ABSTRACT
Telomeric repeats-containing RNA (TERRA) are telomere-derived non-coding RNAs that contribute to telomere function in protecting chromosome ends. We recently identified a cell-free form of TERRA (cfTERRA) enriched in extracellular exosomes. These cfTERRA-containing exosomes stimulate inflammatory cytokines when incubated with immune responsive cells. Here, we report that cfTERRA levels were increased in exosomes during telomere dysfunction induced by the expression of the dominant negative TRF2. The exosomes from these damaged cells also enriched with DNA damage marker γH2AX and fragmented telomere repeat DNA. Purified cfTERRA stimulated inflammatory cytokines, but the intact membrane-associated nucleoprotein complexes produced a more robust cytokine activation. Therefore, we propose cfTERRA-containing exosomes transport a telomere-associated molecular pattern (TAMP) and telomere-specific alarmin from dysfunctional telomeres to the extracellular environment to elicit an inflammatory response. Since cfTERRA can be readily detected in human serum it may provide a useful biomarker for the detection of telomere dysfunction in the early stage of cancers and aging-associated inflammatory disease.
KEYWORDS: cfTERRA, exosomes, inflammation, TAMP (telomere-associated molecular pattern), telomere, TERRA
Telomeres are repetitive DNA elements that protect the ends of linear chromosomes from catastrophic damage.1 Telomeric repeats are assembled into a dynamic nucleoprotein complex, collectively termed Shelterin, which regulates telomere repeat length, stability, and function.2 As cells divide and age, telomere repeat DNA shortens by attrition due to the end-replication problem. Excessive cell divisions or replication stress can lead to critically short telomeres incapable of forming a functional Shelterin complex. These uncapped telomeres elicit a DNA damage response and cell cycle arrest.3 In stem cells and most cancer cells, the telomere repeats are restored by the telomere-specific reverse transcriptase telomerase.4 In the absence of telomerase, these dysfunctional telomeres induce cellular senescence and drive the process of organismal aging.5 Programmed senescence is an important control on tissue homeostasis and an innate barrier to cellular immortalization and carcinogenesis.6 However, excessive or premature senescence can induce a pathogenic tissue microenvironment due to secretion of inflammatory molecules.7 This senescence-associated secretory phenotype (SASP) is characterized by the secretion of proinflammatory cytokines, chemokines, growth factors, and proteases.8 However, the molecular mechanisms driving SASP are not well-understood. A recent study described a 'telomere-associated secretory phenotype' (TASP),9 which produces a distinct inflammatory pattern associated with pre-shortened telomeres, suggesting that telomere dysfunction may be directly linked to senescence-associated inflammation.
Telomere uncapping can result in the increase production of telomere repeat-containing RNA (TERRA).10,11 TERRA is a heterogeneous ensemble of non-coding RNAs generated from multiple different telomeres, and comprises 100 to 9,000 bases of telomere repeats in mammals.12 Due to the unique G-rich sequence, TERRA is folded into G-quadruplex structure to interact with numerous proteins that regulate telomere maintenance.13 We previously found that TERRA interacts with shelterin TRF2 and promotes telomeric heterochromatin formation by mediating the interaction of TRF2 and ORC.14 Others have found that TERRA can inhibit telomerase, bind to hnRNPA and MRE11, and form stable RNA-DNA hybrid R-loops at telomeres.15,16 Elevated TERRA levels also correlate with the alternative lengthening of telomeres (ALT) phenotype,17,18 and chromosome instability syndromes linked to deficiencies in DNMT3b-associated DNA methylation.19 Whether TERRA also participates in telomere-associated inflammatory secretion was not known.
Recently, we found a cell-free form of TERRA (cfTERRA) that constituted a nucleoprotein component of extracellular microvesicular exosomes in cancer cell culture and human blood plasma.20 These cfTERRA-containing exosomes were strong inducers of inflammatory cytokines in peripheral blood mononuclear cells (PBMCs). We also demonstrated that synthetic TERRA was able to stimulate inflammatory cytokines when delivered by liposomes. Here, we describe recent advances in the role of TERRA during telomere dysfunction, and discuss how cfTERRA may mediate the crosstalk between telomere dysfunction and inflammation.
TERRA is highly enriched in human exosomes
TERRA levels increase in response to telomere uncapping.10 To recapitulate telomere uncapping in cell culture models, we examined the dominant negative form of TRF2, TRF2ΔBΔM, shown previously to form telomere-specific DNA damage.21 We compared protein and RNA levels in cellular and exosomal fractions in BJ-hTERT cells with inducible TRF2ΔBΔM expression (Fig. 1). TRF2ΔBΔM was maintained in low expression levels, and was highly induced in presence of doxycycline for 4 d (Fig. 1A). TRF2ΔBΔM proteins were mostly associated with cellular fractions with traceable amount in exosome fractions, while CD63, a marker for exosomes, was found enriched in exosomes. As expected, induction of TRF2ΔBΔM increased the level of DNA damage marker γH2AX in BJ-hTERT cells. Interestingly, γH2AX was also modestly enriched in the exosome fractions of the TRF2ΔBΔM induced cells (Fig. 1A lower panel). Total cellular TERRA levels were measured by Northern blot (Fig. 1B). We found that cells with induced TRF2ΔBΔM expressed elevated levels of TERRA (Fig. 1B), consistent with previous reports showing elevated TERRA after TRF2 depletion.10 We previously demonstrated that exosomal TERRA is smaller than cellular TERRA, and is associated with a small percentage of telomeric DNA and histone protein components.20 Using identical extraction methods described previously, we measured exosome-associated TERRA by dot blot (Fig. 1C). We found high levels of TERRA in all exosome fractions relative to cellular levels. Cells expressing TRF2ΔBΔM showed a modest increase in cellular and exosomal TERRA levels (Fig. 1C). In contrast, 18S RNA and Alu transcripts were highly expressed, but they were mostly in cellular fractions with low amounts in exosome fractions, indicating that they are not selectively enriched in these exosomes (Fig. 1C, lower panel). These findings indicate the dominant negative TRF2-associated telomere uncapping can induce cellular TERRA and increase the relative amount of TERRA in exosomes.
Figure 1.
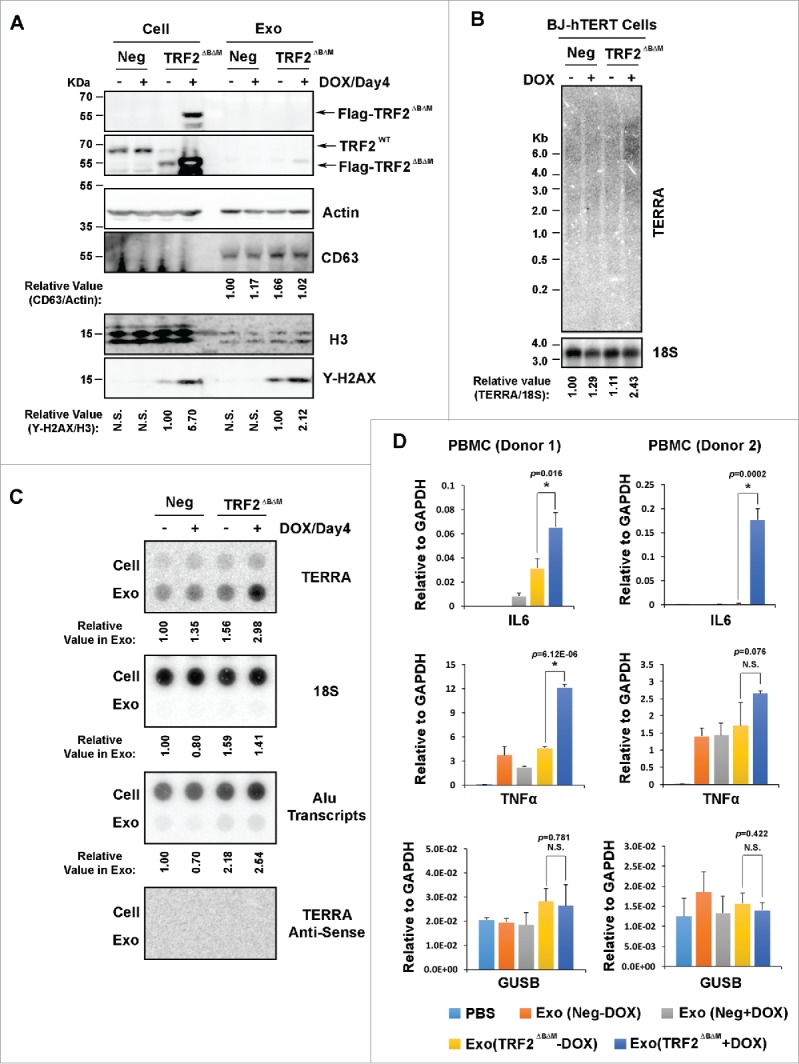
Induction of cellular and cell-free TERRA by the dominant negative TRF2. (A) Western blot analysis of lysates from BJ-hTERT cells (Cell) or purified exosomes (Exo). An inducible form of N-terminal FLAG tagged TRF2ΔBΔM or control vector (Neg) were transduced into BJ-hTERT cells by lentiviral infection. The expression of TRF2ΔBΔM was either induced (+) by 0.5 µg/ml doxycycline (DOX) for 4 days, or maintained uninduced (−) by cultured in Tetracycline-Free medium. Total protein lysates were collected from cells or purified exosomes. Western blotting was performed with equal amounts of total protein lysate (15 µg) for each sample, using antibodies specific for FLAG (Sigma), TRF2 (Rabbit), Actin (Sigma), CD63 (Abcam), H3 (Active Motif) or γH2AX (Millipore). The relative signal intensity for CD63 was quantified and normalized by Actin, and the relative signal intensity for γH2AX was quantified and normalized by H3. (B) Northern blot analysis of cellular TERRA in the BJ-hTERT cells, as shown in (A). Isolated cellular RNA (10 µg) was used for each sample, and hybridized with 32P-labeled probes for TERRA, or 18S RNA as indicated. Numbers on the left show the position of RNA markers in base pairs. The relative signal intensity for TERRA was quantified and normalized by 18S RNA. (C) RNA dot blot analysis of RNA isolated from BJ-hTERT cells (Cell) or purified exosomes (Exo), as shown in (A). Equal amounts of RNA (1 µg) were assayed by dot blotting, and hybridized with probes for TERRA, 18S, Alu transcripts, or TERRA-antisense, as indicated. Quantification of RNA in exosomes was shown below each panel. (D) qRT-PCR for expression of IL6, TNFα, or control GUSB mRNA in PBMCs treated with exosomes, as shown in (A). PBMCs were isolated from 2 donors, and incubated with PBS, Exo (Neg-DOX), Exo (Neg+DOX), Exo (TRF2ΔBΔM -DOX), or Exo (TRF2ΔBΔM +DOX). Bar graphs represent qRT-PCR values relative to GAPDH mRNA (mean ± SD) from 3 independent experiments. P-values were calculated by student t-test.
Inflammatory properties of exosomal TERRA
We previously demonstrated that cfTERRA-containing exosomes were strong inducers of inflammatory cytokines in peripheral blood mononuclear cells (PBMCs). We then examined the inflammatory properties of exosomes from the TRF2ΔBΔM induced BJ-hTERT cells when incubated with PBMCs from 2 donors (Fig. 1D). Exosomes from TRF2ΔBΔM induced cells exhibited stronger activity in stimulating the transcription of IL6 and TNFα, whereas control GUSB mRNA levels were not significantly affected. These results were consistent with our previous findings, suggesting that elevated TERRA levels in exosomes during telomere uncapping can promote the inflammatory properties of exosomes.
Exosomes from human lymphoblastoid cell lines (LCLs) were also found to have elevated levels of TERRA containing exosomes. We found that these exosomes also contain telomeric DNA that may form stable structures, including DNA-RNA hybrids with TERRA. Extraction of DNA or RNA from various fractionations across a density gradient centrifugation revealed that both telomere repeat-containing DNA and RNA could be isolated from exosome-containing fractions that peak in F9 and F10 (Fig. 2A, top panels). G-rich strand DNA and RNA were enriched, but C-rich DNA could also be detected in fractions F9 and F10. We then tested whether either exosomes, or exosome-associated RNA or DNA could induce an inflammatory response when incubated with PBMCs (Fig. 2B and C). Exosomes enriched with TERRA from F10 were found to induce the highest levels of IL6 and TNFα, whereas no significant effect was caused in control GUSB mRNA levels (Fig. 2D). Purified exosome F10-associated RNA containing highest levels of TERRA was found to partly induce IL6 and TNFα, while only trace activities could be found in the purified DNA fractions. Interestingly, others have found that purified telomeric DNA can inhibit innate immune response.22 These findings suggest that the RNA component from exosome is a more potent inflammatory molecule than telomeric DNA. However, neither RNA nor DNA was as active as the complete exosome fraction, suggesting that additional factors, or intact membrane-associated nucleoprotein complexes are required for robust activation of the inflammatory response.
Figure 2.
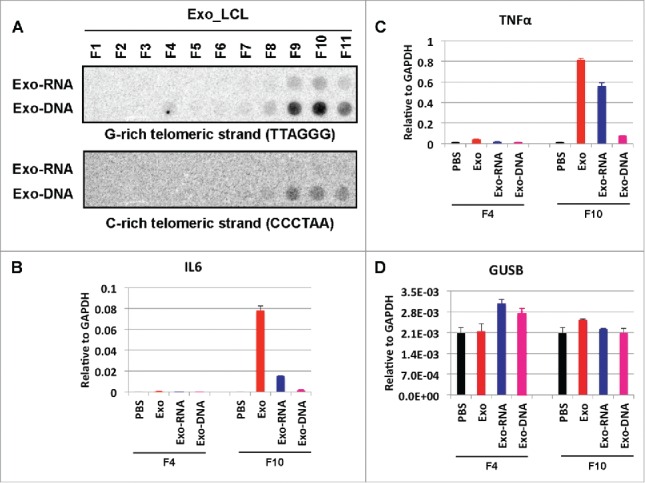
cfTERRA containing exosomal RNA constitutes more activity over DNA in stimulating inflammatory cytokines. (A) Dot blot analysis of telomeric repeats in exosomal RNA (Exo-RNA) and DNA (Exo-DNA) from sucrose gradient fractionation of LCL-derived exosomes. The blot was probed for G-rich telomeric strand (Upper) or C-rich telomeric strand (Lower). (B-D) PBMCs were incubated with PBS, exosomes (Exo), Exo-RNA, or Exo-DNA from indicated sucrose gradient fraction 4 (F4) and fraction 10 (F10), and then assayed by qRT-PCR for expression of IL6 (B), TNFα (C) or control GUSB mRNA (D). Bar graphs represent qRT-PCR values relative to GAPDH mRNA (mean ± SD) from 3 independent experiments.
Based on these data and our recently published study,20 we propose that telomere-induced stress leads to the upregulation of TERRA that is processed into smaller fragments and secreted in exosome-like vesicles. These TERRA containing exosomes are relatively stable and circulate in extracellular spaces, including blood and plasma. We have shown that the TERRA containing exosomes can induce a robust inflammatory response in human PBMCs leading to the production of various cytokines, including IL6 and TNFα (Fig. 2B and C). We propose that TERRA containing exosomes are a significant source of SASP and TASP, as telomere dysfunction is known to occur in senescent cells. The function of this inflammation may be to recruit macrophages to consume and eliminate senescing cells and their byproducts from tissue microenvironments (Fig 3). Excessive SASP can be a source of pathogenic inflammation which may alter the normal physiology of neighboring cells and tissue.23 It is well known the chronic inflammation leads to a tumorigenic environment. Telomere dysfunction induced inflammation is also observed when TRF2 is depleted in mouse embryonic skin24 and alveolar stem cells.25 We suspect that TERRA containing exosomes are a significant component of this inflammatory signaling pathway.
Figure 3.
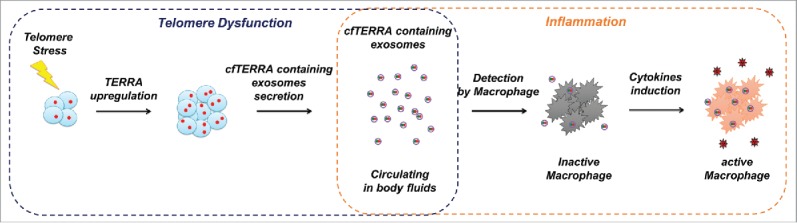
Model of cfTERRA containing exosomes as telomere-specific alarmins containing telomere-associated molecular patterns (TAMP) that mediate the crosstalk of telomere dysfunction with inflammation. Telomere-related stress induces TERRA expression and the secretion of cfTERRA through exosomes. These cfTERRA containing exosomes are released in tissue microenvironment and circulating in body fluids. Immune cells (macrophages) detect cfTERRA containing exosomes as damage signals, and produce inflammatory cytokines to eliminate cells with dysfunctional telomeres.
cfTERRA biogenesis
TERRA is transcribed predominantly by RNA polymerase II26 and regulated by several mechanisms, including developmental status,27 telomere length,11 epigenetic state,28 and stress.29 Numerous studies showed that telomere dysfunction induces changes in cellular TERRA levels.10,30,31 Depletion of TRF2 resulted in up-regulation of TERRA.10 Loss of TRF2 disrupts the telomere T-loop structure,32 activates ATM-mediated DNA damage response,33 and leads to telomere fusions through non-homologous end joining (NHEJ).34 Increase expression of TERRA may be a built-in feedback mechanism to protect telomeres by generating RNA-DNA hybrids and promoting homologous recombination with other telomere repeats.16 This telomeric homologous recombination is observed in telomerase negative ALT cells. As TERRA expression is linked to telomere uncapping and dysfunction, its elevated expression correlates well with telomere damage and stress-response.
How the smaller extracellular forms of cfTERRA are generated is not known. cfTERRA expression levels typically correlate with levels of cellular TERRA, so it is likely that the cfTERRA is co-regulated with cellular TERRA production. We found that cfTERRA was typically ∼200 nt in length, distinctly smaller than cellular forms of TERRA. cfTERRA can be amplified by RT-qPCR primers situated in unique sequences of the adjacent subtelomeres, suggesting that cfTERRA arises from chromosome terminal repeats. As there are many interstitial telomere repeat sequences (ITS) of 200 nucleotide length, it is also possible that some cfTERRA arises from these shorter interstitial repeats in some stress conditions. The shorter RNA forms suggest that cfTERRA are aborted, incomplete transcripts, or post-transcriptionally processed from longer forms of cellular TERRA. cfTERRA is typically detected in higher abundance than cellular TERRA. This may be due to cfTERRA being more stable, a breakdown product of full-length cellular TERRA, or an alternative, aberrant form that is rapidly eliminated through extracellular vesicle transport. We also observed that cfTERRA is co-purified with extracellular telomeric DNA (G and C-rich strands), and is associated with histone, as detected by extracellular ChIP assays with histone H3 specific antibodies.20 Others have reported that nucleosomes and telomeric DNA can be found in cell-free fractions, and are considered contaminants of exosomes. We addressed this concern by demonstrating the exosome-associated cfTERRA is highly resistant to RNAse, while the majority of chromatin DNA can be eliminated by DNase. Thus cfTERRA is either within exosomes, or in a nuclease protected form that is co-purified with exosomes. While cfTERRA may be a jettisoned by-product of stressed cells or part of apoptotic chromatin debris, we are struck by its stability and abundance in cells and tissues. We therefore consider the intriguing possibility that this debris carries inherent telomere-specific signaling information to surrounding cells.
cfTERRA as telomere-specific alarmin, or TAMP
The term “alarmins” has been given to a family of endogenous molecules that signal cellular and tissue damage.35 Many nuclear proteins and DNA structures function as alarmins. Extracellular forms of HMGB1, an otherwise abundant chromatin associated protein, is considered a Danger Associated Molecular Pattern (DAMP) because it can be secreted at high levels in apoptotic cells and trigger macrophage cytokine production.36 Similarly, release of unmethylated CpG DNA is referred to as a Pathogen Associated Molecular Pattern (PAMP) because it appears at high levels during viral and bacterial infections and triggers a robust immunologic response.37 We suggest that cfTERRA is a telomeres-specific DAMP, or TAMP. cfTERRA by itself, or in its endogenous form associated with other exosome components, including histones, is a potent activator of IL6 and TNFα from human PBMCs. This represents a direct and specific communication of telomere dysfunction through DAMP-like signaling. It will be interesting to determine if the cytokine profile and innate immune response to TAMPs are distinct from other DAMPs, such as HMGB1. It is conceivable that a low level of cfTERRA serves as a healthy signal in the absence of other DAMPs, but that elevated levels by themselves, or in association with other DAMPs may initiate pathogenic inflammatory environments, including those observed in telomeropathies, like idiopathic pulmonary fibrosis and bone marrow failure syndromes.
cfTERRA as potential biomarker for telomere-dysfunction and associated human disease
cfTERRA is highly stable, and relatively easy to detect by sequencing or hybridization. We were able to identify cfTERRA foci in human blood and serum by RNA-seq and dot blotting. We also detected elevated levels of TERRA in human tumor tissue, and increase incidence of cfTERRA foci in tumor tissue sections from mouse models of medulloblastoma.20 As cfTERRA can be found at high levels in normal human plasma, it is unlikely that cfTERRA can serve as a single molecular entity for diagnosis. However, it remains possible that cfTERRA in combination with other DAMPs or biomarkers, may reveal early stage pathogenesis associated with telomere-dysfunction in early stage cancers and senescence-associated inflammatory disease.
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Andreas Wiedmer and Zhong Deng for technical support.
Funding
This work was supported by grants from NIH NCI (CA RO1CA140652) to PML and from the NCI Cancer Center Core Grant (P30 CA10815) and the Commonwealth Universal Research Enhancement Program, PA Department of Health.
References
- 1.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015; 350:1193-8; PMID:26785477; http://dx.doi.org/ 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- 2.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science 2012; 336:593-7; PMID:22556254; http://dx.doi.org/ 10.1126/science.1218498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SS, Bohrson C, Pike AM, Wheelan SJ, Greider CW. ATM Kinase Is Required for Telomere Elongation in Mouse and Human Cells. Cell Rep 2015; 13:1623-32; PMID:26586427; http://dx.doi.org/ 10.1016/j.celrep.2015.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol 2013; 14:69-82; PMID:23299958; http://dx.doi.org/ 10.1038/nrm3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Z, Jay KA, Smith DL, Zhang Y, Liu Z, Zheng J, Tian R, Li H, Blackburn EH. Early telomerase inactivation accelerates aging independently of telomere length. Cell 2015; 160:928-39; PMID:25723167; http://dx.doi.org/ 10.1016/j.cell.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15:482-96; PMID:24954210; http://dx.doi.org/ 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 7.Golomb L, Sagiv A, Pateras IS, Maly A, Krizhanovsky V, Gorgoulis VG, Oren M, Ben-Yehuda A. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ 2015; 22:1764-74; PMID:26434982; http://dx.doi.org/ 10.1038/cdd.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6:2853-68; PMID:19053174; http://dx.doi.org/ 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braig M, Pallmann N, Preukschas M, Steinemann D, Hofmann W, Gompf A, Streichert T, Braunschweig T, Copland M, Rudolph KL, et al.. A 'telomere-associated secretory phenotype' cooperates with BCR-ABL to drive malignant proliferation of leukemic cells. Leukemia 2014; 28:2028-39; PMID:24603533; http://dx.doi.org/ 10.1038/leu.2014.95 [DOI] [PubMed] [Google Scholar]
- 10.Porro A, Feuerhahn S, Delafontaine J, Riethman H, Rougemont J, Lingner J. Functional characterization of the TERRA transcriptome at damaged telomeres. Nat Commun 2014; 5:5379; PMID:25359189; http://dx.doi.org/ 10.1038/ncomms6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cusanelli E, Romero CA, Chartrand P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell 2013; 51:780-91; PMID:24074956; http://dx.doi.org/ 10.1016/j.molcel.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 12.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007; 318:798-801; PMID:17916692; http://dx.doi.org/ 10.1126/science.1147182 [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Suzuki Y, Ito K, Komiyama M. Telomeric repeat-containing RNA structure in living cells. Proc Natl Acad Sci U S A 2010; 107:14579-84; PMID:20679250; http://dx.doi.org/ 10.1073/pnas.1001177107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 2009; 35:403-13; PMID:19716786; http://dx.doi.org/ 10.1016/j.molcel.2009.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res 2010; 38:5797-806; PMID:20460456; http://dx.doi.org/ 10.1093/nar/gkq296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balk B, Maicher A, Dees M, Klermund J, Luke-Glaser S, Bender K, Luke B. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol 2013; 20:1199-205; PMID:24013207; http://dx.doi.org/ 10.1038/nsmb.2662 [DOI] [PubMed] [Google Scholar]
- 17.Arora R, Lee Y, Wischnewski H, Brun CM, Schwarz T, Azzalin CM. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commun 2014; 5:5220; PMID:25330849; http://dx.doi.org/ 10.1038/ncomms6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng LJ, Cropley JE, Pickett HA, Reddel RR, Suter CM. Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res 2009; 37:1152-9; PMID:19129228; http://dx.doi.org/ 10.1093/nar/gkn1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Z, Campbell AE, Lieberman PM. TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells. Cell Cycle 2010; 9:69-74; PMID:20016274; http://dx.doi.org/ 10.4161/cc.9.1.10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Deng Z, Dahmane N, Tsai K, Wang P, Williams DR, Kossenkov AV, Showe LC, Zhang R, Huang Q, et al.. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc Natl Acad Sci U S A 2015; 112:E6293-300; PMID:26578789; http://dx.doi.org/ 10.1073/pnas.1505962112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell 1998; 92:401-13; PMID:9476899; http://dx.doi.org/ 10.1016/S0092-8674(00)80932-0 [DOI] [PubMed] [Google Scholar]
- 22.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol 2003; 171:1393-400; PMID:12874230; http://dx.doi.org/ 10.4049/jimmunol.171.3.1393 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Ohsawa S, Igaki T. Mitochondrial defects trigger proliferation of neighbouring cells via a senescence-associated secretory phenotype in Drosophila. Nat Commun 2014; 5:5264; PMID:25345385; http://dx.doi.org/ 10.1038/ncomms6264 [DOI] [PubMed] [Google Scholar]
- 24.Martinez P, Ferrara-Romeo I, Flores JM, Blasco MA. Essential role for the TRF2 telomere protein in adult skin homeostasis. Aging Cell 2014; 13:656-68; PMID:24725274; http://dx.doi.org/ 10.1111/acel.12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A 2015; 112:5099-104; PMID:25840590; http://dx.doi.org/ 10.1073/pnas.1504780112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nergadze SG, Farnung BO, Wischnewski H, Khoriauli L, Vitelli V, Chawla R, Giulotto E, Azzalin CM. CpG-island promoters drive transcription of human telomeres. Rna 2009; 15:2186-94; PMID:19850908; http://dx.doi.org/ 10.1261/rna.1748309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Z, Wang Z, Xiang C, Molczan A, Baubet V, Conejo-Garcia J, Xu X, Lieberman PM, Dahmane N. Formation of telomeric repeat-containing RNA (TERRA) foci in highly proliferating mouse cerebellar neuronal progenitors and medulloblastoma. J Cell Sci 2012; 125:4383-94; PMID:22641694; http://dx.doi.org/ 10.1242/jcs.108118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Z, Wang Z, Stong N, Plasschaert R, Moczan A, Chen HS, Hu S, Wikramasinghe P, Davuluri RV, Bartolomei MS, et al.. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J 2012; 31:4165-78; PMID:23010778; http://dx.doi.org/ 10.1038/emboj.2012.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tutton S, Azzam GA, Stong N, Vladimirova O, Wiedmer A, Monteith JA, Beishline K, Wang Z, Deng Z, Riethman H, et al.. Subtelomeric p53 binding prevents accumulation of DNA damage at human telomeres. The EMBO journal 2016; 35:193-207; PMID:26658110; http://dx.doi.org/ 10.15252/embj.201490880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Z, Kim ET, Vladimirova O, Dheekollu J, Wang Z, Newhart A, Liu D, Myers JL, Hensley SE, Moffat J, et al.. HSV-1 remodels host telomeres to facilitate viral replication. Cell Rep 2014; 9:2263-78; PMID:25497088; http://dx.doi.org/ 10.1016/j.celrep.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer V, Crittin J, Grolimund L, Lingner J. The THO complex component Thp2 counteracts telomeric R-loops and telomere shortening. EMBO J 2013; 32:2861-71; PMID:24084588; http://dx.doi.org/ 10.1038/emboj.2013.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doksani Y, Wu JY, de Lange T, Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell 2013; 155:345-56; PMID:24120135; http://dx.doi.org/ 10.1016/j.cell.2013.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH, de Lange T. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol 2004; 2:E240; PMID:15314656; http://dx.doi.org/ 10.1371/journal.pbio.0020240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribes-Zamora A, Indiviglio SM, Mihalek I, Williams CL, Bertuch AA. TRF2 interaction with Ku heterotetramerization interface gives insight into c-NHEJ prevention at human telomeres. Cell Rep 2013; 5:194-206; PMID:24095731; http://dx.doi.org/ 10.1016/j.celrep.2013.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81:1-5; PMID:17032697; http://dx.doi.org/ 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- 36.Pisetsky D. Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med Wkly 2011; 141:w13256; PMID:21877298; http://dx.doi.org/ 10.4414/smw.2011.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol 2012; 144:32-40; PMID:22659033; http://dx.doi.org/ 10.1016/j.clim.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]


