Abstract
Enzymatic cleavage of the nonsymmetric provitamin A carotenoid α-carotene results in one molecule of retinal (vitamin A), and one molecule of α-retinal, a biologically inactive analog of true vitamin A. Due to structural similarities, α-retinyl esters and vitamin A esters typically coelute, resulting in the overestimation of vitamin A originating from α-carotene. Herein, we present a set of tools to identify and separate α-retinol products from vitamin A. α-Retinyl palmitate (αRP) standard was synthesized from α-ionone following a Wittig-Horner approach. A high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method employing a C30 column was then developed to separate the species. Authentic standards of retinyl esters and the synthesized α-RP confirmed respective identities, while other α-retinyl esters (i.e. myristate, linoleate, oleate, and stearate) were evidenced by their pseudomolecular ions observed in electrospray ionization (ESI) mode, fragmentation, and elution order. For quantitation, an atmospheric pressure chemical ionization (APCI) source operated in positive ion mode was used, and retinol, the predominant in-source parent ion was selected and fragmented. The application of this method to a chylomicron-rich fraction of human plasma is demonstrated. This method can be used to better determine the quantity of vitamin A derived from foods containing α-carotene.
Keywords: α-retinyl palmitate, α-retinol, retinyl esters, provitamin A carotenoids, synthesis, HPLC-MS/MS
1. Introduction
Vitamin A deficiency remains a significant problem worldwide, with deficient regions obtaining a majority of their vitamin A needs from provitamin A carotenoids found in fruits and vegetables [1,2]. The vitamin A capacity of carotenoids is dictated by the presence of an unsubstituted β-ionone ring in conjugation with a polyene chain [3]. While numerous analytical methods have been developed for the determination of vitamin A delivery from a provitamin A rich meal [4–6],most studies focus on the provitamin A carotenoid β-carotene. The symmetric structure of β-carotene yields two molecules of retinal after central cleavage making it the most potent provitamin A precursor. However in nature, β-carotene is often found concurrent with nonsymmetric provitamin A carotenoids like α-carotene. Analogous to β-carotene, α-carotene is enzymatically cleaved at the central double bond, yet only produces one molecule of retinal. The remaining product, α-retinal, contains an ε-ring, and possesses only 2% of the bioactivity of vitamin A in animal models [7–10]. Both α-retinal and retinal are reduced to α-retinol and retinol, respectively, then esterified to fatty acids, before being packaged and released in blood chylomicrons. The structural similarity between the isobars α-retinol and retinol, with only a difference in the placement of a single double bond, causes difficulty resolving α-retinol/α-retinyl esters from analogous retinol/retinyl esters, respectively, using traditional C18 reversed-phase chromatography. Due to this coelution, it is likely that reported levels of newly formed vitamin A after the consumption of an α-carotene-rich meal have been overestimated.
A few articles have reported tentative identification of α-retinyl esters in animal tissues and blood after the feeding of α-carotene or α-retinol [11–15]. Collectively, these studies identified α-retinyl esters based upon anticipated retention time, expected UV-Vis spectra, and disappearance after sample saponification [11–15]. In one study, the presumed resulting α-retinol was isolated from tissues and then used to quantify α-retinol from the same tissues [11]. Another study required double-analysis of both a saponified and unsaponified sample, in addition to a calculation, to estimate α-retinyl ester contribution [12]. Saponification of retinol species prior to analysis requires more preparatory time, increases potential for degradation of these sensitive compounds, and does not allow for the differentiation of the circulating esters [12,14]. Additionally, without authentic standards, reports of intact α-retinyl esters in animal tissues are tentative [15]. Indeed, the lack of authentic standards has prevented unequivocal identification of α-retinyl esters for the past 20 years.
The objective of this work was to provide a tool to better measure the vitamin A potential of α-carotene-containing foods. α-Retinol was synthesized and esterified to palmitic acid (αRP, the presumed predominate acylated form of α-retinol [16]). Together with retinyl palmitate (RP), and other authentic retinyl ester standards, a high-performance liquid chromatography-photodiode array-tandem mass spectrometry (HPLC-PDA-MS/MS) method was developed. The method was then applied to differentiate α-retinyl esters from retinyl esters found in the chylomicron-containing fraction of human blood plasma.
2. Materials and Methods
2.1 Reagents
Ammonium acetate was purchased from J.T. Baker (Phillipsburg, NJ, USA). HPLC grade methyl tert-butyl ether (MTBE),Optima grade water,methanol (MeOH) and formic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.2 Synthesis and purification of all-trans αRP standard
A detailed description of αRP synthesis and purification is provided in the supplementary materials and summarized in Figure S1.
2.3 HPLC-MS/MS method for separation of αRP and RP
Separation of αRP and RP standards was achieved using a C30 column (4.6 mm × 250 mm, 3 μm particle size, YMC).A 1200 SL series HPLC system with a 60 mm path length1260 PDA (Agilent Technologies, Santa Clara, CA) was employed. A gradient of solvent A: 90:10 MeOH/H2O with 0.1% formic acid (v/v), and solvent B: 78:20:2 MTBE/MeOH/H2O with 0.1% formic acid (v/v) was as follows: 30% B, followed by a linear gradient to 50% B over 18 min, holding at 100% B for 2 min, and re-equilibrating at 30% B for 3.5 min. The column was held at 40 °C, with a flow rate of 1.3 mL/min, and 40 μL injection volumes. The HPLC-PDA was interfaced with a QTRAP 5500 mass spectrometer (AB Sciex, Foster City, CA) using an APCI source in positive ion mode for αRP and RP quantitation. The source parameters were as follows: curtain gas: 30 psi, heated nebulizer temperature: 450 °C, nebulizer gas: 45 psi, declustering potential: 100 V, entrance potential: 10 V, and collision cell exit potential: 11 V. Both esters afforded strong in-source retinol fragments that were utilized for MS/MS detection. The parent-daughter transitions which both 1) displayed distinctly different MRM ratios for α-retinyl and retinyl esters and 2) provided optimal intensities for quantitation were chosen (Table 1). Peak areas were integrated with Analyst 1.5.1 (AB Sciex).
Table 1.
MS/MS parameters used for the identification and quantitation of α-retinyl esters and retinyl esters.
| No. | Retention time (min) | Compound identity | HPLC-PDA spectrum (nm) | HPLC-APCI(+)-MS/MS m/z parent ion > m/z daughter ion | APCI collision energies (volts)e | HPLC-ESI(+)-MS/MS m/z parent ion > m/z daughter ion | ESI collision energies (volts)e |
|---|---|---|---|---|---|---|---|
| 1 | 12.8 | α-Retinyl myristate | 298,311a ,325 | 269.2 > 123.1b | 27.5 | 496.6 > 145.0 | 17.5 |
| 2 | 13.2 | Retinyl myristate | 325a | 269.2 > 239.1c,d, 145.1d | 35.0, 25.0 | 496.6 > 197.0 | 40 |
| 3 | 13.2 | α-Retinyl linoleate | 298,311a ,325 | 269.2 > 123.1b | 27.5 | 548.6 > 145.0 | 17.5 |
| 4 | 13.9 | Retinyl linoleate | 325a | 269.2 > 239.1c,d , 145.1d | 35.0, 25.0 | 548.6 > 197.0 | 40 |
| 5 | 15.4 | α-Retinyl oleate | 298,311a ,325 | 269.2 > 123.1b | 27.5 | 550.6 > 145.0 | 17.5 |
| 6 | 15.9 | Retinyl oleate | 325a | 269.2 > 239.1c,d , 145.1d | 35.0, 25.0 | 550.6 > 197.0 | 40 |
| 7 | 16.9 | α-Retinyl palmitate | 298,311a ,325 | 269.2 > 123.1b | 27.5 | 524.6 > 145.0 | 17.5 |
| 8 | 17.3 | Retinyl palmitate | 325a | 269.2 > 239.1c,d , 145.1d | 35.0, 25.0 | 524.6 > 197.0 | 40 |
| 9 | 21.0 | α-Retinyl stearate | 298,311a ,325 | 269.2 > 123.1b | 27.5 | 552.6 > 145.0 | 17.5 |
| 10 | 21.6 | Retinyl stearate | 325a | 269.2 > 239.1c,d , 145.1d | 35.0, 25.0 | 552.6 > 197.0 | 40 |
Denotes λmax
Daughter with the strongest transition for α-retinol derivatives, chosen for quantitation
Daughters unique to retinol derivatives only
Daughters chosen for quantitation of retinol derivatives
Collision energy used for each respective daughter follows the order in which they are listed in the previous column
2.4 Biological Sample Preparation
Triglyceride-rich lipoprotein (TRL) fractions of human plasma containing newly formed chylomicrons were isolated [17] and extracted as published previously [18].
2.5 HPLC-PDA-MS/MS method to separate total α-retinyl esters and retinyl esters in biological samples
To distinguish the non-palmitate esters of α-retinol and retinol found in biological samples (i.e. myristate, linoleate, oleate, and stearate), the HPLC gradient described above was extended as follows: beginning at 30% B, followed by a linear increase to 55.6% B over 23 min, holding at 100% B for 2 min, and re-equilibrating at 30% B for 3.5 min. To detect the intact parent α-retinyl esters and retinyl esters and, the HPLC was interfaced with the mass spectrometer via an ESI probe operated in positive ion mode. Source parameters were as described in section 2.3 were applied, with the exception of the source temperature which was increased to 525 °C and collision energies of 17.5 and 40 V for the α- and retinyl esters, respectively . Identification of αRP and retinyl esters in the TRL sample was based upon parent ion masses, UV-Vis spectra, and retention time coincident with authentic standards (Table 1). The non-palmitate α-retinyl esters were tentatively identified by expected retention order relative to the corresponding retinyl ester, UV-Vis spectra, parent ion mass, and expected daughter ratios of the in-source α-retinol parent. An APCI source (with parameters provided in section 2.3) was used for analyte quantitation. For all MS/MS experiments, MRM mode was used with maximized 70 msec dwell times per transition. All α-retinyl esters were quantitated using αRP, and all retinyl esters were quantitated using RP. The fatty acid moiety of each ester is lost in-source providing the same parent ion (and thus same response) for α-retinol and retinol, respectively (as previously confirmed with the authentic retinyl esters used for identification).
3. Results and Discussion
3.1 HPLC-MS/MS Method Development
Identification and quantitation of α-retinyl ester and retinyl ester pairs presents an analytical challenge due to their structural similarity and isobaric nature. By coupling HPLC and MS/MS, we were able to chromatograph and use unique fragmentation patterns to differentiate the α-retinyl products from their retinyl counterparts.
Chromatographic separation of αRP and RP was achieved by modifying the gradient and increasing the polarity of the non-eluting solvent of the method outlined by Kopec et. al. [18]. As a result, αRP eluted immediately before RP, while still maintaining peak resolution. This elution pattern was observed for all esters of α-retinol and retinol. C30 columns, widely used for carotenoid and retinoid separation, were tested at varying lengths (150 mm, 250 mm) and particle sizes (3 μm, 5 μm) to improve resolution. A column length of 250 mm with 3 μm particle size gave the best separation.
UV-Vis spectral characteristics were also helpful in distinguishing α-retinyl esters from retinyl esters. The movement of a double bond out of conjugation in the ε-ring of αRP causes a hypsochromic shift, resulting in a λmax of 311 nm (as compared a λmax of 325 nm for RP) (Figure S2) [19]. αRP retains the 3-pronged fine-structure characteristic of carotenoids, with shoulders clearly visible at 298 and 325 nm, while RP has no fine structure (as commonly observed for short β-ring apo-carotenoids and other vitamin A derivatives) [19]. Furthermore, fatty acid acylation has no impact on the λmax of α-retinol and retinol [20]. α-Retinol and retinol eluted at 3.62 and 3.76 min respectively.
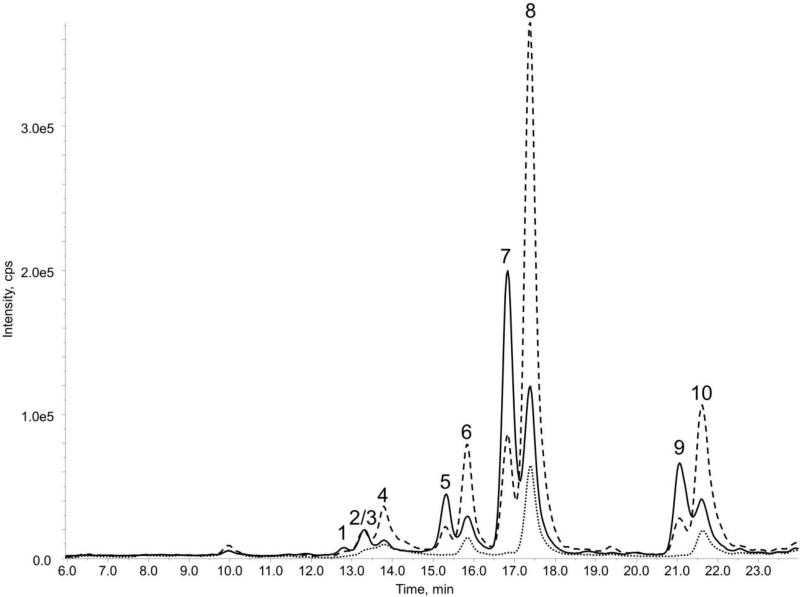
By extending the gradient, we observed and separated myristate, linoleate, oleate, and stearate esters of both α-retinol and retinol in addition to palmitate in the TRL extracts (Figure 1). Identities were confirmed in ESI positive mode (Figure S3), where each of the esters formed a radical cation which fragmented to yield a dehydrated retinol fragment at m/z 269.2. The same pseudomolecular ion has been described previously for retinyl esters [4]. Transitions monitored are listed in Table 1 and a mass chromatogram is shown in the supplementary material.
Figure 1.
HPLC-MS/MS chromatogram of a TRL extract from one representative subject 6 h post-prandial, utilizing the HPLC method separating all esters and the MS equipped with an APCI+ source. MRM transitions specific for α-retinol and α-retinyl esters (269 m/z > 123 m/z —) and retinol and retinyl esters RP (269 m/z >145 m/z ----, 269 m/z > 239 m/z ····) are displayed. Peak numbers correspond to those detailed in Table 1.
An APCI source operated in positive ion mode proved to be more sensitive for the detection of αRP and other α-retinyl esters as compared to an ESI source, confirming previous reports for retinoids [5,18,21]. Further signal enhancement was achieved by replacing the mobile phase additive ammonium acetate with formic acid. αRP and retinyl ester standard solutions confirmed APCI in-source fragmentation to produce a parent ion at 269.2 m/z (representing the neutral loss of the fatty acid moiety and water, [M+H-fatty acid-H2O]+). This observation is consistent with other reports of retinyl palmitate analysis by MS [5,18,21,22]. Notably, the dehydrated α-retinol species (containing an ε-ring) and retinol species (containing a β-ring) have different fragmentation patterns which simplified the identification of the α-analogues in the TRL extracts. In addition, these fragmentation patterns mirror the fragmentation of the parent provitamin A carotenoids α-carotene (containing an ε-ring and a β-ring) and β-carotene (containing two β-rings)[19,23].. The parent-daughter pair m/z 269.2 >123.1, characteristic of ε-ring carotenoids, was the strongest transition observed for α-retinol species and is thought to correspond to the ε-ring moiety itself [19,23]. This daughter was also observed for retinol, but at lower levels, and thus was selected for quantification of αRP. In contrast, m/z 269.2 > 239.1 showed exceptional selectivity for the retinyl esters and was not produced from fragmentation of α-retinol. This fragment was summed with m/z 145.1 (also representative of retinol) for quantitation. Figure 1 demonstrates a mass chromatogram of a TRL extract with these characteristic transitions selected. Using these aforementioned transitions, this method was determined to have a limit of quantitation (LOQ, defined as signal to noise = 10) of 4.8 and 29.5 nM for αRP and RP, respectively, and a limit of detection (LOD, defined as signal to noise = 3) of 1.4 and 8.9 NM for αRP and RP, respectively. In total, the 3 MS/MS transitions chosen were continuously monitored to provide more confidence in the identities of α-retinyl and retinyl esters by comparing ratio intensities.
4. Conclusions
Without proper tools, the contribution of α-retinyl esters to the newly circulating retinyl ester pool can be easily overlooked. Herein, we have provided a descriptive method of α-retinol and αRP synthesis, and detailed HPLC-MS/MS methods that allow for the direct characterization and quantification of α-retinyl esters (both intact and fragmented) in biological samples. We anticipate that these methods will aid in the accurate quantification of vitamin A derived from α-carotene sources, to better assess its vitamin A potential.
Supplementary Material
Highlights.
We provide a detailed method for the synthesis of α-retinyl palmitate standard
An HPLC-MS/MS method to characterize and separate α-retinyl esters from retinyl esters in plasma
We provide a method to more accurately determine vitamin A derived from α-carotene
Acknowledgements
This work was supported by a seed grant from The Ohio State University Food Innovation Center, and by the Nutrient and Phytochemical Shared Resource of The Ohio State University Comprehensive Cancer Center (NIH P30 CA016058). We would also like to thank Dr. Earl H. Harrison for his generous contribution of the retinyl ester standards.
Abbreviations
- αRP
α-retinyl palmitate
- APCI
atmospheric pressure chemical ionization
- ESI
electrospray ionization
- HPLC
high performance liquid chromatography
- MeOH
methanol
- MTBE
methyl tert-butyl ether
- NMR
nuclear magnetic resonance
- PDA
photodiode array
- RP
retinyl palmitate
- MS/MS
tandem mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
The authors have no conflicts of interest to disclose.
References
- 1.Zeitlin MF, Megawangi R, Kramer EM, Armstrong HC. Am. J. Clin. Nutr. 1992;56:136. doi: 10.1093/ajcn/56.1.136. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan U, Martorell R, Latham MC, Abel R. J. Nutr. 1999;129:2021. doi: 10.1093/jn/129.11.2021. [DOI] [PubMed] [Google Scholar]
- 3.Harrison EH. Biochim. Biophys. Acta. 2012;1821:70. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang G. Am. J. Clin. Nutr. 2010;91:1468. doi: 10.3945/ajcn.2010.28674G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleshman MK, Riedl KM, Novotny JA, Schwartz SJ, Harrison EH. J. Lipid Res. 2012;53:820. doi: 10.1194/jlr.D021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Lieshout M, West CE, van Breemen RB. Am. J. Clin. Nutr. 2003;77:12. doi: 10.1093/ajcn/77.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Pitt GAJ. Am. J. Clin. Nutr. 1969;22:1045. doi: 10.1093/ajcn/22.8.1045. [DOI] [PubMed] [Google Scholar]
- 8.Goodman DS, Smith JE, Hembry RM, Dingle JT, Lipid Res J. 1974;15:406. [PubMed] [Google Scholar]
- 9.Ames SR, Swanson WJ, Harris PL. J. Am. Chem. Soc. 1955;77:4136. [Google Scholar]
- 10.Sneider WD, Rosso GC, Rogers a E., Wolf G, Dowling JE, Callahan MJ. J. Nutr. 1974;104:1662. doi: 10.1093/jn/104.12.1662. [DOI] [PubMed] [Google Scholar]
- 11.Tanumihardjo SA, Howe JA, Nutr J. 2005;53706:2622. doi: 10.1093/jn/135.11.2622. [DOI] [PubMed] [Google Scholar]
- 12.Dever JT, Surles RL, Davis CR, a Tanumihardjo S. J. Nutr. 2011;141:42. doi: 10.3945/jn.110.127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riabroy N, Tanumihardjo SA. J. Nutr. 2014 doi: 10.3945/jn.114.191668. [DOI] [PubMed] [Google Scholar]
- 14.Riabroy N, Dever JT, Tanumihardjo S. a. Br. J. Nutr. 2014;111:1373. doi: 10.1017/S0007114513003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap S, Choo Y, Hew N, Goh S. Nutr. Res. 1997;17:1721. [Google Scholar]
- 16.Ross AC, Harrison EH. In: Handb. Vitam. 4th ed. Rucker RB, Zempleni J, Suttie JW, McCormick DB, editors. CRC Press; 2010. pp. 1–40. [Google Scholar]
- 17.Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, Schwartz SJ. J. Nutr. 2014;144:1158. doi: 10.3945/jn.113.187674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopec RE, Schweiggert RM, Riedl KM, Carle R, Schwartz SJ. Rapid Commun. Mass Spectrom. 2013;27:1393. doi: 10.1002/rcm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids, Volume 1B: Spectroscopy. 1996 [Google Scholar]
- 20.Furr HC, Barua AB, Olson JA. In: Retin. Biol. Chem. Med. 2nd editio Sporn MB, Roberts AB, Goodman DS, editors. Raven Press, Ltd.; New York: 1994. pp. 179–209. [Google Scholar]
- 21.van Breemen RB, Nikolic D, Xu X, Xiong Y, van Lieshout M, West CE, Schilling a B. J. Chromatogr. A. 1998;794:245. doi: 10.1016/s0021-9673(97)01138-2. [DOI] [PubMed] [Google Scholar]
- 22.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids Handb. 1996 [Google Scholar]
- 23.van Breemen RB, Dong L, Pajkovic ND. Int. J. Mass Spectrom. 2012;312:163. doi: 10.1016/j.ijms.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.