ABSTRACT
Insulin-Like Growth Factor 2 (IGF-2) is a peptide hormone essential for prenatal growth and development. IGF-2 exerts its mitogenic effects via Insulin-Like Growth Factor 1 Receptor (IGF-1R), and is eliminated by binding to Insulin-Like Growth Receptor 2 (IGF-2R). IGF-2 is also negatively regulated by Phosphatase and Tensin Homolog (PTEN), a phosphatase mutated in various tumors. Not much is known about the interplay between these factors during human odontogenesis. In this study, expression patterns of IGF-2, IGF-1R, IGF-2R and PTEN were analyzed by double immunofluorescence in incisor human tooth germs during the foetal period of development between the 7th and 20th gestational week. Throughout the investigated period, IGF-2 was mostly expressed in enamel organ, whereas mild to moderate expression of PTEN could be seen in dental papilla and parts of enamel organ. Expression of IGF-1R was ubiquitous and displayed strong intensity throughout the entire enamel organ. In contrast, expression of IGF-2R had rather erratic pattern in enamel organ and dental papilla alike. Expression patterns of IGF-2, IGF-1R, IGF-2R and PTEN in highly proliferative cervical loops, as well as in differentiating pre-ameloblasts and pre-odontoblasts of cusp tip region during the early and late bell stages when enamel organ acquires definitive shape, indicate importance of these factors in crown morphogenesis of human incisor. Taken together, our data suggest the involvement of IGF-2, IGF-1R, IGF-2R and PTEN in temporo-spatial patterning of basic cellular processes (proliferation, differentiation) during normal tooth development. They are also relevant for improving knowledge of molecular basis of human odontogenesis.
KEYWORDS: differentiation, human tooth development, IGF-axis, IGF-2, IGF-1R, IGF-2R, proliferation, PTEN
INTRODUCTION
In the course of odontogenesis, human tooth germs undergo successive developmental stages of dental lamina, bud, cap and bell. Along with morphologically distinctive outlook, each of these stages is characterisedby a specific spatial pattern of basic cellular and tissue processes (proliferation, differentiation, apoptosis, epithelial-to-mesenchymal or mesenchymal-to-epithelial induction and transformation).1–3 Various factors have been implicated in molecular regulation of such patterning, in particular, those encoded by homeobox genes which seem to be of paramount importance for initiation of tooth development and reciprocation of odontogenic potential between epithelial and mesenchymal parts of tooth germ during the entire course of odontogenesis.4,5 Additionally, a number of growth factors have been shown to play diverse roles in directing both proliferation and differentiation of specific tooth germ cell populations within the context of each stage of development.6,7 Unfortunately, not much is known about molecular basis of human odontogenesis, since most of the available data come from studies on tooth germs in animal models.4,7,8 To partly address the issue, we set out to analyze expression patterns of Insulin-Like Growth Factor 2 (IGF-2), Insulin-Like Growth Factor 1 Receptor (IGF-1R), Insulin-Like Growth Factor 2 Receptor (IGF-2R), and Phosphatase and Tensin Homolog (PTEN) in human tooth germs during foetal period of development.
IGF-2 is a mitogenic peptide hormone with predominant role in prenatal growth and development. In humans, gain or loss of function of gene encoding IGF-2 may be involved in etiology of development disorders described in Beckwith-Wiedemann and Silver-Russell syndromes. The former is classically identified at birth by macrosomia, macroglossia, and omphalocele, while presence of other features such as asymmetry of the limbs, organomegaly, and neonatal hypoglycemia vary between individual cases.9 In contrast, Silver-Russell syndrome is characterized by intrauterine and/or postnatal growth retardation, typically triangular shaped face and body asymmetry in a subset of affected individuals.10 In mice, paternal inheritance of Igf-2 null allele (due to genomic imprinting) results in foeto-placental growth retardation.11 On a cellular level, the effects of IGF-2 are similar to that of Insulin-Like Growth Factor 1 (IGF-1) by promoting proliferation, differentiation, survival and migration of cells.12,13 Functional versatility of IGF-2 must primarily be viewed within the context of IGF-axis, whose members such as Insulin-Like Growth Factor Binding Proteins (IGFBPs) and receptors IGF-1R and IGF-2R are involved in complex regulation of activity of both IGFs.14 Namely, by binding IGF-1 and −2 in serum, IGFBPs facilitate endocrine delivery of these factors to target tissues in addition to IGFBP-free autocrine/paracrine delivery of IGF-1 and −2 from specific tissues.14 Furthermore, both IGFs exert mitogenic and metabolic effects almost exclusively via IGF-1R (which binds IGF-1 with greater affinity than IGF-2), whereas IGF-2R has no signaling capacity, but rather serves to scuttle IGF-2 away from the cell surface targeting it for lysosomal degradation.15,16
Numerous studies indicate the importance of tumor suppressor PTEN in control of cell proliferation and apoptosis. Germline mutations of PTEN in human syndromes such as Cowden disease and Proteus syndrome (characterised by multiple hamartomas and other tumors), as well as its somatic mutations in a number of primary tumors, show that PTEN loss-of-function is responsible for rampant proliferation and extended cell survival in neoplastic tissues.17–20 Counteracting IGF-1 and −2, PTEN's phosphatase activity was proven crucial for down-regulation of signaling pathways associated with IGFs main receptor IGF-1R, especially PI3K/Akt pathway, which promotes cell survival and progression of cell cycle.21,22 Therefore, as a negative regulator of PI3K-Akt pathway PTEN is able to modulate IGFs action, which was demonstrated by the complex interplay between IGF-2 and PTEN in normal adult tissues and tumors.11,23
So far, the involvement of IGF-axis in embryonic and foetal development of human and animals has been relatively well investigated.12,24–26 In developing human tooth germs, IGF-axis has been implicated in amelogenesis and root formation, but there are only few accounts on particular members' roles (most notably IGF-1 and IGF-1R) during the earlier stages of tooth development.27–31 On the other hand, involvement of PTEN in developing odontogenic tissues still remains to be investigated, even though the total mapping of PTEN expression patterns has been performed for developing human and mouse embryos.32,33 Significant inter-species differences of PTEN expression patterns reported in those studies, support the occurrence of diverse phenotypes yielded by germline loss-of-function mutations of PTEN in humans and knockout mice, as much as they imply distinctive roles PTEN might play during normal development in various species.
Given the overall importance of IGF-axis in embryonic and foetal development and its intricate regulatory association with PTEN, the aim of this study was to analyze expression patterns of IGF-2, IGF-1R, IGF-2R and PTEN in developing human tooth germs. This should improve our insight into the molecular background of temporal and spatial patterning of basic cellular processes during human odontogenesis.
RESULTS
By the end of the 5th week of development, thickening of embryonic oral epithelium (dental lamina) heralds development of human tooth germs. This is followed by consecutive developmental stages (bud, cap and bell) in preparation for synthesis, secretion and maturation of enamel and dentin in tooth crown. In this study, the development of human incisor tooth germs was investigated in period between the 7th week and the 20th week of development.
Co-localization of IGF-2 and PTEN by double immunofluorescence
Co-localization of IGF-2 and PTEN by double immunofluorescence was performed on incisor tooth germs aged 7, 10, 12, 14 and 20 weeks of development (Fig. 1, Fig. 2, Fig. 3, Table 1).
FIGURE 1.
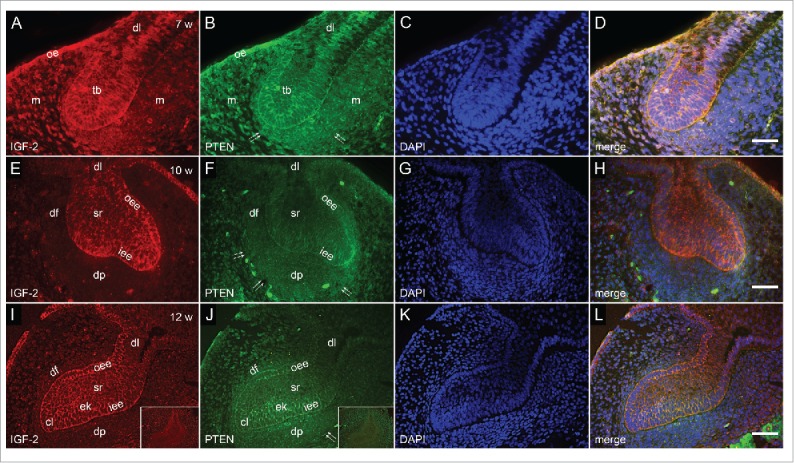
Co-localization of IGF-2 and PTEN by double immunofluorescence in human incisor tooth germs between 7th and 12th week of development. (a-d) Human incisor tooth germ in the 7th week of development (bud stage); (a) Moderate to strong expression of IGF-2 in the tooth bud. Jaw mesenchyme is negative to IGF-2; (b) Moderate to strong expression of PTEN in the tooth bud. Jaw mesenchyme is moderately positive to PTEN. Forefront of mesenchymal expression (arrows); (c) DAPI staining of nuclei; (d) Merging of a+b+c (Magnification: ×40, scale bar: 25 µm). (e-h) Human incisor tooth germ in the 10th week of development – (cap stage); (e) Moderate expression of IGF-2 in outer and inner enamel epithelia, and stellate reticulum. Strong expression of IGF-2 in prospective cervical loop. Dental papilla and dental follicle are negative to IGF-2; (f) Moderate expression of PTEN in outer enamel epithelium, stellate reticulum and dental papilla. Strong expression of PTEN in prospective cervical loop. Forefront of expression in dental papilla (arrows); (g) DAPI staining of nuclei; (h) Merging of e+f+g (Magnification: ×20, scale bar: 40 µm). (i-l) Human incisor tooth germ in the 12th week of development (late cap stage); (i) Slight increase of expression of IGF-2 in stellate reticulum (Inset: negative control staining to IGF-2); (j) Inner enamel epithelium and primary enamel knot express PTEN moderately. Slight increase of expression of PTEN in restricted area of dental papilla underlying the enamel knot (Inset: negative control staining to PTEN); (k) DAPI staining of nuclei; (l) Merging of i+j+k (Magnification: ×20, scale bar: 40 µm)Designations: oral epithelium (oe), dental lamina (dl), tooth bud (tb), jaw mesenchyme (m), outer enamel epithelium (oee), inner enamel epithelium (iee), primary enamel knot (ek), stellate reticulum (sr), stratum intermedium (si), cervical loop (cl), enamel knot (ek), preameloblasts (pa), dental papilla (dp), dental follicle (df), pre-odontoblasts (po), forefront of marker's expression in tissue (double arrows); individual cells expressing marker (thick arrows).
FIGURE 2.
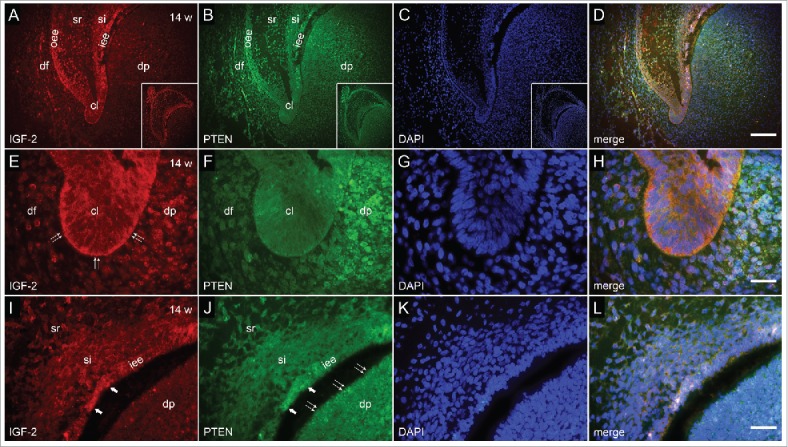
Co-localization of IGF-2 and PTEN by double immunofluorescence in human incisor tooth germ in the 14th week of development (early bell stage). (a-d) Human incisor tooth germ in the 14th week of development (early bell stage) (a) Expression of IGF-2 is mostly restricted to enamel organ (Inset: human incisor tooth germ in early bell stage, IGF-2 staining); (b) Expression of PTEN in enamel organ, dental papilla and dental follicle (Inset: Human incisor tooth germ in early bell stage, PTEN staining); (c) DAPI staining of nuclei (Inset: Human incisor tooth germ in early bell stage, DAPI staining of nuclei); (d) Merging of a+b+c (Magnification: ×20, scale bar: 40 µm). (e-h) Detail of cervical loop (e) Moderate expression of IGF-2 in the cervical loop and adjacent parts of enamel epithelia. Mild expression of IGF-2 in adjacent area of dental papilla; (f) Mild expression of PTEN in the cervical loop. Moderate expression of PTEN in adjacent area of dental papilla; (g) DAPI staining of nuclei; (h) Merging of e+f+g (Magnification: ×100, scale bar: 10 µm). (i-l) Detail of the site of future cusp tip (i) Moderate expression of IGF-2 in the inner enamel epithelium (prospective pre-ameloblasts) and mild expression in stratum intermedium; (j) Moderate expression of PTEN in the inner enamel epithelium. Moderate to strong expression of PTEN in prospective pre-odontoblasts of dental papilla; (k) DAPI staining of nuclei; (l) Merging of i+j+k (Magnification: ×100; scale bar: 10 µm).
FIGURE 3.
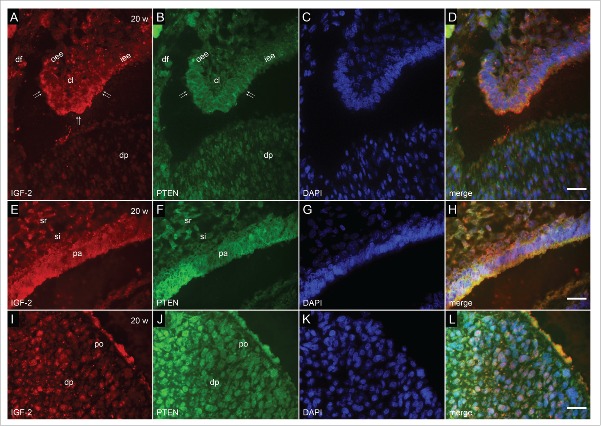
Co-localization of IGF-2 and PTEN by double immunofluorescence in human incisor tooth germ in the 20th week of development (late bell stage). (a-d) Detail of cervical loop (a) Moderate expression of IGF-2 in the cervical loop and adjacent parts of enamel epithelia; (b) Mild expression of PTEN in the cervical loop; (c) DAPI staining of nuclei; (d) Merging of a+b+c (Magnification: ×100; scale bar: 10 µm). (e-h) Detail of enamel organ at the site of future cusp tip (e) Moderate to strong expression of IGF-2 in pre-ameloblasts. Moderate expression of IGF-2 in stratum intermedium; (f) Moderate to strong expression of PTEN in pre-ameloblasts. Mild expression of PTEN in stratum intermedium; (g) DAPI staining of nuclei; (h) Merging of e+f+g (Magnification: ×100; scale bar: 10 µm). (i-l) Detail of dental papilla at the site of future cusp (i) Mild expression of IGF-2 in pre-odontoblasts; (j) Moderate expression of PTEN in pre-odontoblasts; (k) DAPI staining of nuclei; (l) Merging of i+j+k (Magnification: ×100; scale bar: 10 µm).
TABLE 1.
Expression of IGF-2 and PTEN in epithelial and mesenchymal parts of human incisor tooth germ between the 7th and 20th week of development.
| Tooth germ parts |
Epithelial |
Mesenchymal |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (weeks) | Factor | tb | dl | oee | iee | cl | sr | si | pa | dp | df | po |
| 7 | IGF-2 | ++ | + | / | / | / | / | / | / | − | / | / |
| PTEN | ++ | ++ | / | / | / | / | / | / | + | / | / | |
| 10 | IGF-2 | / | + | ++ | ++ | +++ | + | / | / | − | − | / |
| PTEN | / | − | ++ | + | +++ | + | / | / | ++ | ++ | / | |
| 12 | IGF-2 | / | + | ++ | ++ | ++ | ++ | / | / | − | − | / |
| PTEN | / | − | ++ | + | ++ | ++ | / | / | ++ | ++ | / | |
| 14 | IGF-2 | / | ++ | ++ | ++ | ++ | + | + | / | + | − | / |
| PTEN | / | − | ++ | ++ | + | + | + | / | ++ | ++ | / | |
| 20 | IGF-2 | / | + | ++ | ++ | ++ | + | ++ | +++ | + | − | + |
| PTEN | / | − | + | ++ | + | − | + | +++ | ++ | ++ | ||
Legends: tb – tooth bud; dl – dental lamina; oee – outer enamel epithelium; iee – inner enamel epithelium; cl – cervical loop; sr – stellate reticulum; si – stratum intermedium; pa – pre-ameloblasts; dp – dental papilla; df – dental follicle; po – pre-odontoblasts
Reactivity:
− (absent);
+ (mild);
++ (moderate);
+++ (strong);
/(absence of tissue)
In the 7th week of development, incisor tooth germ is in the bud stage of development. Moderate to strong expression of IGF-2 was observed in the whole tooth bud, especially in the cells at the bud's margin (prospective inner and outer enamel epithelia) and tip (prospective primary enamel knot) (Fig. 1a, d). The expression of IGF-2 in particular portions of dental lamina was either moderate or none, whereas oral epithelium expressed IGF-2 strongly. No expression of IGF-2 could be observed in mesenchymal tissue surrounding the bud (prospective dental papilla and dental follicle). The expression of PTEN in tooth bud, oral epithelium and dental lamina was similar to that described for IGF-2, with exception of moderate expression of PTEN throughout the dental lamina. Furthermore, narrow belt of mesenchymal tissue surrounding the tip of the bud, expressed PTEN moderately (Fig. 1b, d).
In the 10th week of development, incisor tooth germ is in the cap stage of development. The epithelial parts are now comprised of several distinctive tissues including inner and outer enamel epithelia enclosing the inner portion of prospective stellate reticulum. At this stage, dental papilla and dental follicle are discernible in mesenchymal parts of the tooth germ. Moderate expression of IGF-2 was observed in both enamel epithelia, however, at their confluence (prospective cervical loops) it was of strong intensity (Fig. 1e, h). IGF-2 was only mildly expressed in prospective stellate reticulum, whereas no expression of IGF-2 could be detected in dental papilla and dental follicle. PTEN displayed intensity of expression similar to that of IGF-2 with regard to outer enamel epithelium, prospective cervical loops and stellate reticulum and was mildly expressed in the inner enamel epithelium. However, throughout dental papilla and dental follicle PTEN was expressed moderately (Fig. 1f, h).
In the 12th week of development, incisor tooth germ is in the late cap stage of development. The expression pattern of IGF-2 remained the same as described in previous section with exception of slight increase of intensity in stellate reticulum, and decreased intensity in cervical loops (Fig. 1i, l). PTEN was still mildly expressed in the inner enamel epithelium, and moderately in enamel knot. Furthermore, area of dental papilla underlying the enamel knot expressed PTEN a bit more intensely than the rest of dental papilla cells (Fig. 1j, l).
In the 14th week of development, incisor tooth germ is in the early bell stage of development. Located between the inner enamel epithelium and stellate reticulum, newly differentiated cell layer of stratum intermedium can now be seen. Due to completion of its developmental role, enamel knot disintegrates by this stage. IGF-2 was moderately expressed by the inner enamel epithelium at the site of future cusp tip (location of prospective pre-ameloblasts), whereas its expression in stratum intermedium at the same site was mild (Fig. 2a, i). Also, moderate expression of IGF-2 could be seen in cervical loops and parts of outer enamel epithelium, while stellate reticulum barely expressed IGF-2 (Fig. 2a, d, e). Although the expression of PTEN displayed mostly similar pattern in enamel organ, only mild expression of PTEN was observed in cervical loops, whereas parts of dental papilla closely adjacent to inner enamel epithelium and cervical loops, expressed PTEN moderately (Fig. 2b, d, f, h). Furthermore, moderate to strong expression of PTEN was observed in cells of dental papilla at the site of future cusp tip (prospective pre-odontoblasts) (Fig. 2j, l).
In the 20th week of development, incisor tooth germ is in the late bell stage of development. The enamel organ significantly increases in size. Cells of the inner enamel epithelium at the site of future cusp tip differentiate into pre-ameloblasts, whereas the opposing cells of dental papilla begin to differentiate in pre-odontoblasts. While stratum intermedium is now compressed to only few layers of spindle-shaped cells, cervical loops continue to ingress into the underlying mesenchyme. IGF-2 and PTEN displayed the same expression pattern in proliferating cervical loops with a bit less intense expression of PTEN in surrounding and underlying mesenchyme (Fig. 3a, b, d) Both IGF-2 and PTEN were strongly expressed by future cusp tip pre-ameloblasts, whereas moderate expression of PTEN was predominant in pre-odontoblasts of dental papilla (Fig. 3i, j, l).
IGF-1R immunofluorescent staining
Immunofluorescent staining to IGF-1R was performed on human incisor tooth germs aged 7, 10, 12, 14 and 20 weeks of development (Fig. 4, Fig. 5, Table 2).
FIGURE 4.
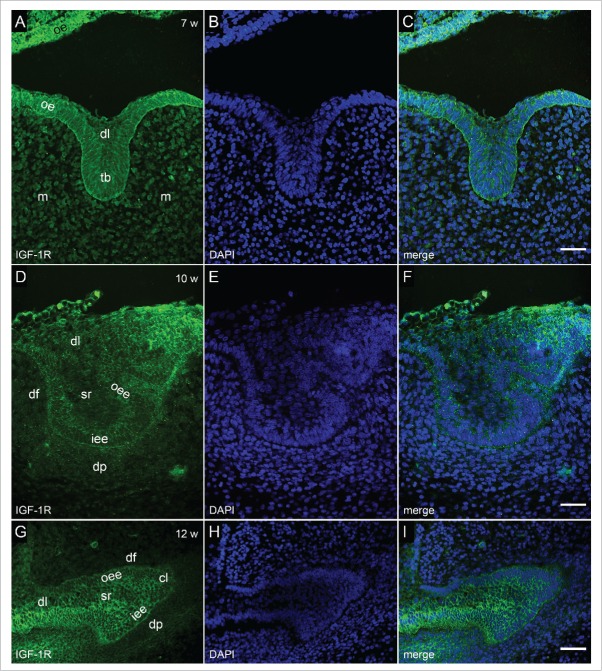
Immunofluorescent staining to IGF-1R in human incisor tooth germ between 7th and 12th week of development. (a-c) Human incisor tooth germ in the 7th week of development (bud stage) (a) Moderate expression of IGF-1R in tooth bud. Strong expression of IGF-1R in oral epithelium. Jaw mesenchyme is mildly positive to IGF-1R; (b) DAPI staining of nuclei; (c) Merging of a+b (Magnification ×40; scale bar: 25 µm). (d-f) Human incisor tooth germ in the 10th week of development (cap stage) (d) Moderate expression of IGF-1R in inner and outer enamel epithelia. Dental lamina, stellate reticulum and dental papilla are mildly positive to IGF-1R; (e) DAPI staining of nuclei; (f) Merging of d+e (Magnification: ×40; scale bar: 25 µm). (g-i) Human incisor tooth germ in the 12th week of development (late cap stage) (g) Strong expression of IGF-1R in the inner and outer enamel epithelia and prospective cervical loops. Stellate reticulum is moderately positive to IGF-1R, whereas dental expression of IGF-1R in dental lamina is mild; (h) DAPI staining of nuclei; (i) Merging of g+h (Magnification: ×40; scale bar: 25 µm).
FIGURE 5.
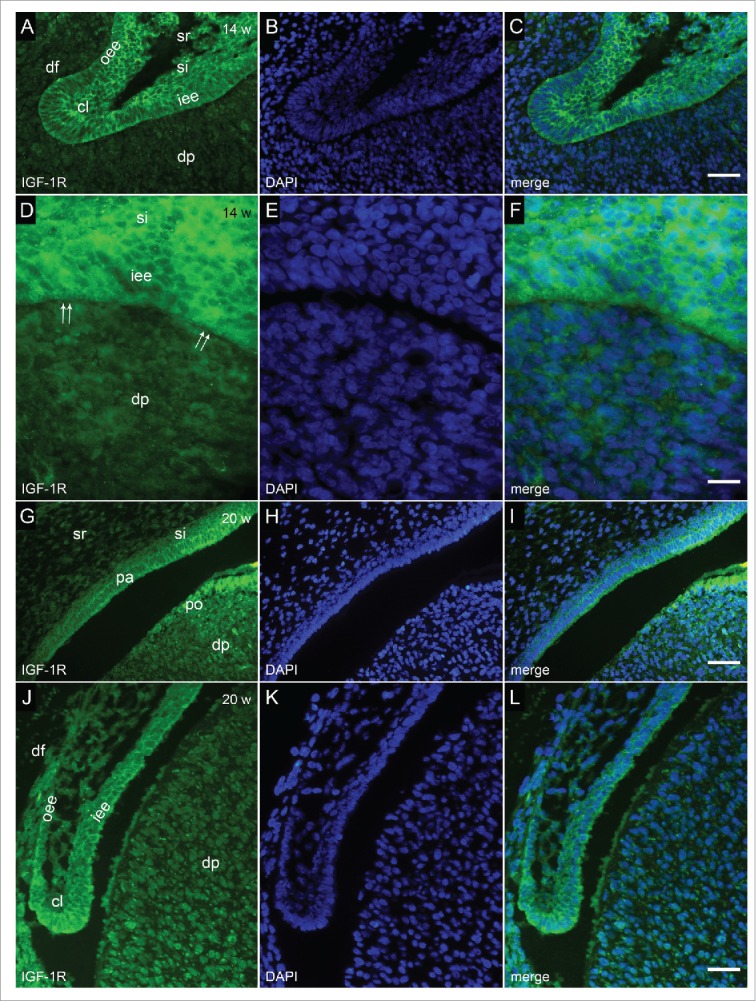
Immunofluorescent staining to IGF-1R in human incisor tooth germ in the 14th and 20th week of development (early and late bell stage). (a-c) Human incisor tooth germ in the 14th week of development (early bell stage) (detail of cervical loop) (a) Strong expression of IGF-1R in the cervical loop, adjacent portions of the inner and outer enamel epithelia and stratum intermedium; (b) DAPI staining of nuclei; (c) Merging of a+b (Magnification: ×40; scale bar: 25 µm). (d-f) Human incisor tooth germ in the 14th week of development (early bell stage) (detail at the site of future cusp tip) (d) Strong expression of IGF-1R in the cells of inner enamel epithelium (prospective pre-ameloblasts) and stratum intermedium. Underlying cells of dental papilla (prospective odontoblasts) are mildly positive to IGF-1R; (e) DAPI staining of nuclei; (f) Merging of d+e (Magnification: ×100; scale bar: 10 µm). (g-i) Human incisor tooth germ in the 20th week of development (late bell stage (detail at the site of future cusp tip) (g) Strong expression of IGF-1R in pre-ameloblasts and pre-odontoblasts. Moderate expression of IGF-1R in stratum intermedium and dental papilla. Stellate reticulum is mildly positive to IGF-1R; (h) DAPI staining of nuclei; (i) Merging of g+h (Magnification: ×20; scale bar: 40 µm). (j-l) Human incisor tooth germ in the 20th week of development (late bell stage) (detail of cervical loop) (j) Strong expression of IGF-1R in the cervical loop, adjacent portions of the inner and outer enamel epithelia and stratum intermedium. Dental papilla is moderately positive to IGF-1R; (k) DAPI staining of nuclei; (l) Merging of j+k (Magnification: ×40; scale bar: 25 µm).
TABLE 2.
Expression of IGF-1R and IGF-2R in epithelial and mesenchymal parts of human incisor tooth germ between the 7th and 20th week of development.
| Tooth germ parts |
Epithelial |
Mesenchymal |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (weeks) | Factor | tb | dl | oee | iee | cl | sr | si | pa | dp | df | po |
| 7 | IGF-1R | ++ | + | / | / | / | / | / | / | + | / | / |
| IGF-2R | ++* | + | / | / | / | / | / | / | ++* | / | / | |
| 10 | IGF-1R | / | + | ++ | ++ | ++ | + | / | / | + | − | / |
| IGF-2R | / | + | +* | ++* | ++* | – | / | / | ++* | + | / | |
| 12 | IGF-1R | / | + | +++ | +++ | +++ | + | / | / | + | − | / |
| IGF-2R | / | + | +* | ++* | +* | + | / | / | ++* | + | / | |
| 14 | IGF-1R | / | + | +++ | +++ | +++ | + | ++ | / | + | − | / |
| IGF-2R | / | ++* | +* | ++* | ++* | + | +* | / | ++* | + | / | |
| 20 | IGF-1R | / | + | ++ | +++ | +++ | + | ++ | +++ | ++ | − | +++ |
| IGF-2R | / | ++* | +* | ++* | +++* | + | ++* | ++* | ++* | + | +* | |
Legends: tb – tooth bud; dl – dental lamina; oee – outer enamel epithelium; iee – inner enamel epithelium; cl – cervical loop; sr – stellate reticulum; si – stratum intermedium; pa – pre-ameloblasts; dp – dental papilla; df – dental follicle; po – pre-odontoblasts
Reactivity:
– (absent);
+ (mild);
++ (moderate);
+++ (strong);
/(absence of tissue);
(expression in individual cells)
In the 7th week of development (bud stage), IGF-1R was moderately expressed throughout the incisor tooth bud, mildly in dental lamina, whereas its expression in oral epithelium gradually increased from moderate to strong and very strong as the epithelium stretches away from the tooth bud (Fig. 4a, c). In jaw mesenchyme surrounding the bud, IGF-1R was expressed mildly.
In the 10th week of development (cap stage), IGF-1R displayed moderate expression in inner and outer enamel epithelia, being mildly expressed in stellate reticulum and dental lamina (Fig. 4d, f). Slight increase in the intensity of expression could be observed in area of dental papilla closely adjacent to the inner enamel epithelium.
In the 12th week of development (late cap stage), IGF-1R increased the expression in both enamel epithelia, whereas moderate to strong expression of IGF-1R was present in enamel knot area and prospective cervical loops (Fig. 4g, i). The intensity of expression remained the same in dental lamina, stellate reticulum and dental papilla as observed in the 10th week of development.
In the 14th week of development (early bell stage), IGF-1R was strongly expressed by cervical loops, adjacent portions of inner and outer enamel epithelia, as well as in the inner enamel epithelium at the site of future cusp tip (prospective pre-ameloblasts) (Fig. 5a, c, d, f). IGF-1R was moderately expressed in stratum intermedium, while cells of dental papilla in the region of future cusp tip (prospective pre-odontoblasts), expressed IGF-1R mildly.
In the 20th week of development (late bell stage), similar pattern of expression of IGF-1R could be observed in inner enamel epithelium and stratum intermedium as described for the early bell stage (Fig. 5g, i). Mild expression of IGF-1R was seen in stellate reticulum, whereas it was strongly expressed in cervical loops (Fig. j, l). It should be noted that the overall expression of IGF-1R was increased throughout the dental papilla in comparison to previous developmental stage, being of moderate to strong intensity in pre-odontoblasts, and thus reflecting the intensity of expression of IGF-1R in pre-ameloblasts.
IGF-2R immunofluorescent staining
Immunofluorescent staining to IGF-2R was performed on human incisor tooth germs aged 7, 10, 12, 14 and 20 weeks of development (Fig. 6, Table 2).
FIGURE 6.
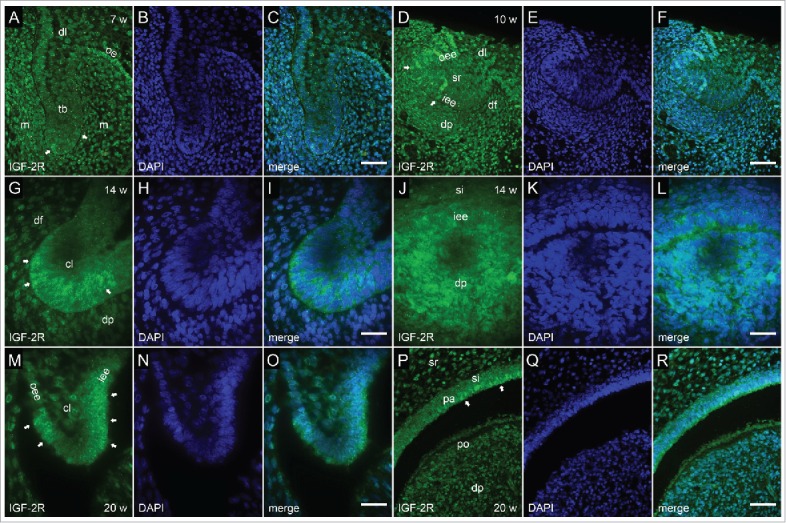
Immunofluorescent staining to IGF-2R in human incisor tooth germ between the 7th and 20th week of development (bud to late bell stage). (a-c) Human incisor tooth germ in the 7th week of development (bud stage) (a) Moderate expression of IGF-2R in sparsely distributed cells of the tooth bud and jaw mesenchyme; (b) DAPI staining of nuclei; (c) Merging of a+b (Magnification: ×40; scale bar: 25 µm). (d-f) Human incisor tooth germ in the 10th week of development (cap stage) (d) Moderate expression of IGF-2R in sparsely distributed cells of enamel organ and dental papilla; (e) DAPI staining of nuclei; (f) Merging of d+e (Magnification: ×40; scale bar: 25 µm). (g-i) Human incisor tooth germ in the 14th week of development (early bell stage) (detail of cervical loop) (g) Moderate expression of IGF-2R in the cervical loop; (h) DAPI staining of nuclei; (i) Merging of g+h (Magnification: ×100; scale bar: 10 µm). (j-l) Human incisor tooth germ in the 14th week of development (early bell stage) (detail at the site of future cusp tip) (j) Mild to moderate expression of IGF-2R by individual cells of the inner enamel epithelium and underlying dental papilla; (k) DAPI staining of nuclei; (l) Merging of j+k (Magnification: ×100; scale bar: 10 µm). (m-o) Human incisor tooth germ in the 20th week of development (late bell stage) (detail of cervical loop) (m) Moderate to strong expression of IGF-2R in cervical loop. Individual cells at the very tip of the cervical loop are negative to IGF.2R; (n) DAPI staining of nuclei; (o) Merging of m+n (Magnification: 100×; scale bar: 10 µm). (p-r) Human canine tooth germ in the 20th week of development (late bell stage) (detail at the site of future cusp tip) (p) Moderate expression of IGF-2R in small clusters of pre-ameloblasts. Pre-odontoblasts are mostly negative to IGF-2R; (q) DAPI staining of nuclei; (r) Merging of p+q (Magnification: 100×; scale bar: 10 µm).
In the 7th week of development (bud stage), IGF-2R was moderately expressed by individual cells sparsely distributed throughout the incisor tooth bud and surrounding jaw mesenchyme. Expression of IGF-2R was mild in dental lamina, whereas its expression in a strip of adjacent oral epithelium was of moderate intensity (Fig. 6a, c).
In the 10th week of development (cap stage), IGF-2R was expressed either mildly or moderately by individual cells located in inner and outer enamel epithelia, dental papilla and dental follicle (Fig. 6d, f). Similar scattered expression pattern of IGF-2R was observed in epithelial and mesenchymal parts of human incisor tooth germ in the 12th week of development (data not shown).
In the 14th and 20th week of development (early and late bell stage) IGF-2R displayed more cohesive expression patterns in cervical loops (Fig. 6g, i; Fig. 6m, o). At the site of future cusp tip, it was expressed moderately by individual cells of inner enamel epithelium and underlying dental papilla (Fig. 6j, l), as well as by some pre-ameloblasts (Fig. 6p, r). Pre-odontoblasts of dental papilla were mostly negative to IGF-2R.
DISCUSSION
Opposing functional roles of IGF-2 and PTEN with regard to promotion or suppression of cell proliferation and survival, led us to hypothesis that these factors might have mutually exclusive expression patterns in developing human tooth germs. Thus, IGF-2 was not expected to be co-expressed with PTEN in highly proliferative odontogenic tissues (i.e. tooth bud, cervical loops), or regions of odontogenic tissues which display transient proliferative activity (both in enamel organ and mesenchymal parts of the tooth germ). Present findings do not support such hypothesis with exception of those on expression patterns of IGF-2 and PTEN in dental papilla. Throughout the investigated period, dental papilla was positive to PTEN, while almost no expression of IGF-2 could be observed. This might reflect the notion that proliferative spurt in dental papilla significantly lags behind the proliferative spurt occurring in enamel organ.1,34 However, continuous co-expression of these factors in enamel organ seems to be a common feature of developing human tooth germ. In development and malignant alteration, IGF-2 and PTEN have been reported to co-express as they act in tandem through a negative feedback loop, where differences in intensity of either of these 2 factors' expression affect the overall proliferative activity in particular tissue.11 Similar effects can be seen in enamel organ's primary enamel knot, pre-ameloblasts at the site of future cusp tip and cervical loops, as all of these tissues co-express IGF-2 and PTEN while displaying either none, low or high proliferative activity, respectively.
While expressing IGF-2 with the same intensity as the inner enamel epithelium, cells of the primary enamel knot increased expression of PTEN during the late cap stage. These are among the first cells in enamel organ to arrest the cell cycle as they anchor at the site of inner enamel epithelium folding (future cusp tip). During the late cap stage, removal of the primary enamel knot is already underway in order to be completed by the beginning of the early bell stage. It is still unclear whether this occurs exclusively due to apoptosis, cell migration or combination of the 2 processes.35–37 There are indications that apoptosis might be a prime mechanism of the primary enamel knot removal. Firstly, in addition to anti-proliferative effects, PTEN is also known to attenuate cellular migration.17,38, 39 Thus, the increase of expression of PTEN in the primary enamel knot prior to its removal might also be suggestive of reduced migratory potential of the primary enamel knot cells. Secondly, even though the primary enamel knot expressed IGF-2, it was negative to IGF-1 as previously described.28 Since IGF-1 and −2 promote cell survival, this might point to an increasing susceptibility of the primary enamel knot cells to apoptosis.25,40,41 Thirdly, in contrast to developing premolars and molars, incisor tooth germs have only a single site of inner enamel epithelium folding provided by the primary enamel knot. Absent need of secondary enamel knots (which serve as sites of additional epithelial folding and are believed to be partly populated by the migrating cells of the primary enamel knot), it is plausible that the primary enamel knot of incisor tooth germ could be terminated exclusively by apoptosis.35
Highly proliferative confluence regions of inner and outer enamel epithelia (prospective cervical loops) and cervical loops co-expressed IGF-2 and PTEN in somewhat different manner than the primary enamel knot. Namely, cervical loops displayed slight increase of expression of IGF-2 with comparable decrease of expression of PTEN. These alterations in expression of IGF-2 and PTEN might be required for directed ingrowth of cervical loops into the underlying mesenchymal tissue, which ultimately causes elongation of enamel organ during the course of early and late bell stage. Similar example of negative feedback loop between IGF-2 and PTEN has been proposed to be crucial for regulation of terminal length of developing mammary gland ducts.23 On the other hand, differentiating pre-ameloblasts of the future cusp tip started to express IGF-2 and PTEN with equally strong intensity by the late bell stage, whereas the population of PTEN-positive cells in dental papilla directly opposed to pre-ameloblasts, began to express IGF-2. Such co-expression pattern of IGF-2 and PTEN is indicative to delayed onset of differentiation of pre-odontoblasts.
The expression of IGF-1R in human incisor tooth germ was ubiquitous throughout the investigated period, meaning that both epithelial and mesenchymal tissues of the human incisor tooth germ possess the ability to harness the effects of IGF-1 and −2.42 Although IGFs are exclusively expressed in enamel organ, the intensity of expression of IGF-1R can still be positively correlated with that, since IGF-1R was strongly expressed in enamel organ in contrast to only mild expression in dental papilla and dental follicle. It should also be noted that during the bud stage, non-odontogenic oral epithelium expressed IGF-1R more strongly than the tooth bud itself. The scope of this study enables us only to speculate whether downregulation of IGF-1R normally takes place at the onset of tooth development in particular regions of embryonic oral epithelium with odontogenic potential. Similarly to IGF-1R, IGF-2R was expressed in all tissues of human tooth germ. However, the distribution and intensity of its expression in enamel organ were rather erratic throughout the investigated period and could not be closely correlated with that of IGF-2. These findings indicate that although most tissues in human tooth germ do possess the ability to suppress IGF-2 via IGF-2R, negative regulation of IGF-2 is simultaneously executed by alternative mechanisms.14,25
In conclusion, described features of expression of IGF-2 and PTEN imply versatile roles and synchronous involvement of these factors in stage-specific spatial patterning of basic cellular processes (proliferation, differentiation, cell survival) during human tooth development. Expression pattern of IGF-1R seemed to be more context-dependent than that of IGF-2R, and was, in contrast, well correlated with the presence of IGF-1 and −2 in tissues of developing human incisor tooth germs. Unfortunately, data presented here allow us only to speculate about the acting modes of delivery of IGFs (autocrine/paracrine) in odontogenic tissues. Therefore, further studies on distribution of IGFs mRNA transcripts and IGFBPs in human tooth germs are still needed in order to fully disclose the nature of involvement of IGF-axis during human odontogenesis.
MATERIALS AND METHODS
Tissue procurement and processing
For this study, 10 human fetuses aged between 7 and 20 weeks of development were obtained after spontaneous abortions and tubal pregnancies from the Department of Pathology, University Hospital in Split, Croatia. The samples were stored in the form of histological sections and kept at −24°C as a part of archive human collection of the human material belonging to the Department of Anatomy, Histology and Embryology, School of Medicine, University of Split. Approval for tissue processing was given by the Ethical and Drug Committee of University Hospital in Split (Class: 033–081/11–03/0005, No: 2181-198-03-04/10-11-0024, 2011) in accordance with Helsinki Declaration.43 For assessment of gestational age of the human fetuses, external measurements were used.44 Analysis was performed only on foetal tissues from head areas and/or parts of jaws containing tooth germs. Following tissue fixation with 4% paraformaldehyde in phosphate-buffered saline (PBS) and dehydration in graded ethanol dilutions, foetal tissues were paraffin-embedded and cut in frontal or transversal planes (serial 7 µm sections). Tissue sections were then mounted on glass slides for microscopic examination using Olympus BX51 light microscope (Olympus, Tokyo, Japan). Once the adequate tissue preservation was confirmed by the examination of control sections stained with haematoxylin and eosin, sections were selected for single and double immunofluorescent staining.
Double immunofluorescent staining
Tissue sections were deparaffinised in xylene and descending ethanols, rehydrated in distilled water and left incubating for 30 min in 0.1% H2O2 for suppression of endogenous peroxidase activity. After that, sections were washed in PBS and put in sodium citrate buffer for 15 min heating at 95°C in microwave oven. Prior the 24 hrs incubation in dark chamber with primary antibodies, sections were left to cool down to room temperature. Primary antibodies used for double immunofluorescent staining were as follows: goat monoclonal anti-human IGF-2 antibody (1:500; ab123812 Abcam, UK), and mouse monoclonal anti-human PTEN antibody (1:200; MAB3847, R&D Systems, MN, USA). Rabbit polyclonal anti-human IGF-1R antibody (1:50; ab39398 , Abcam, UK) and mouse monoclonal anti-human IGF-2R antibody (1:100; ab2733, Abcam, UK) were used for single immunofluorescent staining. Once the incubation with primary antibodies was done, sections were primed for 1 hr incubation in dark chamber with secondary antibodies as follows: donkey anti-goat Alexa Fluor 594 (1:300; ab150136, Abcam, UK), donkey anti-mouse Alexa Fluor 488 (1:300; ab150105, Abcam, UK), donkey anti-mouse Alexa Fluor 488 (1:300; ab150105, Abcam, UK), and donkey anti-rabbit Streptavidin Alexa Fluor 488 (1:300; Invitrogen Molecular Probes Inc., Eugene, OR, USA). When the incubation in secondary antibody/antibodies was over, sections had to be washed in PBS and counterstained with 4′6-diamidino-2-phenylindole (DAPI) for 2 min to stain nuclei. Sections were once again rinsed in PBS, air-dried, mounted and cover-slipped (Immuno-Mount; Shandon, Pittsburgh, PA). Antibody specificity control was also performed by omission of primary antibodies from the staining procedure.
Immunofluorescence images were made by SPOT Insight camera (Diagnostic Instruments, USA), mounted on Olympus BX61 microscope (Olympus, Tokyo, Japan). Acquisition and merging of images were performed by CellA® and SpotAdvanced® software followed by the image-plate assembly in Adobe Photoshop® CS6. Expression patterns of all antibodies used in the present study were analyzed semi-quantitatively (Table 1, Table 2).
ABBREVIATIONS
- IGF-2
Insulin-Like Growth Factor 2
- IGF-1R
Insulin-Like Growth Factor 1 Receptor
- IGF-2R
Insulin-Like Growth Factor 2 Receptor
- PTEN
Phosphatase and Tensin Homolog
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENT
The authors wish to thank Assist. Prof. Natalija Filipovic, MD PhD, for expert technical assistance.
Funding
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (grant no. 021-2160528-0507, main investigator prof. Mirna Saraga-Babic, MD PhD).
AUTHOR CONTRIBUTIONS
Darko Kero designed the study, performed selection and double immunofluorescent staining of tissue sections (IGF-2/PTEN), acquired, interpreted and processed data, assessed the literature review reports and wrote the manuscript. Livia Cigic performed double immunofluorescent staining (IGF-2/PTEN), acquired and interpreted data, reviewed part of the literature (IGF-2, PTEN/functional studies) and wrote Materials and Methods and Results sections of the manuscript. Ivana Medevedec Mikic performed single immunofluorescent staining (IGF-1R, IGF-2R), acquired and interpreted data, reviewed part of the literature (IGF-1R, IGF-2R/functional studies) and wrote Results section of the manuscript. Tea Galic performed single immunofluorescent staining (IGF-1R), reviewed part of the literature (IGF-2 and PTEN in organogenesis and odontogenesis), and was in charge for proofreading of the manuscript. Mladen Cubela performed single immunofluorescent staining (IGF-2R), reviewed part of the literature (IGF-1R and IGF-2R in organogenesis and odontogenesis), and was in charge for prrofreading of the manuscript. Katarina Vukojevic and Mirna Saraga Babic co-designed the study, provided inputs on staining procedures and data interpretation, revised and edited the manuscript.
REFERENCES
- [1].Kalibovic Govorko D, Becic T, Vukojevic K, Mardesic-Brakus S, Biocina-Lukenda D, Saraga-Babic M. Spatial and temporal distribution of Ki-67 proliferation marker, Bcl-2 and Bax proteins in the developing human tooth. Arch Oral Biol 2010; 55:1007-16; PMID:20732674; http://dx.doi.org/ 10.1016/j.archoralbio.2010.07.024 [DOI] [PubMed] [Google Scholar]
- [2].Kero D, Kalibovic Govorko D, Vukojevic K, Cubela M, Soljic V, Saraga-Babic M. Expression of cytokeratin 8, vimentin, syndecan-1 and Ki-67 during human tooth development. J Mol Histol 2014; 45:627-40; PMID:25120060; http://dx.doi.org/ 10.1007/s10735-014-9592-1 [DOI] [PubMed] [Google Scholar]
- [3].Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 2000; 92:19-29; PMID:10704885; http://dx.doi.org/ 10.1016/S0925-4773(99)00322-6 [DOI] [PubMed] [Google Scholar]
- [4].Bei M. Molecular genetics of tooth development. Curr Opin Genet Dev 2009; 19:504-10; PMID:19875280; http://dx.doi.org/ 10.1016/j.gde.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tucker AS, Sharpe PT. Molecular genetics of tooth morphogenesis and patterning: the right shape in the right place. J Dental Res 1999; 78:826-34; PMID:10326726; http://dx.doi.org/ 10.1177/00220345990780040201 [DOI] [PubMed] [Google Scholar]
- [6].Petrovic D. The role of vascular endothelial growth factor gene as the genetic marker of atherothrombotic disorders and in the gene therapy of coronary artery disease. Cardiovasc Hematol Agents Med Chem 2010; 8:47-54; PMID:20210775; http://dx.doi.org/ 10.2174/187152510790796183 [DOI] [PubMed] [Google Scholar]
- [7].Thesleff I, Mikkola M. The role of growth factors in tooth development. Int Rev Cytol 2002; 217:93-135; PMID:12019566; http://dx.doi.org/ 10.1016/S0074-7696(02)17013-6 [DOI] [PubMed] [Google Scholar]
- [8].Lesot H, Brook AH. Epithelial histogenesis during tooth development. Arch Oral Biol 2009; 54 Suppl 1:S25-33; PMID:18656852; http://dx.doi.org/ 10.1016/j.archoralbio.2008.05.019 [DOI] [PubMed] [Google Scholar]
- [9].Witte DP, Bove KE. Beckwith-Wiedemann syndrome and the insulin-like growth factor-II gene. Does the genotype explain the phenotype? Am J Pathol 1994; 145:762-5; PMID:7943167 [PMC free article] [PubMed] [Google Scholar]
- [10].Abu-Amero S, Monk D, Frost J, Preece M, Stanier P, Moore GE. The genetic aetiology of Silver-Russell syndrome. J Med Genet 2008; 45:193-9; PMID:18156438; http://dx.doi.org/ 10.1136/jmg.2007.053017 [DOI] [PubMed] [Google Scholar]
- [11].Church DN, Phillips BR, Stuckey DJ, Barnes DJ, Buffa FM, Manek S, Clarke K, Harris AL, Carter EJ, Hassan AB. Igf2 ligand dependency of Pten(+/-) developmental and tumour phenotypes in the mouse. Oncogene 2012; 31:3635-46; PMID:22120709; http://dx.doi.org/ 10.1038/onc.2011.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agrogiannis GD, Sifakis S, Patsouris ES, Konstantinidou AE. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol Med Rep 2014; 10:579-84; PMID:24859417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 2013; 59:240-9; PMID:23257688; http://dx.doi.org/ 10.1159/000343995 [DOI] [PubMed] [Google Scholar]
- [14].Livingstone C. IGF2 and cancer. Endocrine-related cancer 2013; 20:R321-39; PMID:24080445; http://dx.doi.org/ 10.1530/ERC-13-0231 [DOI] [PubMed] [Google Scholar]
- [15].Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev 2005; 16:421-39; PMID:15936977; http://dx.doi.org/ 10.1016/j.cytogfr.2005.04.004 [DOI] [PubMed] [Google Scholar]
- [16].Matsumoto A, Harada H, Saito M, Taniguchi A. Induction of insulin-like growth factor 2 expression in a mesenchymal cell line co-cultured with an ameloblast cell line. In Vitro Cell Dev Biol Anim 2011; 47:675-80; PMID:21959847; http://dx.doi.org/ 10.1007/s11626-011-9456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci 2014; 39:183-90; PMID:24656806; http://dx.doi.org/ 10.1016/j.tibs.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patel M, Gomez NC, McFadden AW, Moats-Staats BM, Wu S, Rojas A, Sapp T, Simon JM, Smith SV, Kaiser-Rogers K, et al.. PTEN deficiency mediates a reciprocal response to IGFI and mTOR inhibition. Mol Cancer Res 2014; 12:1610-20; PMID:24994750; http://dx.doi.org/ 10.1158/1541-7786.MCR-14-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zu K, Martin NE, Fiorentino M, Flavin R, Lis RT, Sinnott JA, Finn S, Penney KL, Ma J, Fazli L, et al.. Protein expression of PTEN, insulin-like growth factor I receptor (IGF-IR), and lethal prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 2013; 22:1984-93; http://dx.doi.org/ 10.1158/1055-9965.EPI-13-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Angadi PV, Krishnapillai R. Evaluation of PTEN immunoexpression in oral submucous fibrosis: role in pathogenesis and malignant transformation. Head Neck Pathol 2012; 6:314-21; PMID:22392409; http://dx.doi.org/ 10.1007/s12105-012-0341-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem 1998; 273:29864-72; PMID:9792703; http://dx.doi.org/ 10.1074/jbc.273.45.29864 [DOI] [PubMed] [Google Scholar]
- [22].Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet 2002; 70:829-44; PMID:11875759; http://dx.doi.org/ 10.1086/340026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moorehead RA, Hojilla CV, De Belle I, Wood GA, Fata JE, Adamson ED, Watson KL, Edwards DR, Khokha R. Insulin-like growth factor-II regulates PTEN expression in the mammary gland. J Biol Chem 2003; 278:50422-7; PMID:14517213; http://dx.doi.org/ 10.1074/jbc.M306894200 [DOI] [PubMed] [Google Scholar]
- [24].Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development 1991; 111:105-15; PMID:2015788 [DOI] [PubMed] [Google Scholar]
- [25].Ferguson MW, Sharpe PM, Thomas BL, Beck F. Differential expression of insulin-like growth factors I and II (IGF I and II), mRNA, peptide and binding protein 1 during mouse palate development: comparison with TGF beta peptide distribution. J Anat 1992; 181 (Pt 2):219-38; PMID:1284245 [PMC free article] [PubMed] [Google Scholar]
- [26].Han VK, D'Ercole AJ, Lund PK. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science 1987; 236:193-7; PMID:3563497; http://dx.doi.org/ 10.1126/science.3563497 [DOI] [PubMed] [Google Scholar]
- [27].Bellone C, Barni T, Pagni L, Balboni GC, Vannelli GB. [Growth factors in human tooth development]. Boll Soc Ital Biol Sper 1990; 66:231-8; PMID:2165788 [PubMed] [Google Scholar]
- [28].Kero D, Kalibovic Govorko D, Medvedec Mikic I, Vukojevic K, Cigic L, Saraga-Babic M. Analysis of expression patterns of IGF-1, caspase-3 and HSP-70 in developing human tooth germs. Arch Oral Biol 2015; 60:1533-44; PMID:26276267; http://dx.doi.org/ 10.1016/j.archoralbio.2015.07.004 [DOI] [PubMed] [Google Scholar]
- [29].Beck F, Samani NJ, Penschow JD, Thorley B, Tregear GW, Coghlan JP. Histochemical localization of IGF-I and -II mRNA in the developing rat embryo. Development 1987; 101:175-84; PMID:3449366 [DOI] [PubMed] [Google Scholar]
- [30].Caton J, Bringas P Jr., Zeichner-David M. IGFs increase enamel formation by inducing expression of enamel mineralizing specific genes. Arch Oral Biol 2005; 50:123-9; PMID:15721138; http://dx.doi.org/ 10.1016/j.archoralbio.2004.11.012 [DOI] [PubMed] [Google Scholar]
- [31].Joseph BK, Harbrow DJ, Sugerman PB, Smid JR, Savage NW, Young WG. Ameloblast apoptosis and IGF-1 receptor expression in the continuously erupting rat incisor model. Apoptosis 1999; 4:441-7; PMID:14634328; http://dx.doi.org/ 10.1023/A:1009600409421 [DOI] [PubMed] [Google Scholar]
- [32].Gimm O, Attie-Bitach T, Lees JA, Vekemans M, Eng C. Expression of the PTEN tumour suppressor protein during human development. Human Mol Genet 2000; 9:1633-9; PMID:10861290; http://dx.doi.org/ 10.1093/hmg/9.11.1633 [DOI] [PubMed] [Google Scholar]
- [33].Luukko K, Ylikorkala A, Tiainen M, Makela TP. Expression of LKB1 and PTEN tumor suppressor genes during mouse embryonic development. Mech Dev 1999; 83:187-90; PMID:10381580; http://dx.doi.org/ 10.1016/S0925-4773(99)00050-7 [DOI] [PubMed] [Google Scholar]
- [34].Kero D, Novakovic J, Vukojevic K, Petricevic J, Kalibovic Govorko D, Biocina-Lukenda D, Saraga-Babic M. Expression of Ki-67, Oct-4, gamma-tubulin and alpha-tubulin in human tooth development. Arch Oral Biol 2014; 59:1119-29; PMID:25062118; http://dx.doi.org/ 10.1016/j.archoralbio.2014.05.025 [DOI] [PubMed] [Google Scholar]
- [35].Coin R, Lesot H, Vonesch JL, Haikel Y, Ruch JV. Aspects of cell proliferation kinetics of the inner dental epithelium during mouse molar and incisor morphogenesis: a reappraisal of the role of the enamel knot area. Int J Dev Biol 1999; 43:261-7; PMID:10410906 [PubMed] [Google Scholar]
- [36].Lesot H, Vonesch JL, Peterka M, Tureckova J, Peterkova R, Ruch JV. Mouse molar morphogenesis revisited by three-dimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int J Dev Biol 1996; 40:1017-31; PMID:8946249 [PubMed] [Google Scholar]
- [37].Matalova E, Sharpe PT, Lakhani SA, Roth KA, Flavell RA, Setkova J, Misek I, Tucker AS. Molar tooth development in caspase-3 deficient mice. Int J Dev Biol 2006; 50:491-7; PMID:16586350; http://dx.doi.org/ 10.1387/ijdb.052117em [DOI] [PubMed] [Google Scholar]
- [38].Hopkins BD, Parsons RE. Molecular pathways: intercellular PTEN and the potential of PTEN restoration therapy. Clin Cancer Res 2014; 20:5379-83; PMID:25361917; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci 2001; 114:2375-82; PMID:11559746 [DOI] [PubMed] [Google Scholar]
- [40].Delaney CL, Cheng HL, Feldman EL. Insulin-like growth factor-I prevents caspase-mediated apoptosis in Schwann cells. J Neurobiol 1999; 41:540-8; PMID:10590177; http://dx.doi.org/ 10.1002/(SICI)1097-4695(199912)41:4%3c540::AID-NEU9%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- [41].Joseph BK, Savage NW, Harbrow DJ, Young WG. Non-expression of insulin-like growth factor-I receptor is associated with apoptosis: an ultrastructural study on rat ameloblasts. Apoptosis 1997; 2:471-7; PMID:14646530; http://dx.doi.org/ 10.1023/A:1026474128296 [DOI] [PubMed] [Google Scholar]
- [42].Rother KI, Accili D. Role of insulin receptors and IGF receptors in growth and development. Pediatric Nephrol 2000; 14:558-61; PMID:10912518; http://dx.doi.org/ 10.1007/s004670000351 [DOI] [PubMed] [Google Scholar]
- [43].Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ 2008; 86:650-2; PMID:18797627; http://dx.doi.org/ 10.2471/BLT.08.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].O'Rahilly R. Guide to the staging of human embryos. Anat Anz 1972; 130:556-9; PMID:5048213 [PubMed] [Google Scholar]