Abstract
Background
As a pleiotropic cytokine, interleukin-10 (IL-10) plays a regulatory role in carcinogenesis and tumor growth. The aim of this meta-analysis was to assess the susceptibility of the IL-10 gene C-819T polymorphism to gastric cancer.
Material/Methods
Study identification and data extraction were independently completed by 2 authors. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated and summarized.
Results
In total, 11 articles including 1960 gastric cancer patients and 3705 controls were qualified. Overall analyses revealed a 13% reduced risk of gastric cancer conferred by the −819T allele relative to the −819C allele (OR=0.87; 95% CI: 0.77–0.97; P=0.016), without heterogeneity (I2=35.1%). In subgroup analyses, a significant difference was identified in East Asian populations (OR=0.85; 95% CI: 0.73–0.98; P=0.029, I2=43.6%), for gastric adenocarcinoma (OR=0.80; 95% CI: 0.66–0.96; P=0.017, I2=0.0%), and in population-based studies (OR=0.81; 95% CI: 0.70–0.93; P=0.003, I2=0.0%). The visual funnel plots and Egger’s tests suggested no evidence of publication bias.
Conclusions
Extending previous findings, we demonstrate a protective role of the IL-10 gene −819T allele in susceptibility to gastric cancer, and this role was more evident for gastric adenocarcinoma.
MeSH Keywords: Interleukin-10; Meta-Analysis; Polymorphism, Genetic; Stomach Neoplasms
Background
Interleukin-10 (IL-10) is a pleiotropic cytokine, and a wealth of evidence supports its regulatory role in carcinogenesis and tumor growth [1,2]. Observational studies have shown that there were high levels of serum IL-10 in patients with a variety of solid tumors, including gastric cancer [3–5]. In addition, experimental studies indicated that IL-10 expression at early tumor sites in mice could cause systemic suppression of antitumor immunity [6]. It is therefore rational to list the IL-10 gene as a cancer-susceptibility candidate.
The IL-10 gene (Gene ID: 3586) is mapped on chromosome 1q31–q32, and includes 5 exons. Several polymorphic loci have been identified and characterized in the IL-10 gene, and one of the most widely evaluated is C-819T (rs1800871) in the promoter region. Many association studies have examined the association of the IL-10 gene C-819T polymorphism with cancer risk, but no firm conclusions have yet been reached. It is well recognized that genetic heterogeneity in carcinogenesis is recognized as a major reason for this inconclusiveness [7]. Other possible reasons include insufficient statistical power and varied linkage patterns across ancestries. In this study, we summarize available published data to assess the susceptibility of the IL-10 gene C-819T polymorphism to gastric cancer risk via a comprehensive meta-analysis.
Material and Methods
Guideline
The conduct of this meta-analysis is in agreement with the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Article identification
We searched Medline and Embase to retrieve potential articles that assessed the relationship between the IL-10 gene C-819T polymorphism and gastric cancer as of 30 March 2015. The key terms included ‘gastric or stomach or cancer or carcinoma or tumor’ and ‘interleukin 10 or interleukin-10 or IL 10 or IL-10’, in combination with ‘polymorphism or variant or SNP or genetic or genotype or allele’. To avoid missing relevant studies, the reference lists of some major articles and reviews were manually checked.
Inclusion criteria
Articles were included if they met the following criteria: (1) being published in English language; (2) following a retrospective or nested case-control study design; (3) involving gastric cancer as the clinical outcome; (4) using validated genotyping platforms; (5) providing detailed genotype data of C-819T polymorphism in gastric cancer patients and cancer-free controls. We excluded conference abstracts/proceedings or posters because they contained insufficient data to make a complete evaluation.
Data abstraction
Baseline characteristics and genotype distributions from each qualified article were abstracted independently by 2 authors (Qingxian Huang and Fang Liu) according to a predetermined protocol formulated by all authors (Table 1). We resolved all disagreements in data abstraction through discussion and consensus.
Table 1.
Baseline characteristics and genotype distributions of IL-10 gene C-819T polymorphism of all eligible populations in this meta-analysis.
| Author (year) | Ethnicity | Cancer type | Matched | Source of controls | Sample size | Age (yrs) | Males (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | ||||||||||
| Wu (2003) | East Asian | Gastric cancer | Yes | Hospital | 220 | 230 | 60.9 | 60.7 | 61.82 | 61.74 | |||||
| Alpizar-Alpizar (2005) | Latinos | Gastric cancer | Yes | Hospital | 45 | 45 | 59.8 | 61.4 | 79.30 | 79.30 | |||||
| Zambon (2005) | Caucasian | Gastric noncardia adenocarcinoma | NA | Hospital | 129 | 644 | NA | NA | 60.47 | 45.71 | |||||
| Kamangar (2006) | Caucasian | Gastric adenocarcinoma | Yes | Population | 98 | 152 | 58.5 | 59.0 | NA | NA | |||||
| Sugimoto (2007) | East Asian | Gastric adenocarcinoma | No | Hospital | 105 | 168 | 66.8 | 45.9 | 80.95 | 66.67 | |||||
| Crusius (2008) | Caucasian | Gastric adenocarcinoma | Yes | Population | 229 | 1094 | NA | NA | NA | NA | |||||
| Ko (2009) | East Asian | Gastric cancer | Yes | Population | 58 | 233 | NA | NA | 70.00 | 70.00 | |||||
| Liu (2011) | East Asian | Gastric cancer | Yes | Population | 234 | 243 | 61.2 | NA | 69.23 | NA | |||||
| He (2012) | East Asian | Gastric cancer | Yes | Hospital | 196 | 248 | NA | NA | 70.40 | 68.50 | |||||
| Kim (2012) | East Asian | Gastric noncardia adenocarcinoma | Yes | Hospital | 495 | 495 | 54.9 | 54.3 | 68.10 | 68.10 | |||||
| Zeng (2012) | East Asian | Gastric cancer | Yes | Population | 151 | 153 | 59.4 | 57.1 | 64.20 | 66.70 | |||||
| Author (year) | Smokers (%) | Drinkers (%) | Family cancer history (%) | HP infection (%) | Patients | Controls | P for HWE | ||||||||
| Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | CC | CT | TT | CC | CT | TT | ||
| Wu (2003) | 51.36 | 34.35 | NA | NA | 68.64 | 55.65 | NA | NA | 27 | 105 | 88 | 20 | 83 | 127 | 0.231 |
| Alpizar-Alpizar (2005) | NA | NA | NA | NA | NA | NA | 82.80 | 91.40 | 25 | 16 | 4 | 18 | 24 | 3 | 0.180 |
| Zambon (2005) | NA | NA | NA | NA | NA | NA | NA | NA | 70 | 42 | 17 | 353 | 245 | 46 | 0.696 |
| Kamangar (2006) | NA | NA | NA | NA | NA | NA | NA | NA | 58 | 35 | 5 | 80 | 62 | 10 | 0.663 |
| Sugimoto (2007) | NA | NA | NA | NA | NA | NA | 100.00 | 0.00 | 6 | 57 | 42 | 9 | 73 | 86 | 0.194 |
| Crusius (2008) | NA | NA | NA | NA | NA | NA | NA | NA | 145 | 72 | 12 | 636 | 378 | 80 | 0.024 |
| Ko (2009) | 69.00 | 58.00 | 55.00 | 57.00 | NA | NA | 86.00 | 86.00 | 0 | 33 | 25 | 0 | 111 | 122 | 0.000 |
| Liu (2011) | NA | NA | NA | NA | NA | NA | NA | NA | 39 | 96 | 99 | 28 | 106 | 109 | 0.773 |
| He (2012) | 31.60 | 35.90 | 22.50 | 27.90 | NA | NA | 63.78 | 47.58 | 18 | 96 | 82 | 28 | 128 | 92 | 0.095 |
| Kim (2012) | 63.00 | 58.00 | 66.20 | 70.30 | 44.40 | 45.50 | 89.90 | 66.60 | 50 | 214 | 231 | 56 | 191 | 248 | 0.042 |
| Zeng (2012) | 24.50 | 22.22 | 62.25 | 42.48 | 23.84 | 11.77 | 68.20 | 45.10 | 11 | 80 | 60 | 10 | 65 | 78 | 0.467 |
HP – Helicobacter pylori; HWE – Hardy-Weinberg equilibrium; NA – not available.
Abstracted data included the first author’s last name, year of publication, ethnicity, gastric cancer subtype, case-control matched status, source of controls, sample size, the genotype numbers of the IL-10 gene C-819T polymorphism between gastric cancer patients and controls, as well as age, sex, smoking, drinking, family history of cancer, and Helicobacter pylori infection status.
Statistics
Statistical calculations were done with STATA software v11.2 (StataCorp, Texas, USA) for Windows.
The magnitude of association between IL-10 gene C-819T polymorphism and gastric cancer risk was expressed as odds ratio (OR) and its 95% confidence interval (95% CI), which were calculated in a random-effects model using the DerSimonian and Laird method [8].
Publication bias was examined using the Begg’s funnel plot and Egger’s test. The trim-and-fill method was also used to infer the existence of unpublished hidden articles from a filled funnel plot, and correct the analysis by imputing the presence of missing studies to yield an unbiased pooled estimate.
Heterogeneity between studies was weighted by inconsistency index (I2) statistic (range: 0% to 100%), which is defined as the percentage of the observed between-study variability that is due to heterogeneity rather than chance. In this meta-analysis, I2 exceeding 50% was selected as a threshold to indicate significant heterogeneity.
Two steps were taken to explore possible causes of heterogeneity: (a) for categorical covariates, subgroup analyses were performed according to the degree of Hardy-Weinberg equilibrium, ethnicity of study groups, specific site of gastric cancer, case-control matched status, source of cancer-free controls, and sample size, respectively; and (b) for continuous covariates, meta-regression analyses were undertaken with restricted maximum likelihood estimates.
In addition, sensitivity analysis was conducted to evaluate the contribution of individual studies to pooled effect estimate by sequentially omitting each study one at a time and computing differential estimates for remaining studies.
Results
Eligibility
Out of 315 initially identified relevant articles, 11 articles were qualified for the final analysis according to the inclusion criteria [5,9–18] including 1960 gastric cancer patients and 3705 cancer-free controls in total.
Characteristics
The baseline characteristics of the 11 qualified articles are shown in Table 1. Seven articles enrolled study subjects of East Asian ancestry, 3 articles of Caucasian ancestry, and 1 article of Latino ancestry. Gastric adenocarcinoma was investigated in 3 articles and gastric noncardia adenocarcinoma in 2 articles. Controls were enrolled from hospitals in 6 articles and from populations in 5 articles. Three of 11 qualified articles had a total sample size of ≥500. The genotype distributions of IL-10 gene C-819T polymorphism deviated from Hardy-Weinberg equilibrium in 3 articles.
Publication bias
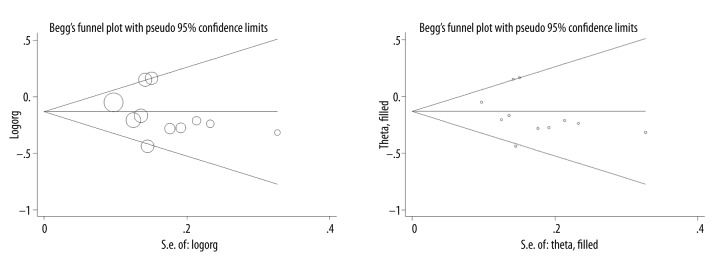
From a visual inspection (Figure 1), the Begg’s funnel plot was symmetrical, and no missing study was annotated in the filled funnel plot. As indicated by the Egger’s test, there was no indication of publication bias (P=0.333).
Figure 1.
Begg’s (the upper panel) and filled (the lower panel) funnel plots of IL-10 gene C-819T polymorphism for gastric cancer risk.
Pooled estimates
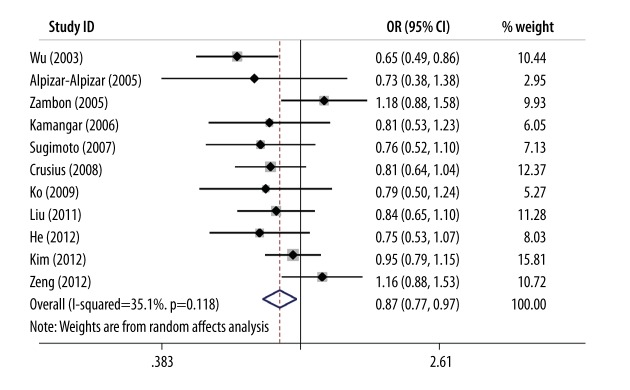
Pooling the results of 11 qualified articles together detected a 13% reduced risk of gastric cancer conferred by the −819T allele relative to the −819C allele (OR=0.87; 95% CI: 0.77–0.97; P=0.016), and evidence for heterogeneity was relatively week (I2=35.1%) (Figure 2).
Figure 2.
Forest plots of IL-10 gene C-819T polymorphism for gastric cancer risk.
Stratified estimates
In spite of the nonsignificant heterogeneity for the overall estimate, an assessment of its diverse sources is still necessary. Subgroup analyses according to a panel of predetermined categorical variables are presented in Table 2.
Table 2.
Subgroup analyses of IL-10 gene C-819T polymorphism for gastric cancer risk.
| Subgroup | Number: Studies (patients/controls), n (n/n) | OR; 95% CI; P | I2 (%) | P |
|---|---|---|---|---|
| Ethnicity | ||||
| East Asian | 7 (1459/1770) | 0.85; 0.73–0.98; 0.029 | 43.6 | 0.100 |
| Caucasian | 3 (456/1890) | 0.93; 0.72–1.19; 0.548 | 50.7 | 0.132 |
| Gastric cancer site | ||||
| Adenocarcinoma | 3 (432/1414) | 0.80; 0.66–0.96; 0.017 | 0.0 | 0.954 |
| Noncardia adenocarcinoma | 2 (624/1139) | 1.03; 0.84–1.26; 0.805 | 31.2 | 0.228 |
| Matched status | ||||
| Yes | 9 (1726/2893) | 0.85; 0.75–0.95; 0.006 | 25.7 | 0.216 |
| Hardy-Weinberg equilibrium | ||||
| Satisfied | 8 (1178/1883) | 0.86; 0.73–1.02; 0.080 | 50.5 | 0.049 |
| Unsatisfied | 3 (782/1822) | 0.88; 0.77–1.02; 0.092 | 0.0 | 0.538 |
| Source of controls | ||||
| Hospital | 6 (1190/1830) | 0.91; 0.74–1.11; 0.338 | 61.7 | 0.023 |
| Population | 5 (770/1875) | 0.81; 0.70–0.93; 0.003 | 0.0 | 0.991 |
| Sample size | ||||
| <500 | 8 (1575/2226) | 0.82; 0.71–0.94; 0.006 | 25.6 | 0.225 |
| ≥500 | 3 (385/1479) | 0.96; 0.79–1.15; 0.625 | 44.3 | 0.166 |
OR – odds ratio; 95% CI – 95% confidence interval.
According to the degree of Hardy-Weinberg equilibrium at a significance level of 5%, effect estimates were comparable between articles with C-819T genotypes in and not in Hardy-Weinberg equilibrium. By ethnicity, significance was only obtained in East Asians (OR=0.85; 95% CI: 0.73–0.98; P=0.029), without heterogeneity (I2=43.6%). The effect estimate was potentiated (OR=0.80; 95% CI: 0.66–0.96; P=0.017) when gastric adenocarcinoma was specified, and there was no observable heterogeneity. After restricting analysis to articles with matched patients and controls on age or sex, effect estimate was slightly strengthened (OR=0.85; 95% CI: 0.75–0.95; P=0.006).
In subgroup analyses by source of controls, the effect estimate was significant only in population-based studies (OR=0.81; 95% CI: 0.70-0.93; P=0.003) relative to the hospital-based studies (OR=0.91; 95% CI: 0.74–1.11; P=0.338). By sample size, the magnitude of effect estimates was even stronger in small studies (total sample size <500) (OR=0.82; 95% CI: 0.71–0.94; P=0.006) than in large studies (total sample size ≥500) (OR=0.96; 95% CI: 0.79–1.15; P=0.625), with weak evidence of heterogeneity.
Meta-regression analysis
Other sources of heterogeneity were explored by meta-regression analyses. After regressing age, sex, smoking, drinking, family history of cancer, and Helicobacter pylori infection, none of the regression coefficients differed significantly from zero (data not shown).
Sensitivity analysis
With regard to the comparison of −819T allele with −819C allele, pooled effect estimates of the other studies confirmed the overall difference in both direction and magnitude after removing each eligible study one at a time (Figure 3).
Figure 3.
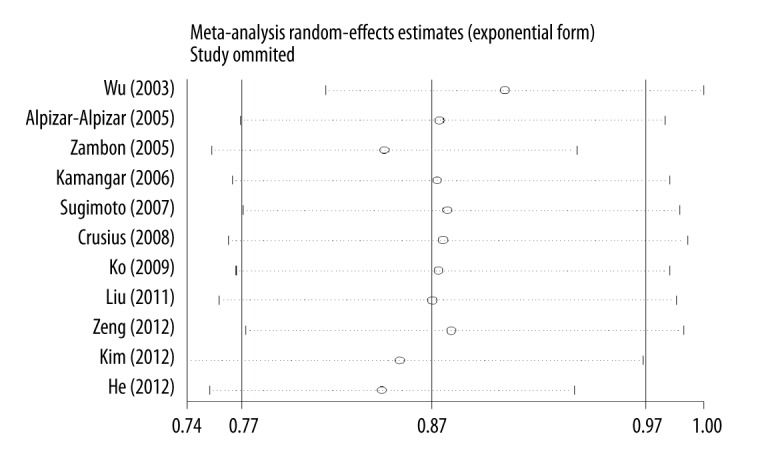
Sensitivity analysis of IL-10 gene C-819T polymorphism for gastric cancer risk.
Discussion
Gathering data from 11 qualified studies in a meta-analysis, we demonstrate a protective role of IL-10 gene −819T allele in susceptibility to gastric cancer, and this role was more evident for gastric adenocarcinoma. Moreover, the results of this meta-analysis were unlikely to have been biased by heterogeneity or publication bias.
The candidate gene approach is increasingly being used to tease out susceptible genes that may potentially trigger the initiation and progression of various types of cancer. One of these genes that might be associated with gastric cancer is the IL-10 gene. However, such candidate gene studies are often criticized due to the repeated failure to validate results. As meta-analysis is a powerful tool to address the discrepancies in genetic association studies, we decided to evaluate the association of a promoter polymorphism C-819T in the IL-10 gene with the development of gastric cancer by a comprehensive meta-analysis. Also, we conducted subgroup and meta-regression analyses to identify and control various sources of heterogeneity.
The promoter region of IL-10 gene is polymorphic, and another 2 promoter polymorphisms, A-592C and A-1082G, also attract much interest and have been summarized by several meta-analyses on susceptibility to gastric cancer [19–21]. Extending previous findings and a recent meta-analysis by Xue et al. [20], the current meta-analysis demonstrated a significant association between the IL-10 gene −819T allele and a reduced risk for gastric cancer, especially for gastric adenocarcinoma. There is functional evidence supporting a marked correlation between −819T allele and increased IL-10 expression in both serum [19] and monocytic THP1 cells [22]. An understanding of how IL-10 expression is regulated is therefore essential in unraveling the pathogenesis of gastric cancer. Thus far, the functional relevance of IL-10 gene C-819T polymorphism has not been elucidated; it is reasonably expected that if involved, C-819T polymorphism might precipitate gastric cancer by altering the expression of the IL-10 gene.
A note of caution should be sounded when interpreting our findings, especially in subgroup analyses, given the limited sample sizes involved. This is well exemplified in comparison of the small with the large studies, as significance was only attained in small studies. It has been suggested that to achieve satisfactory power, at least 1000 subgroups are required and in most cases depending on the prevalence of polymorphism examined in the general population [23]. We therefore must regard our findings as preliminary, which should be viewed as hypothesis-generating and call for further validation in large-scale and well-design studies.
There were several limitations to this meta-analysis. Firstly, this meta-analysis is based on the summary estimates of each qualified case-control study, which rarely establishes causal relationship, and it is encouraging to incorporate the concept of Mendelian randomization into observational association studies [24]. Secondly, only 1 promoter polymorphism, C-819T in the IL-10 gene, was evaluated in this meta-analysis, which might not be sufficient to address the complex genetic architecture of gastric cancer. Thirdly, only published articles written in English language were retrieved for inclusion and some unpublished small and/or negative articles might be missing, leading to the potential existence of publication bias. Fourthly, it is essential to examine the gene-environment and gene-gene interactions at the levels of individual studies and meta-analysis. To achieve this goal, one usually needs to perform a meta-analysis of individual participant data, which is not always practical for the majority of available meta-analyses.
Conclusions
To sum up, this meta-analysis of 11 qualified studies and on 5665 subjects demonstrated a protective role of the IL-10 gene −819T allele in susceptibility to gastric cancer, and this role was more evident for gastric adenocarcinoma. Further genetic and functional investigations are required to elucidate the mechanisms of IL-10 gene and gastric cancer, and the relationship between the IL-10 gene and other types of cancer.
Footnotes
Author disclosure
The authors declare they have no conflicts of interest.
Source of support: Yantai Scientific Development Project (2014WS020), Taishan Scholars Construction Engineering; National Natural Science Foundation of China (81400771 and 81171303), Shandong Provincial Natural Science Foundation (ZR2014HL028 and ZR2010HM091), A Project of Shandong Province Higher Educational Science and Technology Program (J14LE01), and Binzhou Medical University Scientific Research Funds (BY2013KYQD17 and BY2013KYQD18)
References
- 1.Holan V, Zajicova A, Javorkova E, et al. Distinct cytokines balance the development of regulatory T cells and interleukin-10-producing regulatory B cells. Immunology. 2014;141:577–86. doi: 10.1111/imm.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanikawa T, Wilke CM, Kryczek I, et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–29. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rad R, Dossumbekova A, Neu B, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082–89. doi: 10.1136/gut.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szkaradkiewicz A, Karpinski TM, Drews M, et al. Natural killer cell cytotoxicity and immunosuppressive cytokines (IL-10, TGF-beta1) in patients with gastric cancer. J Biomed Biotechnol. 2010;2010:901564. doi: 10.1155/2010/901564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Cho YA, Choi IJ, et al. Effects of interleukin-10 polymorphisms, Helicobacter pylori infection, and smoking on the risk of noncardia gastric cancer. PLoS One. 2012;7:e29643. doi: 10.1371/journal.pone.0029643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo N, Hayakawa S, Takigawa M, et al. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–57. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbach D, Lupien M, Karagas MR, et al. Cancer heterogeneity: origins and implications for genetic association studies. Trends Genet. 2012;28:538–43. doi: 10.1016/j.tig.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu MS, Wu CY, Chen CJ, et al. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer. 2003;104:617–23. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- 10.Zambon CF, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141–52. doi: 10.1016/j.cyto.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Alpizar-Alpizar W, Perez-Perez GI, Une C, et al. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med. 2005;5:169–76. doi: 10.1007/s10238-005-0082-3. [DOI] [PubMed] [Google Scholar]
- 12.Kamangar F, Abnet CC, Hutchinson AA, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland) Cancer Causes Control. 2006;17:117–25. doi: 10.1007/s10552-005-0439-7. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M, Furuta T, Shirai N, et al. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol. 2007;22:1443–49. doi: 10.1111/j.1440-1746.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- 14.Crusius JB, Canzian F, Capella G, et al. Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST) Ann Oncol. 2008;19:1894–902. doi: 10.1093/annonc/mdn400. [DOI] [PubMed] [Google Scholar]
- 15.Ko KP, Park SK, Cho LY, et al. Soybean product intake modifies the association between interleukin-10 genetic polymorphisms and gastric cancer risk. J Nutr. 2009;139:1008–12. doi: 10.3945/jn.108.101865. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Song B, Wang JL, et al. Polymorphisms of interleukin-10 promoter are not associated with prognosis of advanced gastric cancer. World J Gastroenterol. 2011;17:1362–67. doi: 10.3748/wjg.v17.i10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He B, Pan Y, Xu Y, et al. Increased risk for gastric cancer in carriers of the lymphotoxin-alpha+252G variant infected by Helicobacter pylori. Genet Test Mol Biomarkers. 2012;16:9–14. doi: 10.1089/gtmb.2011.0078. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Li Y, Liu T, et al. Diverse H. pylori strains, IL-10 promoter polymorphisms with high morbidity of gastric cancer in Hexi area of Gansu Province, China. Mol Cell Biochem. 2012;362:241–48. doi: 10.1007/s11010-011-1149-y. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, Sturgis EM, Cao X, et al. Interleukin-10 promoter variants predict HPV-positive tumors and survival of squamous cell carcinoma of the oropharynx. FASEB J. 2013;27:2496–503. doi: 10.1096/fj.12-226803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue H, Lin B, An J, et al. Interleukin-10–819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z, Liu Q, Huang C, et al. The interleukin 10–819C/T polymorphism and cancer risk: A HuGE review and meta-analysis of 73 studies including 15,942 cases and 22,336 controls. OMICS. 2013;17:200–14. doi: 10.1089/omi.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuss E, Fimmers R, Kruger A, et al. Differential regulation of interleukin-10 production by genetic and environmental factors – a twin study. Genes Immun. 2002;3:407–13. doi: 10.1038/sj.gene.6363920. [DOI] [PubMed] [Google Scholar]
- 23.Salanti G, Sanderson S, Higgins JP. Obstacles and opportunities in meta-analysis of genetic association studies. Genet Med. 2005;7:13–20. doi: 10.1097/01.gim.0000151839.12032.1a. [DOI] [PubMed] [Google Scholar]
- 24.Sleiman PM, Grant SF. Mendelian randomization in the era of genomewide association studies. Clin Chem. 2010;56:723–28. doi: 10.1373/clinchem.2009.141564. [DOI] [PubMed] [Google Scholar]