35:555–565. doi: 10.1016/s0278-6915(97)00021-5. [DOI] [PubMed] [Google Scholar]
- Skog K, Solyakov A. Heterocyclic amines in poultry products: A literature review. Food Chem. Toxicol. 2002;40:1213–1221. doi: 10.1016/s0278-6915(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Skog K, Solyakov A, Arvidsson P, Jagerstad M. Analysis of nonpolar hetrocyclic amines in cooked foods and meat extracts using gas chromatography–mass spectrometry. J. Chromatogr. A. 1998;803:227–233. doi: 10.1016/s0021-9673(97)01266-1. [DOI] [PubMed] [Google Scholar]
- Solyakov A, Skog K. Screening for heterocyclic amines in chicken cooked in various ways. Food Chem. Toxicol. 2002;40:1205–1211. doi: 10.1016/s0278-6915(02)00054-6. [DOI] [PubMed] [Google Scholar]
- Solyakov A, Skog K, Jagerstad M. Heterocyclic amines in process flavours, process flavour ingredients, bouillon concentrates and a pan residue. Food Chem. Toxicol. 1999;37:1–11. doi: 10.1016/s0278-6915(98)00098-2. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayaski K. Quantification of the co-mutagenic B-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Zetler G, Singbartl G, Schlosser L. Cerebral pharmocokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Guan Y, Louis E. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal. Biochem. 2000;279:125–129. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]