Abstract
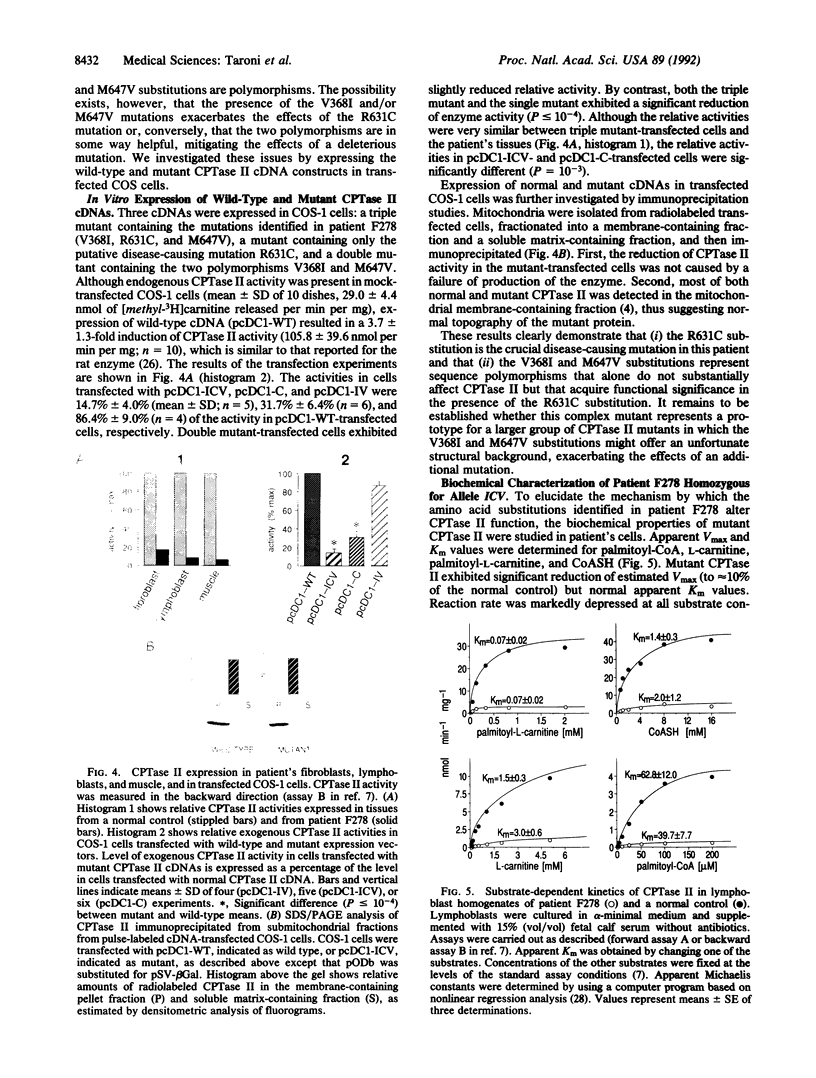
Deficiency of carnitine palmitoyltransferase II (CPTase II; palmitoyl-CoA:L-carnitine O-palmitoyltransferase, EC 2.3.1.21) is a clinically heterogeneous autosomal recessive disorder of energy metabolism. We studied the molecular basis of CPTase II deficiency in an early-onset patient presenting with hypoketotic hypoglycemia and cardiomyopathy. cDNA and genomic DNA analysis demonstrated that the patient was homozygous for a mutant CPTase II allele (termed ICV), which carried three missense mutations: a G-1203----A transition, predicting a Val-368----Ile substitution (V368I); a C-1992----T transition, predicting an Arg-631----Cys substitution (R631C); and an A-2040----G transition, predicting a Met-647----Val substitution (M647V). Genomic DNA analysis of family members showed that the mutations cosegregated with the disease in the family. However, screening of 59 healthy controls demonstrated that both the V368I and M647V mutations are sequence polymorphisms with allele frequencies of 0.5 and 0.25, respectively. By contrast, the R631C substitution was not detected in 22 normal individuals or in 12 of 14 CPTase II-deficient patients with the adult muscular form. Notably, 2 adult CPTase II-deficient patients were heterozygous for the ICV allele, thus suggesting compound heterozygosity for this and a different mutant allele. The consequences of the three mutations on enzyme activity were investigated by expressing normal and mutated CPTase II cDNAs in COS cells. The R631C substitution drastically depressed the catalytic activity of CPTase II, thus confirming that this is the crucial mutation. Interestingly, the V368I and M647V substitutions, which did not affect enzyme activity alone, exacerbated the effects of the R631C substitution. Biochemical characterization of mutant CPTase II in patient's cells showed that the mutations are associated with (i) severe reduction of Vmax (approximately 90%), (ii) normal apparent Km values, and (iii) decreased protein stability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Demaugre F., Bonnefont J. P., Colonna M., Cepanec C., Leroux J. P., Saudubray J. M. Infantile form of carnitine palmitoyltransferase II deficiency with hepatomuscular symptoms and sudden death. Physiopathological approach to carnitine palmitoyltransferase II deficiencies. J Clin Invest. 1991 Mar;87(3):859–864. doi: 10.1172/JCI115090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaugre F., Bonnefont J. P., Mitchell G., Nguyen-Hoang N., Pelet A., Rimoldi M., Di Donato S., Saudubray J. M. Hepatic and muscular presentations of carnitine palmitoyl transferase deficiency: two distinct entities. Pediatr Res. 1988 Sep;24(3):308–311. doi: 10.1203/00006450-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Liver mitochondria contain an ATP-dependent, vanadate-sensitive pathway for the degradation of proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1869–1873. doi: 10.1073/pnas.79.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato S., Castiglione A., Rimoldi M., Cornelio F., Vendemia F., Cardace G., Bertagnolio B. Heterogeneity of carnitine-palmitoyltransferase deficiency. J Neurol Sci. 1981 May;50(2):207–215. doi: 10.1016/0022-510x(81)90167-2. [DOI] [PubMed] [Google Scholar]
- DiDonato S., Cornelio F., Pacini L., Peluchetti D., Rimoldi M., Spreafico S. Muscle carnitine palmityltransferase deficiency: a case with enzyme deficiency in cultured fibroblasts. Ann Neurol. 1978 Nov;4(5):465–467. doi: 10.1002/ana.410040513. [DOI] [PubMed] [Google Scholar]
- DiDonato S., Gellera C., Peluchetti D., Uziel G., Antonelli A., Lus G., Rimoldi M. Normalization of short-chain acylcoenzyme A dehydrogenase after riboflavin treatment in a girl with multiple acylcoenzyme A dehydrogenase-deficient myopathy. Ann Neurol. 1989 May;25(5):479–484. doi: 10.1002/ana.410250510. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro G., Taroni F., Rocchi M., Liras Martin A., Colombo I., Tarelli G. T., DiDonato S. cDNA cloning, sequence analysis, and chromosomal localization of human carnitine palmitoyltransferase. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10981–10981. doi: 10.1073/pnas.88.23.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro G., Taroni F., Rocchi M., Martin A. L., Colombo I., Tarelli G. T., DiDonato S. cDNA cloning, sequence analysis, and chromosomal localization of the gene for human carnitine palmitoyltransferase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):661–665. doi: 10.1073/pnas.88.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug G., Bove K. E., Soukup S. Lethal neonatal multiorgan deficiency of carnitine palmitoyltransferase II. N Engl J Med. 1991 Dec 26;325(26):1862–1864. doi: 10.1056/NEJM199112263252607. [DOI] [PubMed] [Google Scholar]
- Isaya G., Fenton W. A., Hendrick J. P., Furtak K., Kalousek F., Rosenberg L. E. Mitochondrial import and processing of mutant human ornithine transcarbamylase precursors in cultured cells. Mol Cell Biol. 1988 Dec;8(12):5150–5158. doi: 10.1128/mcb.8.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Taroni F., Rosenberg L. E. The precursor of the biotin-binding subunit of mammalian propionyl-CoA carboxylase can be translocated into mitochondria as apo- or holoprotein. J Biol Chem. 1991 Jul 15;266(20):13267–13271. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner R., Tilmans I., Mischke D. Avoiding strand reassociation in direct sequencing of double-stranded PCR products with thermolabile polymerases. PCR Methods Appl. 1992 Feb;1(3):193–194. doi: 10.1101/gr.1.3.193. [DOI] [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Cox W. F., Schroeder J. G., Liao S. T., Foster D. W., McGarry J. D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J Biol Chem. 1990 Jun 25;265(18):10714–10719. [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Sen A., Cox W. F., McPhaul M. J., Slaughter C. A., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver mitochondrial carnitine palmitoyltransferase II. J Biol Chem. 1990 Jun 25;265(18):10720–10725. [PubMed] [Google Scholar]
- Zierz S., Engel A. G. Regulatory properties of a mutant carnitine palmitoyltransferase in human skeletal muscle. Eur J Biochem. 1985 May 15;149(1):207–214. doi: 10.1111/j.1432-1033.1985.tb08913.x. [DOI] [PubMed] [Google Scholar]