Abstract
While many intertidal animals exhibit circatidal rhythms, the nature of the underlying endogenous clocks that control these rhythms has been controversial. In this study American horseshoe crabs, Limulus polyphemus, were used to test the circalunidian hypothesis by exposing them to four different tidal regimes. Overall, the results obtained support the circalunidian hypothesis: each of the twice-daily rhythms of activity appears to be controlled by a separate clock, each with an endogenous period of approximately 24.8h. First, spontaneous “skipping” of one of the daily bouts was observed under several different conditions. Second, the presence of two bouts of activity/day, with different periods, was observed. Lastly, we were able to separately synchronize bouts of activity to two artificial tidal regimes with different periods. These results, taken together, argue in favor of two separate circalunidian clocks in Limulus, each of which controls one of the two bouts of their daily tidal activity rhythms.
Keywords: rhythms, circatidal, endogenous, circadian, entrainment, period
2. Introduction
Many marine organisms that visit or live in the intertidal zone exhibit tidally- organized behavioral rhythms that are, in most cases, endogenous and capable of being entrained by a number of different natural cues (DeCoursey 1983; Palmer 1995a; Chabot et al. 2010). For example, the shore crab (Carcinus maenas; Naylor 1958), fiddler crabs (Uca pugnax; Bennett et al., 1957; Uca minax; Palmer 1989), and the American horseshoe crab (Limulus polyphemus; Chabot and Watson 2010) generally express two bouts of activity per day that are associated with high or low tides and can be entrained by water pressure changes (Naylor and Atkinson 1972; Chabot et al. 2008). Because of their association with the tides, these periodic bouts of activity are known as tidal rhythms and occur approximately every 12.4 h. However, while rhythms have been shown to be under the control of an endogenous timing system in many species, the underlying clock systems that drive these circatidal rhythms are poorly understood and remain controversial (Palmer 1995a; 1995b; 1997a; 1997b; 2000; Naylor 1996; 1997; Aldrich 1997).
The two primary competing theories that have been put forth to explain the underlying clock systems that give rise to these 12.4 h rhythms are: 1) The circatidal oscillator theory, which states that two bouts of activity per day are controlled by one, ~ 12.4 h, circatidal clock and; 2) the circalunidian theory, which states that the two bouts of activity are controlled by two separate, ~24.8 hr, circalunidian (the time between successive moonrises) oscillators (Palmer and Williams 1986). Throughout the years, Palmer, Naylor, and their associates and others gathered evidence that appear to support their respective hypotheses (Palmer and Williams 1986; Palmer 1995; Naylor 1996). However, the results to date are sufficiently unclear that each has reached opposite conclusions even when using the same data sets (Palmer, 1997a;b). In general, these earlier studies made use of data that were often heavily extracted because, it seemed at the time, that circatidal rhythms did not persist for long in constant tidal conditions and were notoriously “noisy”. Further, because of the issue of a lack of persistence in green crabs, Naylor’s results were generally derived from pooled, rather than individual, animals (Naylor 1996). Fortunately, the more recent discovery of clear, persistent circatidal rhythms in individuals from four intertidal species (Limulus polyphemus - Chabot et al. 2007; Eurydice pulchra - Zhang et al. 2013; Apteronemobius ashania - Satoh and Numata 2014; Dimorphostylis asiatica, Akiyama 2014) may allow for more definitive evidence.
Recent studies have demonstrated that horseshoe crabs express robust circatidal rhythms that both persist in constant conditions in the laboratory and can be entrained by artificial tides (Chabot et al., 2008; 2010). While some preliminary observations have indicated that their tidal rhythms might be under the control of circalunidian clocks (Chabot and Watson, 2014), the results were primarily descriptive. Because of the promising preliminary results, they might serve as a good model system in which to test the circalunidian theory in a more quantified way. To accomplish this goal, we measured the locomotor activity rhythms of horseshoe crabs exposed to several types of environmental “perturbations”. Overall, we observed a number of characteristics that support the circalunidian oscillator hypothesis in this species: 1) the presence of two peaks of activity per day, each with a different period (Palmer 2000); 2) “skipping”, or the sudden switch from unimodal to bimodal patterns; 3) “splitting”, or the separation of one component or bout of activity into two components (Pittendrigh and Daan 1976; Palmer 1997a; Johnson 2004) and; 4) the ability of animals to entrain to two bouts of activity to two different artificial tides, each with a different period, and further, to anticipate these tidal cues.
3. Materials and Methods
3.1 General Experimental Conditions
Adult horseshoe crabs, Limulus polyphemus, were collected from the Great Bay estuary, near the Jackson Estuarine Laboratory (JEL) in Durham, New Hampshire, either when they approached the beaches during breeding season or by SCUBA divers. The crabs were then either transported to a laboratory at Plymouth State University, Plymouth, New Hampshire (Experiments 1 and 2) or housed in outdoor tanks at Jackson Estuarine Laboratory, Durham, NH (Experiment 3). In total, 48 crabs were used (all males; 172 g–511 g). Crabs in Experiments 1 and 2 were not fed during experimentation to prevent feeding disturbances of activity patterns and to maintain water quality in the recirculating tanks. Animals used for Experiment 3 were fed approximately 5 g of frozen clam every 7–10 days.
3.2 Experiment 1: Two artificial tides per day with different periods
Horseshoe crabs were placed in “Running Wheels” (Chabot and Watson 2007) in 110 liter recirculating tanks (three crabs/tank; each tank was 50 cm × 95 cm, and 45 cm deep) located in a temperature and light controlled room. Water temperature was 17 ± 2°C, pH was 8.0 ± 0.2, nitrates were always < 100 mg/L and salinity was 27 ± 2 psu. Lighting was provided by a single 40-W fluorescent bulb (Coralife 10,000K) suspended above the tanks. During the simulated daytime (or during constant light, LL), the light intensity at water level was 3.3–5.0 photons/m2/s, while at night, and during DD, it was 0 photons/m2/s. When exposed to a photoperiod, it was 14 hrs light and 10 hrs dark (LD 14:10) and the L to D and D to L transitions were instantaneous.
Tidal cycles were established using water pumps controlled by timers. Water flow rates and timing were adjusted so that, if exposed to 24.8 h/24.8 hr tidal cycles, every 12.4 hr each crab was exposed to a “high tide” that alternated with “low tides”. The depth of water above the carapace of the crab during high tide was 40 cm while the depth during low tide was approximately 3 cm. The timing of the pumps was adjusted, depending on the tidal cycles, to produce ~ 4 h of rising (or falling) water levels followed by ~2.2 hr of constant water levels.
In this experiment, crabs were exposed to either 14:10 LD cycles (n=13) or constant light (LL; n=14). While LD cycles can affect circatidal rhythms (Saigusa 1992; Chabot et al. 2007), constant conditions (such as LL) disrupt circadian rhythms and seem to disrupt circatidal rhythms (Palmer 1995a). Thus, animals were exposed to either of these conditions to increase the opportunities to observe possible un-disrupted effects of either of the two cycles. Since the behavior observed in this experiment under either condition was similar in both cases, LD was used only in subsequent experiments. Crabs were first subjected, for 10–14 days, to two high tides per day, separated by 12.4 hr. Then the artificial tides were adjusted so that one of the high tides occurred every 24.8 hr and the other every 24.2 hr. This mimicked a circalunidian model with clocks with different periods controlling each high tide. These tides were terminated either 13 (LL) or 36 (LD) days later and the crabs were then exposed to constant water levels that were half way between high and low tides for two weeks.
3.3 Experiment 2: One 24.8 hr tide per day
Horseshoe crabs (n=12) were placed in running wheels and subjected to a 15:9 LD cycle. Other lighting and water quality parameters were the same as described for Experiment 1. Running wheels were placed in the bottom of recirculating “tidal tanks” where the water level could be raised or lowered by 0.6 m at a rate of approximately 0.2 m/h using Rio 50 pumps controlled by timers. The timing of the pumps was adjusted to produce ~ 4 h of rising (or falling) water levels followed by ~8.4 hr of constant water levels (depending on the period).
Crabs were first subjected to a single, 12.4 hr long, high tide per day with a period of 24.8 hr. Two weeks later, in an attempt to cause instability in the underlying oscillator system, the single tide was delayed so that the crabs experienced the tide 12 hr later on that day; this shifted cycle persisted for the next four weeks until the artificial tide was terminated. The crabs were then held in water of a constant depth for at least ten days in order to determine if underlying oscillators were entrained.
3.4 Experiment 3: Natural photoperiod and limited tidal cues
Horseshoe crabs (n=7) were exposed to a natural photoperiod and constant water levels in outdoor tanks with constantly replenished estuarine water at the Jackson Estuarine Laboratory (JEL, Durham, NH). Accelerometers (Onset Computer, Pocasset, MA), configured to measure acceleration (g) in the three orthogonal axes, were affixed to the prosoma of each crab using cable ties, duct tape, and cyanoacrylate glue (Schaller et al., 2010). Each crab was placed in a cylindrical wire enclosure (70 cm diameter × 48 cm height) that was inside a larger tank (183 cm × 92 cm × 50 cm, 1–2 crabs per tank, four tanks total). The bottom of each tank contained approximately 15cm of sand so that crabs could bury themselves. Sea water continuously flowed (~4 liters/min) through the 850 liter tanks, keeping salinity and temperature consistent with that of the Great Bay estuary and the tanks remained uncovered, so they were exposed to the natural light/dark cycle (approximate sunrise: 5:01–5:15; sunset: 20:00–20:28). The horseshoe crabs were allowed access to 2% of their body weight in diced quahogs three times a week by placing the food in the bottom of the tanks. These crabs were handled approximately once/14 days in order to download the accelerometers.
3.5 Data Analysis
RW activity data were collected in 5-min intervals on a computer-based data collection system (ClockLab, Actimetrics, Evanston, IL). The accelerometers provided acceleration in the three orthogonal axes per minute for each horseshoe crab. The difference between successive x, y, and z coordinates was calculated to obtain the net acceleration change in the three coordinate planes, and these differences were used to calculate the net magnitude of acceleration each minute. These values were then used to generate actograms and Lomb-Scargle periodograms (Ruf 1999).
Both RW and accelerometer data were analyzed via the ClockLab suite of time series data analysis programs (Actimetrics, Evanston, IL). Significance of rhythmicity was determined both visually (Chabot and Menaker 1992; Chabot et al. 2004) and by Lomb-Scargle periodogram analysis (P<.05). Since circular statistics are not sufficiently robust to pick up multiple rhythmic components or complex periods, visual analyses were used solely when different T cycles were administered (i.e. - the second component of Experiment 1) or when different free-running periods were observed (Experiment 3). The Lomb-Scargle method (Ruf 1999) was used because it reduces the occurrence of artificial harmonics created by the major periodicities of the data compared to the Chi-Square method, while calculating periods that are virtually that same (Chabot et al. 2007) as those calculated using the more traditional Chi-square method (Sokolove and Bushell 1975). This analysis was used to determine the maximal value of any primary component of rhythmicity in the circadian (between 22 and 26 hr) or circatidal range (between 10.4 and 14.4 hr) for each crab during each experimental condition. In general, at least 10 days of data were used to calculate Lomb-Scargle periodograms. To determine the phase angle of activity to environmental cues (LD or water depth changes), best eye-fit lines were drawn through the onsets of activity when possible using a single-blind protocol. We also examined all of our data using the “onset” and “offset” plots in ClockLab (Actimetrics) and compared them to our best-fit lines. They were virtually the same as long as we extrapolated between missing onsets and offsets.
4. Results
4.1 Experiment 1: Two Artificial Tides per Day
When horseshoe crabs in LD were exposed to two high tides per day, most (11/13) synchronized their activity to the tides (Fig. 1 – top, bottom panels). When, in an attempt to destabilize the phase relationship between the two bouts of activity, they were subsequently exposed to one tide cycle with a period of 24.8 hr and one with a period of 24.2 hr, all of the crabs continued to exhibit two bouts of activity/day for variable durations of time, generally one-two weeks. Interestingly, when the two tidal cycles became coincident with one another (approximately Day 38), only one bout of activity was expressed by six of the crabs (Fig. 1, middle, bottom panels). Five crabs showed an additional weaker bout of reduced activity (Figure 1 – top panel – sparse activity near gray line) that appeared to “free-run” with a period of approximately 24.8 hr. When later held in water that remained at a constant depth, over half of the crabs (7/13) exhibited two bouts of activity (Figure 1 – top and bottom panels) and in six of these crabs their activity bouts appeared to be entrained to the previous tidal cycles (Figure 1 – top and bottom panel). The remaining crabs only expressed one bout of activity that appeared to be entrained to one of the previous cycles.
Figure 1.
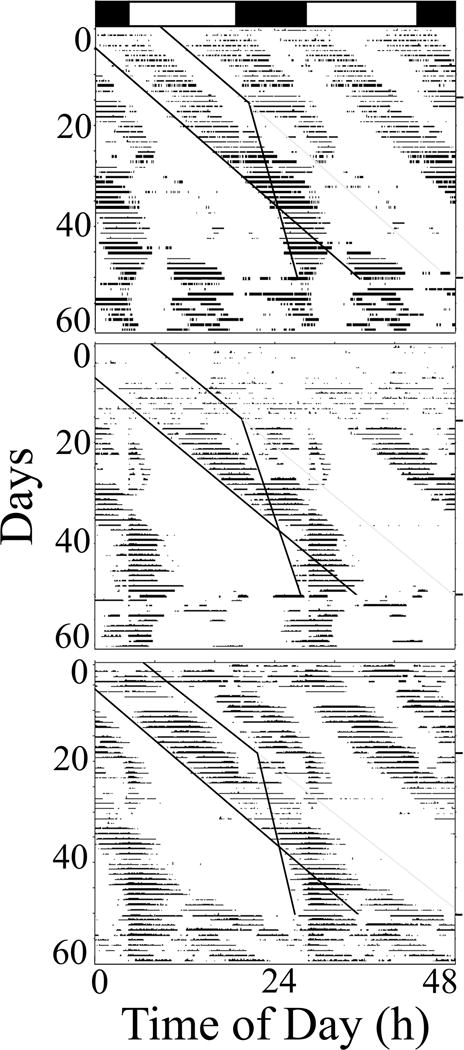
The effects of circalunidian tidal cues on the activity of three representative horseshoe crabs in LD. During the first 18 days crabs were exposed to two identical 24.8 hr periods of water level changes that were 12.4 hr apart (sloping black lines indicate beginning of water level rise; sloping gray lines – predicted period of locomotor activity if 24.2 hr tide had no effect). During the next ~40 days they were exposed to two artificial tides with two different periods (24.8 and 24.4 hr). Then, during the last 15 days, they were not exposed to any changes in water depth. Note that two of three crabs (top, bottom panels) appear to synchronize to the tides during both phases. Note also that two of three crabs exhibit relatively robust bimodal free-running rhythms (top, bottom panels) while one (middle panel) exhibits primarily unimodal rhythms, or “skipping”.
A number of crabs also exhibited some “masking” in response to the LD cycles. Eleven of 13 crabs altered their activity during the “sunrise” and “sunset” transitions: ten often started activity at the D to L transition (Figure 1 – middle, bottom panels), while four stopped activity at these times (data not shown). While many of the clearest LD “masking” events took place when tidal cues were absent (Fig 1 – middle, bottom panels), there were also several instances of LD masking during the tidal cycles (Fig. 1 – middle, bottom panels).
When crabs in LL were exposed to two high tides per day, 12 of 14 crabs clearly synchronized their activity to these cycles (Figure 2; top and middle panel), while the synchronization was less apparent in two of the crabs (Figure 2 – bottom panel). When they were subsequently exposed to artificial tides with different periods (24.8/24.2 hr), eight of thirteen crabs appeared to be synchronized to both cycles (Fig. 2; top, middle panels). Interestingly, some animals appeared to both synchronize to the faster (24.2h) tide, and maintain a remnant of the original 24.8 hour cycle (Fig. 2, top, note gray line). The remaining five crabs exhibited two bouts of activity/day but synchronized either poorly (Fig. 2 –bottom panel) or only to the 24.8 hr or the 24.2 hr cycle (data not shown). Later, when no artificial tides were present, just over half of the crabs (7/13) exhibited free-running circatidal rhythms of activity and one of them expressed a generally unimodal free-running rhythm in the circalunidian (24.8 hr) range (Fig. 2 – bottom panel). When these exact conditions were repeated on the same crabs 45 days later in the summer, similar results were seen: 12 crabs synchronized to 24.8/24.8 hr cycles, four crabs synchronized to 24.8/24.2 hr cycles, an additional four crabs exhibited unimodal rhythms that appeared to be synchronized to the 24.8 hr cycle only and, in constant water level conditions, six crabs exhibited circatidal (bimodal) rhythms while six exhibited free-running rhythms in the circalunidian (unimodal) range.
Figure 2.
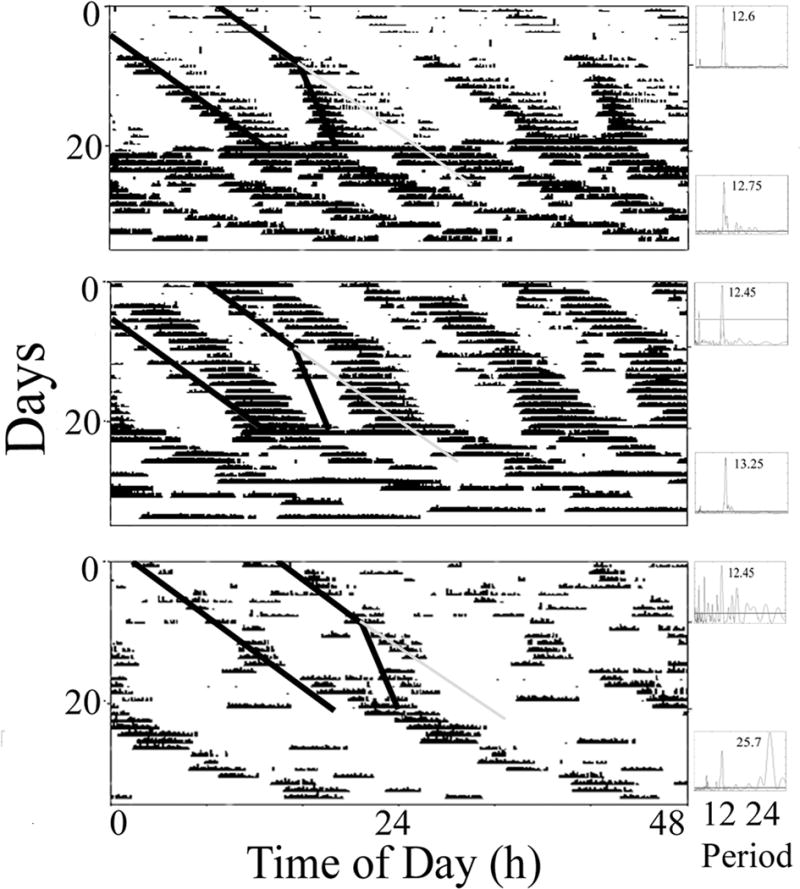
The effects of circalunidian tidal cues on the activity of three representative horseshoe crabs in LL. Large panels (actograms): During the first 10 days crabs were exposed to two identical artificial tides with periods of 24.8 hr. During the second 10 days they were exposed to artificial tides with two different periods (24.8 and 24.4 hr). Then, during the last 13 days, they were in water of a constant depth. Small panels: Lomb-Scargle periodogram analyses of the first and last 10 days of each actogram. Highest significant (P<.001; horizontal lines) peaks in the circatidal (10.4–14.4 hr) or circalunidian (22.8–26.8 hr) range are noted. Note that two crabs (top, middle panels) appear to synchronize to the tides during both phases Bottom panel – an individual that does not synchronize as clearly and displays a primarily unimodal free-running rhythm (~25.7 hr) with some indication of a second, weaker bout.
4.2 Experiment 2: One tidal cycle/day
When one tide/day was delivered to crabs in order to assess the ability of the clock system to respond to this novel perturbation, six of twelve crabs clearly synchronized their activity to the imposed tide (Fig. 3; top and middle panels) for much of the first 18 days. Three of these crabs exhibited two bouts of activity/day (Fig. 3; top and middle panels), even though there was only one artificial high tide/day, while the remaining three crabs exhibited one activity bout/day (data not shown). Additional crabs exhibited single bouts for shorter time periods (Fig. 3 – top – single days are occasionally “skipped” during the L portion; middle panel – see the single bout during days 29–35). Two weeks later, in another attempt to perturb the underlying clock system, the single high tide event/day was delayed by 12.4 hr (yielding a phase change of the tidal cycle of 180°). Under these conditions eight crabs expressed a tidal rhythm and seven of them exhibited bouts of locomotion that were synchronized to the beginning of the imposed tides (Fig. 3; top and bottom panels). When the artificial tides were stopped, six crabs exhibited one bout/day (“skipping”; Fig 3; bottom panel). Of the remaining six crabs, five crabs exhibited clear circatidal rhythms (Fig. 3, top, middle panels), four of which were clearly entrained to the previous tidal cycle (Fig 3; top panel). In addition, some occasional LD masking was clear in all 12 crabs during all three phases of this experiment (Figure 3 – all panels).
Figure 3.
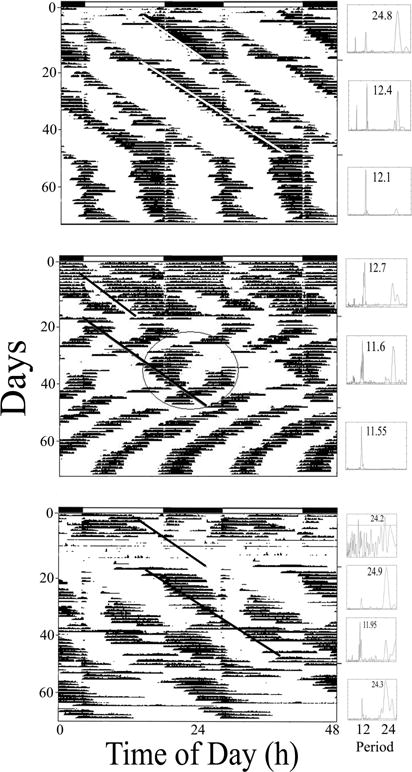
The effects of one 24.8 hr tide per day on the activity patterns expressed by three representative Limulus. Large panels: double-potted actograms. LD cycles are indicated at the top of each actogram. Sloping white or black lines indicate the beginning of water level increases. Small panels: Lomb-Scargle periodogram analyses of the sections of actograms separated by horizontal bars. Highest significant (P<.001) peaks in the circatidal (10.4–14.4 hr) or circalunidian (22.8–26.8 hr) range are noted. Note both bimodal and unimodal expression of activity (“skipping”). Note also the expression of two concurrent bouts of activity with two different periods (highlighted by gray circle).
4.3 Experiment 3: Natural photoperiod and limited tidal cues
Horseshoe crabs that were kept in outdoor flow-through tanks with a constant water level for 45 days exhibited bouts of tidal rhythmicity for time periods that ranged from 10–45 days. Interestingly, three of seven crabs exhibited two bouts of activity, each with different free-running periods (Fig. 4), which appeared to commence after they were handled. Four crabs also exhibited unimodal circalunidian rhythms that, again, appeared to commence (in two of them) after they were handled (data not shown). These rhythms were often interrupted by periods of relatively constant activity (Fig. 4 – top panel, first four days) or inactivity (data not shown). Interestingly, there was far less indication of LD masking in these crabs compared to the laboratory groups, perhaps because the transitions from L to D, and D to L, were gradual.
Figure 4.
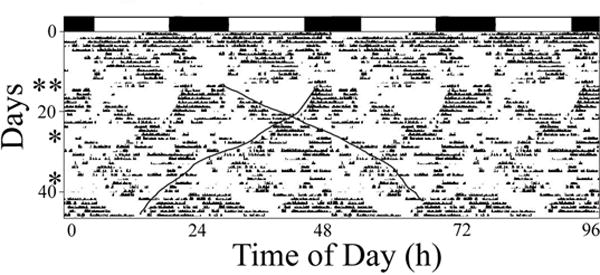
Quadruple-plotted actograms illustrating spontaneous changes in period in crabs exposed to flow-through estuarine water, natural photoperiods, and constant water levels. Approximate photoperiods are indicated at the top of each panel. * - crab were handled (accelerometers downloaded); ** crabs were handled and small blood samples were taken for another study. Dark lines - hand drawn lines indicating the apparent onset or end of activity and indicating two separate bouts of activity that “free-run” at different periods, one shorter than 24h and one longer than 24h).
5. Discussion
5.1 Two clocks appear to control locomotor activity in Limulus
The results presented here provide support for the presence of two oscillators, each controlling one of the two (circatidal) bouts of locomotion often expressed by American horseshoe crabs each day. The activity patterns presented both in this, and other studies involving American horseshoe crabs (Chabot et al. 2007; Chabot and Watson 2010), fulfill all of the criteria necessary for a multi-oscillator system (Pittendrigh and Daan 1976; Palmer 1997a): 1) Evidence of two rhythmic bouts that scan the day with different periodicities in both entraining (Figs. 1, 2) and constant (Figs. 3, 4) conditions; 2) “Splitting”, or the separation of one bout into two bouts (Chabot et al. 2007); and 3) “skipping”, where the expression of one of the two daily bouts of activity ceases for one to several days (Figs. 1, 2, 3). Additionally, some horseshoe crabs appear to be able to synchronize two bouts of activity to two different artificial tidal cues with two different periods (Fig. 1). Furthermore, the bouts of some of these crabs also anticipated each of these two tidal cues (Fig. 2). All of these data, taken together, argues in favor of two separate clocks in Limulus, each of which controls one of the two daily bouts of their tidal activity rhythm (Fig. 5). Moreover, these data are consistent with the circalunidian hypothesis that was first proposed by Williams and Palmer (1986) and later refined by Palmer (1995b). It states that the two oscillators that control locomotor activity in intertidal species are coupled oscillators, and each has a period of approximately 24.8 hr (thus, a circalunidian period). Furthermore, the output from these clocks is manifested by two daily bouts of activity coupled at 12.4 hr antiphase (Fig. 5). This model has been used to explain the variety of activity patterns seen in intertidal species, such as several species of fiddler crabs (Palmer 1995a), green crabs (Palmer 1997a), a cumacean crustacean (Akiyama 2014), and an intertidal fish (tidepool cottids - Green 1971).
Figure 5.
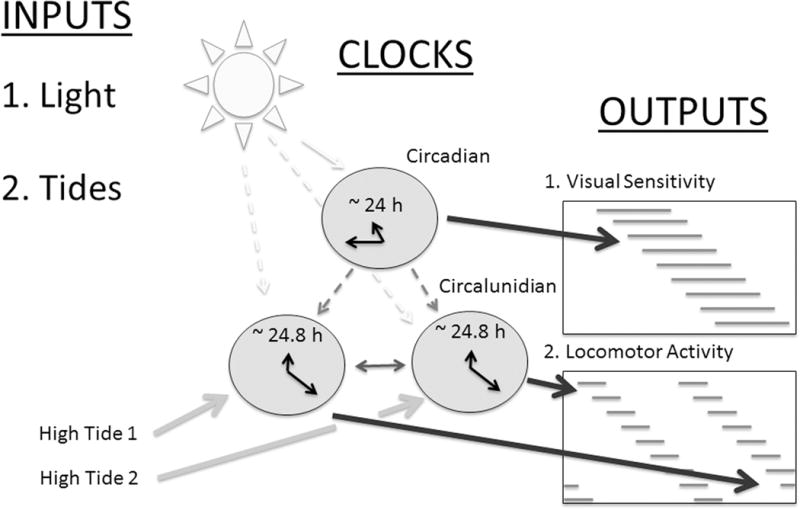
Proposed model of the circalunidian system of the American horseshoe crab. Solid lines – demonstrated influences; dashed lines – hypothesized influences. While the day/night light cycles entrains the circadian clock (Solid white arrow: Barlow, 1983; Watson et al., 2008), each circalunidian clock is entrained by the two different daily tides (Solid gray arrow: Chabot et al., 2011; Figure 1). In turn, the circadian clock drives a single cycle of eye sensitivity each day (Solid black arrow: Watson et al., 2008) while each circalunidian clock drives a separate daily bout of (circatidal) activity ((Solid black arrows: Chabot and Watson, 2010; Figs. 1–4). In this model, LD cycles (or circadian clocks) are hypothesized to influence circatidal rhythms by influencing the circalunidian clocks. Circatidal rhythms are affected by LD cycles in a number of intertidal species (Sesarma pictum, Saigusa, 1992; Uca sp., Stillman and Barnwell, 2004; Limulus, Chabot and Watson, 2004; Chabot et al., 2007), but it is currently unclear if these are direct effects of LD or are mediated through inputs from circadian clocks. The LD (or circadian)-driven silencing of one, but not the other, bout of activity (Chabot and Watson, 2010) may be responsible for the appearance of “skipping” (Fig. 1 – middle panel; Figs. 2 and 3, bottom panels, last several days; Fig. 4 – all panels – last several days).
5.2 Strong Coupling between the Circaluidian Clocks
In horseshoe crabs, the two circalunidian oscillators appear to be strongly coupled. In the first experiment, while the animals often synchronized to both tides/day when the periods of each was 24.8 hr, when one of the tidal periods was changed to 24.2 hr, synchronization was often less clear (Fig. 1). In addition, although some anticipation of the tidal cycle was observed (Fig. 2), activity was not clearly entrained to this signal and the results appeared to be primarily caused in many instances by “masking”. While, on their own, these results may suggest the control of activity by a single 12.4 hr clock, when the other results of this (Figs. 3, 4) and previous studies (Chabot et al., 2008; Chabot and Watson, 2010) instead suggest that the two 24.8 hr clocks are very difficult to de-couple. While we have observed circatidal rhythms of locomotion in many dozens of individuals, we have relatively few examples of the kind of de-coupling that we observed elsewhere in this study (Figs 3, 4; Chabot et al. 2010). Some de-coupling is also seen in the cumacean crustacean Dimorphostylis asiatica (Akiyama 2014). In contrast, the circatidal clock model for the control of circatidal rhythms is based on a single oscillator with a period of 12.4 hr that controls tidal rhythms (Naylor 1996). However, most of the support for this particular model comes from C. maenas that were frequently only tested for 24 (Naylor, 1996) to 72 hr (Naylor 1958) immediately following capture and removal from the estuary. In addition, raw data were not subjected to analysis, but instead the data were first heavily processed. Also noteworthy is that reen crabs do occasionally exhibit skipping. While this phenomenon has been attributed to gating by a circadian clock or by the LD cycle of one of the daily bouts by other investigators (Williams 1991; Palmer 1995b), it might be better explained by the circalunidian hypothesis, as described below.
While there are some aspects of the behavioral patterns exhibited by intertidal animals that could be explained by the circatidal clock model, the observed behavioral patterns appear to better fit the circalunidian hypothesis. As stated above, skipping can possibly be explained by the circatidal model if, for instance, a circadian clock suppresses (or allows) the expression of behavioral activity at certain circadian phases. However, the results in Limulus do not fit this model well because we observe skipping during both light and dark portions of LD cycles (diurnal examples: Fig. 1 – bottom panel; Fig. 3 – bottom panel; nocturnal examples: Chabot et al., 2010). Similar results are seen in talitrids (Rossano et al. 2009) and the grapsid crab, Helice crassa (Williams and Palmer 1988). In addition, the splitting of one component into two (or the consolidation of two components into one; Chabot and Watson 2010) would be predicted by the circalunidian, but not the circatidal, hypothesis. While splitting appears to be uncommon in Limulus, it appears to be less uncommon in the fiddler crab, Uca pugnax (Palmer 1988) and an intertidal brachyuran, Macropthalmus hiritipes (Williams and Palmer 1988). Exposure to constant light induces splitting in some nocturnal rodents and is taken as evidence of control of circadian rhythms by two (~24) clocks (Pittendrigh and Daan 1976). Once split, the rodent rhythms generally remain phase locked at 180 degrees and separated by approximately 12 hr, creating behavioral patterns nearly identical to circatidal patterns observed in intertidal organisms. These two circadian clocks have been termed “evening” and “morning” oscillators because of their tendency to track dusk and dawn respectively and evidence for this type of a circadian oscillatory system has also been found in cockroaches (Page, 1978), fish (Erikson 1973), birds (Gwinner 1974), crickets (Weidenmann 1983), and Drosophila. In Drosophila, these separate clocks have been identified as separate and distinct clusters of neurons that control the dawn and dusk bouts of activity (Stoleru et al. 2004; Picot et al., 2007). The primary differences between these two clock systems then would be a slight difference in the fundamental periods of each of these clocks (24 vs. 24.8 hr) and the different input pathways that entrain them (light vs. tidal cues).
5.3 Advantages of circalunidian clocks
The characteristics of L. polyphemus behavioral rhythms suggest that dual circalunidian oscillators, and not a single circatidal oscillator, may control their locomotor activity (Chabot and Watson 2010). Why might this control system have evolved in intertidal organisms? While the control by one circatidal clock may seem simplest, the control by two circalunidian clocks actually seems more parsimonius because of the complexity of tidal cycles throughout the world. An excellent illustration of this complexity comes from Palmer’s (1990) analysis of the tide-to-tide deviation from the average tidal period of 12.4 hr. He argues that, because the variance between successive tides is nearly ten times as great as the variance between every other tide (174 vs. 10 min), the possession of two separate circalunidian clocks is more adaptive. Two clocks, each tracking one of the daily tides (which vary between successive tides by at most 10 min) would have much more predictive value than one circatidal clock attempting to track each and every tide (which vary by up to 174 min). Another compelling reason for the presence of two circalunidian clocks comes from the fact that many intertidal species, such as Limulus, fiddler crabs and green crabs, often live across a wide geographical range and may be exposed to several different tidal regimes ranging from microtides (essentially atidal environments), to unitidal or bitidal, and even a combination of these (mixed/unequal). Possession of two clocks instead of one would allow flexibility, so individuals can modify the expression of each bout of activity to the local conditions. Interestingly, the behavioral activity of several species of fiddler crabs that can be found in each of these varied habitats has been shown to match the local tidal regimen (Barnwell 1968; Dugaw et al. 2009; Weaver, 2002; Stillman and Barnwell 2004). For example, in Uca larvae, the number of activity bouts expressed per day matches the number of tides per day, not the (sub-species) genotype (Lopez-duarte et al. 2011). Further, Barnwell (1968) found that Uca transplanted from areas with one tide to areas with two tides (or vice versa) had the behavioral flexibility to adjust to the new tidal regimen. Similar results were found in two other Uca species (Palmer 1989; Dugaw et al. 2009; Weaver 2002) as well as blue crabs (Darnell et al. 2010). This type of flexibility may be particularly important for Limulus, since adults can migrate up to 200 km (Swan 2005), and thus may, through their lifetime, be exposed to environments with either one or two tides/day. This behavioral plasticity may be achieved, for example, through independent modulation or silencing of one of the two circalunidian oscillators or their outputs (Green 1971; Palmer 1995a; Thurman 2004). Mathematical modelling of fiddler crab behavior indicates that a circalunidian system can explain many of the observed spontaneous changes in behavior such as phase shifting, splitting, peak disappearance and variations in amplitude (Dugaw et al. 2009).
5.4 Possible interactions between light inputs, circadian, and circatidal rhythms
While the evidence for the control of locomotor rhythms in Limulus appears to favor the circalunidian model, it is also clear that Limulus possesses a circadian clock, and it is possible that this clock might influence the expression of tidal rhythms (Fig. 5). Circadian rhythms are ubiquitous among eukaryotes and Limulus has a robust rhythm of eye sensitivity that is driven by a circadian oscillatory system (Barlow 1983; Barlow et al. 2001). However, Watson et al. (2008) showed that circadian eye sensitivity rhythms and circatidal activity rhythms in this species are controlled by separate oscillators. Nevertheless, there is also evidence that LD cycles, which entrain circadian rhythms (Watson et al. 2008), can influence their locomotor activity as well as heart and respiration rate (Chabot and Watson 2014). Nearly 50% of Limulus in the lab express a diurnal pattern of activity, while 10% exhibit a nocturnal pattern (Chabot et al. 2007). However, while these effects are likely due to the direct masking of activity (Figs. 1 and 3), we cannot rule out the possibility that the circadian clock may have some influence on activity as well (Fig. 5; Dubofsky et al. 2013). Similar masking effects are seen in a cumacean crustacean, D. asiatica (Akiyama 2014) where one of the two daily bouts of circatidal activity is suppressed in in a 12:12 LD cycle but not in a 6:18 LD cycle. Interestingly, LD cycles can also affect the phasing of circatidal activity, such that the rhythms appear to be entrained for several days followed by subsequent drift from the LD cycle in both adults (Chabot et al. 2008, 2010) and juveniles (Dubosky et al. 2013). This type of “weak entrainment” by LD is also seen in the intertidal “true” crabs, Sesarma pictum. (Saigusa 1992) and Hemigrapsis sanguineus (Saigusa and Kawagoye 1997). The circatidal rhythms of an intertidal cricket (A. ashania - Satoh and Numata 2014) and a cumacean crustacean (D. asiatica - Akiyama 2014) are also affected by LD and, likely, by their circadian systems;. Interestingly, ovigerous blue crabs (Callinectes sapidus) from a non-tidal region appear to exhibit circadian, LD-synchronized rhythms, a contrast to the circatidal and circalunidian rhythms expressed by C. sapidus from semidiurnal and diurnal tidal regimes (Darnell et al. 2010). Darnell et al. (2010) hypothesized that a phenotypically plastic circalunidian clock, capable of entraining to a diurnal tidal (24.8 hr) or 24 hour light-dark cycle, may control activity in this species. Whether the clock system that controls circatidal rhythms in Limulus is light sensitive, or is modulated by the circadian system (Fig. 5), will require additional investigations.
5.5 Molecular basis of rhythms
Interestingly, while the basic molecular mechanisms underlying circadian rhythms have been known for a few decades (Konopka et al. 1971; Dunlop 2004), little is known about the molecular mechanisms of circatidal clocks. Interestingly, as mentioned previously, in Drosophila there appear to be two separate circadian clocks, each of which is composed of two groups of neurons in the brain and are known to be responsible for controlling the two daily bouts of circadian rhythms of activity that occur at dawn and dusk (Stoleru et al. 2004). Thus, the behavior in Drosophila can appear bimodal like circatidal rhythms and, like our proposed mechanism for the control of circatidal rhythms, also seem to be composed of two separate clocks with periods of ~24h and phase locked 12h apart. Importantly, this overall clock structure may not be limited to Drosophila since similar behavioral patterns are seen in other insects (Tomioka et al, 2012), mammals (Robinson et al. 2014) and fish (Idda et al. 2012). Therefore, it is plausible that a circalunidian clock system could be similarly composed, with ~24.8 hr molecular clocks instead of ~ 24 hr clocks, and each set of neurons controlling the activity during a given tide. While the interaction of circadian transcription factors can theoretically give rise to ~12h rhythms (Westermark and Herzel 2013), a recent study on the isopod Eurydice pulchra by Zhang et al. (2013) suggests that, like circadian rhythms (Bae and Edery 2006; Lowrey and Takahashi 2011), phosphorylation may be a key mechanism. However, disruption of the output of some of the ”core” circadian genes did not clearly affect circatidal rhythms in this species, while it did alter the expression of circadian rhythms (Zhang et al. 2013). A gene expression analysis of the intertidal mussel, Mytulus edulis, indicated robust circatidal rhythms of numerous physiological mechanisms involved in metabolism, respiration, cell-divisions, and stress responses (Gracey et al. 2008). Interestingly, the only canonical clock gene that was on their expression array, timeless, was not clearly correlated with the time of tide or day. Two other clock genes (CRY1 and RORB) also did not cycle by tide (but did cycle by time of day) in a congener, Mytilus californianus (Connor and Gracey 2011). With the advent of modern molecular techniques such as deep sequencing of mRNA, this clock system, like the circadian clock system, may also be soon unwound.
Acknowledgments
The authors wish to thank Becky Anderson, Katherine Fondo, Alexandria Santry, Kyle Kenyon, Tyler Remillard, and Alicia Franklin for assistance with some of the experiments described within and to the staff at Jackson Estuarine Laboratory, especially Dave Shay, for help with technical aspects of the experiments. This work was supported by the National Science Foundation Integrative and Organismal Systems [Grants 0517229 and 0920342] to CCC and WHW; New Hampshire IDeA Network of Biomedical Research Excellence (NH-INBRE) with an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health [Grant P20GM103506].
Footnotes
Competing interests: The authors declare that they have no competing interests for this manuscript.
Literature Cited
- Akiyama T. Circatidal and circadian rhythms in crustacean swimming behavior. In: Numata H, Helm B, editors. Annual, Lunar, and Tidal Clocks: patterns and Mechanisms of Nature’s Enigmatic Rhythms. New York: Springer; 2014. pp. 65–80. [Google Scholar]
- Aldrich JC. Crab clocks sent for recalibration. Chronobiol Int. 1997;14:435–437. doi: 10.3109/07420529709001464. [DOI] [PubMed] [Google Scholar]
- Bae K, Edery I. Regulating a circadian clock’s period, phase and amplitude by phosphorylation: insights from Drosophila. J Biochem. 2006;140(5):609–17. doi: 10.1093/jb/mvj198. [DOI] [PubMed] [Google Scholar]
- Barlow RB. Circadian rhythms in the Limulus visual system. J Neuroscience. 1983;3:856–870. doi: 10.1523/JNEUROSCI.03-04-00856.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Hitt JM, Dodge FA. Limulus vision in the marine environment. Biol Bull. 2001;200:169–176. doi: 10.2307/1543311. [DOI] [PubMed] [Google Scholar]
- Barnwell FH. The role of rhythmic systems in the adaptation of fiddler crabs to the intertidal one. Am Zoologist. 1968;8:569–583. [Google Scholar]
- Bennett MF, Shriner J, Brown RA. Persistent tidal cycles of spontaneous motor activity in the fiddler crab, Uca pugnax. Biol Bull. 1957;112:267–275. [Google Scholar]
- Chabot CC, Menaker M. Effects of physiological cycles of infused melatonin on circadian rhythmicity in pigeons. J Comp Physiol. 1992;170:615–622. doi: 10.1007/BF00199337. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Kent J, Watson WH. Daily, circadian and tidal rhythms of locomotor activity in the horseshoe crab Limulus polyphemus. Biol Bull. 2004;207:72–75. doi: 10.2307/1543630. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Betournay SH, Braley N, Watson WH. Circadian and circatidal rhythms of locomotion in the horseshoe crab, Limulus polyphemus. JEMBE. 2007;345:79–89. [Google Scholar]
- Chabot CC, Skinner SJ, Watson WH. Locomotion rhythms expressed by the horseshoe crab, Limulus polyphemus: I. Synchronization by artificial tides. Biol Bull. 2008;215:34–45. doi: 10.2307/25470681. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Yelle JF, O’Donnell CB, Watson WH. The effects of water pressure, temperature and current cycles on circatidal rhythms expressed by the American horseshoe crab, Limulus polyphemus. Mar Fresh Behav Physiol. 2010;44:43–60. [Google Scholar]
- Chabot CC, Watson WH. Circatidal rhythms of locomotion in the American horseshoe crab Limulus polyphemus: Underlying mechanisms and cues that influence them. Curr Zool. 2010;56:499–517. [Google Scholar]
- Chabot CC, Watson WH., III . Daily and circatidal rhythms in intertidal marine invertebrates. In: Numata H, Helm B, editors. Annual, Lunar, and Tidal Clocks: patterns and Mechanisms of Nature’s Enigmatic Rhythms. New York: Springer; 2014. pp. 41–64. [Google Scholar]
- Connor KM, Gracey AY. Circadian cycles are the dominant transcriptional rhythm in the intertidal mussel Mytulus californius. Proc Nat Acad Sci. 2011;108:16110–16115. doi: 10.1073/pnas.1111076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell MZ, Rittschoff D, Forward RB. Endogenous swimming rhythms underlying the spawning migration of the blue crab, Callinectes sapidus: Ontogeny and variation with ambient tidal regime. Mar Biol. 2010;157:2415–2425. [Google Scholar]
- DeCoursey PJ. Biological timing. In: Vernberg FJ, Vernberg WB, editors. The Biology of Crustacea. San Diego: Academic Press; 1983. pp. 107–162. [Google Scholar]
- Dubosky BA, Watson WH, Simpson SD, Chabot CC. Patterns of activity in juvenile American horseshoe crabs. Biol Bull. 2013;225:42–49. doi: 10.1086/BBLv225n1p42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaw CJ, Honeyfield R, Taylor CM, Verzi DW. Modeling activity rhythms in fiddler crabs. Chrono Int. 2009;26:1355–1368. doi: 10.3109/07420520903421872. [DOI] [PubMed] [Google Scholar]
- Dunlop J. Molecular biology of circadian pacemaker systems. In: Dunlap J, Loros JJ, DeCoursey PJ, editors. Chronobiology: Biological timekeeping. Sunderland, MA: Sinauer Associates; 2004. pp. 213–254. [Google Scholar]
- Erikson LO. Spring inversion of the diel rhythm of locomotor activity in young sea going trout and Atlantic salmon. Aquilo Ser Zoologica. 1973;14:9–79. As cited in Pittendrigh CS. 1981. Circadian systems: Entrainment. In Aschoff J (Ed). Handbook of behavioral neurobiology; Biological rhythms. New York: Plenum Press, 95–124. [Google Scholar]
- Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN. Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol. 2008;18:1501–1507. doi: 10.1016/j.cub.2008.08.049. [DOI] [PubMed] [Google Scholar]
- Green JM. Field and laboratory activity patterns of the tidepool cottid, Oligocottus maculosus. Girard Can J Zool. 1971;49:255–264. doi: 10.1139/z71-036. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Testosterone induces “splitting” of circadian locomotor activity rhythms in birds. Science. 1974;185:72–74. doi: 10.1126/science.185.4145.72. [DOI] [PubMed] [Google Scholar]
- Idda ML, Bertolucci C, Vallone D, Gothilf Y, Sanchez-Vazquez FJ, Foulkes NS. Circadian clocks: Lessons from fish. In: Kalsbeek A, Merrow M, Roenneberg T, Foster RG, editors. Neurobiology of Circadian Timing. Vol. 199. Progress in Brain Research; 2012. pp. 41–57. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Gwinner E, Karsch FJ, Saunders D, Zucker I, Ball GF. Fundamental properties of circadian rhythms. In: Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology: Biological timekeeping. Sunderland, MA: Sinauer Associates; 2004. pp. 67–106. [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duarte PC, Christy JH, Tankersley RA. A behavioral mechanism for dispersal in fiddler crab larvae (genus Uca) varies with adult habitat, not phylogeny. Limnol Oceanogr. 2011;56:1879–1892. [Google Scholar]
- Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organsims. In: Brody S, editor. Advances in Genetics. Vol. 74. Elsevier; London: 2011. pp. 175–237. (The Genetics of Circadian Rhythms). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E. Tidal and diurnal rhythms of locomotory activity in Carcinus maenas (L.) J Exp Biol. 1958;35:602–610. [Google Scholar]
- Naylor E. Crab Clockwork: the case for interactive circatidal and circadian oscillators controlling rhythmic locomotor activity of Carcinus maenas. Chronobiol Int. 1996;13:153–161. doi: 10.3109/07420529609012649. [DOI] [PubMed] [Google Scholar]
- Naylor E. Crab clocks rewound. Chronobiol Intl. 1997;14:427–430. doi: 10.3109/07420529709001462. [DOI] [PubMed] [Google Scholar]
- Naylor E, Atkinson RJA. Pressure and the rhythmic behavior of inshore marine crabs. Symp Soc Exp Biol. 1972;26:395–415. [PubMed] [Google Scholar]
- Page TL. Interactions between bilaterally paired components of the cockroach circadian system. J Comp Physiol. 1978;124:225–236. [Google Scholar]
- Palmer JD. Comparative studies of tidal rhythms. VI. Several clocks govern the activity of two species of fiddler crabs. Mar Behav Physiol. 1988;13:201–219. [Google Scholar]
- Palmer JD. Comparative studies of tidal rhythms. VIII. A translocation experiment involving circalunidian rhythms. Mar Behav Physiol. 1989;14:231–243. [Google Scholar]
- Palmer JD. Comparative studies of tidal rhythms. X. A dissection of the persistent activity rhythms of Sesarma. Mar Behav Physiol. 1990;17:177–187. [Google Scholar]
- Palmer JD. The Biological Rhythms and Clocks of Intertidal Crabs. Oxford University Press; New York, NY: 1995a. [Google Scholar]
- Palmer JD. Review of the dual-clock control of tidal rhythms and the hypothesis that the same clock governs both circatidal and circadian rhythms. Chronobiol Int. 1995b;12:299–310. [Google Scholar]
- Palmer JD. Dueling hypotheses: Circatidal versus circalunidian battle basics. Chronobiol Intl. 1997a;14:337–346. doi: 10.3109/07420529709001455. [DOI] [PubMed] [Google Scholar]
- Palmer JD. Dueling hypotheses: Circatidal versus circalunidian battle basics-second engagement. Chronobiol Intl. 1997b;14:431–433. doi: 10.3109/07420529709001463. [DOI] [PubMed] [Google Scholar]
- Palmer JD. The clocks controlling the tide-associated rhythms of intertidal crabs. Bioessays. 2000;22:32–37. doi: 10.1002/(SICI)1521-1878(200001)22:1<32::AID-BIES7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Palmer JD, Williams BG. Comparative studies of tidal rhythms II. The dual clock control of the locomotor rhythms of two decapod crustaceans. Mar Behav Physiol. 1986;12:269–278. [Google Scholar]
- Picot M, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biology. 2007;5:2513–2521. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- Robinson I, Reddy AB. Molecular mechanisms of the circadian clockwork in mammals. FEBS Letts. 2014;588:2477–2483. doi: 10.1016/j.febslet.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Rossano C, Gambineri S, Fanini L, Durier V, Rivault C, Scapini F. Behavioral adaptations in talitrids from two Atlantic beaches. Est Coastal Shelf Sci. 2009;85:573–584. [Google Scholar]
- Ruf T. The Lomb-Scargle periodogram in biological rhythm research: Analysis of incomplete and unequally spaced time-series. Biol Rhythms Res. 1999;30:178–201. [Google Scholar]
- Saigusa M. Phase shift of a tidal rhythm by light-dark cycles in the semi-terrestrial crab, Sesarma pictum. Biol Bull. 1992;182:257–264. doi: 10.2307/1542119. [DOI] [PubMed] [Google Scholar]
- Saigusa M, Kawagoye O. Circatidal rhythm of an intertidal crab, Hemigrapsus sanguineus?: synchrony with unequal tide height and involvement of a light-response mechanism. Mar Biol. 1997;129:87–96. [Google Scholar]
- Satoh A, Numata H. Circatidal rhythms and their entrainment to the tidal cycles in insects. In: Numata H, Helm B, editors. Annual, Lunar, and Tidal Clocks: patterns and Mechanisms of Nature’s Enigmatic Rhythms. New York: Springer; 2014. pp. 25–40. [Google Scholar]
- Sokolove PG, Bushell WN. The chi-square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;74:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Stillman JH, Barnwell FH. Relationship of daily and circatidal activity rhythms of the fiddler crab, Uca princeps, to the harmonic structure of semidiurnal and mixed tides. Mar Biol. 2004;144:473–482. [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Roshbash M. Coupled oscillators control morning and evening locomotor behavior of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Swan BL. Migrations of adult horseshoe crabs, Limulus polyphemus, in the middle Atlantic bight: A 17 year tagging study. Estuaries. 2005;28:28–40. [Google Scholar]
- Thurman CL. Unravelling the ecological significance of endogenous rhythms in intertidal crabs. Biol Rhythm Res. 2004;35:43–67. [Google Scholar]
- Tomioka K, Urya O, Kamae Y, Umezaki Y, Yoshii T. Peripheral circadian rhythms and their regulatory mechanism in insects and some other arthropods: a review. J Comp Physiol B. 2012;182:729–740. doi: 10.1007/s00360-012-0651-1. [DOI] [PubMed] [Google Scholar]
- Watson WH, Bedford L, Chabot CC. Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: II. Relationship to circadian rhythms of visual sensitivity. Biol Bull. 2008;215:46–56. doi: 10.2307/25470682. [DOI] [PubMed] [Google Scholar]
- Weaver A, Salmon M. Hatching rhythms of Uca thayeri; evidence for phenotypic plasticity. J Crustac Biol. 2002;22:429–438. [Google Scholar]
- Westermark PO, Herzel H. Mechanism for 12 hr generation by the circadian clock. Cell Reports. 2013;3:1228–1238. doi: 10.1016/j.celrep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Wiedenmann G. Splitting in a circadian activity rhythm: The expression of bilaterally paired oscillators. J Comp Phys. 1983;150:51–60. [Google Scholar]
- Williams BG. Comparative studies of tidal rhythms. V. Individual variation in the rhythmic behavior of Carcinus maenas (L.) Mar Behav Physiol. 1991;19:97–112. [Google Scholar]
- Williams BG, Palmer JD. Comparative study of tidal rhythms: the dual clock control of the locomotor rhythms of two decapod crustaceans. Mar Behav Physiol. 1986;12:269–278. [Google Scholar]
- Williams BG, Palmer, JD Comparative studies of tidal rhythms IV. Spontaneous frequency changes and persistence in the locomotor rhythm of an intertidal crab. Mar Behav Physiol. 1988;13:315–332. [Google Scholar]
- Zhang L, Hastings M, Green EW, Tauber E, Sladek M, Webster SG, Kyiacou CP, Wilcockson DC. Dissociation of circadian and circatidal timekeeping in the marine crustacean Eurydice pulchra. Curr Biol. 2013;23:1–11. doi: 10.1016/j.cub.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


