ABSTRACT
Dedifferentiated fat cells show great promises as a novel cell source for stem cell research. It has many advantages when used for cell–based therapeutics including abundance, pluripotency, and safety. However, there are many obstacles researchers need to overcome to make the next big move in DFAT cells research. In this review, we summarize the current main challenges in DFAT cells research including cell culture purity, phenotypic properties, and dedifferentiation mechanisms. The common methods to produce DFAT cells as well as the cell purity issue during DFAT cell production have been introduced. Current approaches to improve DFAT cell purity have been discussed. The phenotypic profile of DFAT cells have been listed and compared with other stem cells. Further studies on elucidating the underlying dedifferentiation mechanisms will dramatically advance DFAT cell research.
KEYWORDS: adult stem cells, culture purity, dedifferentiated fat cells, dedifferentiation, surface marker
INTRODUCTION
Dedifferentiated fat (DFAT) cells, derived from mature adipocytes, show great promises as a novel cell source for stem cell research. Accumulating evidence has shown that lipid–filled adipocytes, which are the most abundant cell type in any adipose tissue, can be reprogramed into multipotent stem cells named DFAT cells through a traditional ceiling culture method.1 So far, DFAT cells have been derived from many species including mouse, rat, pig, and human.1-3 DFAT cells exhibit robust proliferation capacity and multi–lineage differentiation potentials. Kishimoto N et al. showed that human DFAT cells have higher osteoblastic differentiation ability than adipose derived stem cells (ASCs).4 Jumabay M et al. demonstrated that mouse and human DFAT cells, derived from adipose tissue and lipospirate, respectively, could differentiate into vascular endothelial cells (ECs) both in vitro and in vivo.5 In a rat acute myocardial infarction model, transplanted rat DFAT cells expressed cardiac sarcomeric actin and differentiated to cardiomyocyte–like cells.2 Ohta Y et al. reported that transplanted rat DFAT cells expressed neuronal marker, β III tubulin, and led to marked functional recovery from spinal cord injury (SCI)–induced motor dysfunction in rats.6 Additional studies also reported that DFAT cells could differentiate into chondrocytes, skeletal myocytes, and smooth muscle cells under appropriate culture conditions.7-9
DFAT cells have unique advantages when used as cell source for the treatment of many clinical diseases. First, the abundance of mature adipocytes in adipose tissue makes DFAT cells easy to scale up. Unlike ASCs, which are derived from the small stromal–vascular fraction (SVF) of adipose tissue, DFAT cells are converted directly from adipocytes that constitute more than 90% of adipose tissue volume. The relatively less invasive harvest method and high yield make DFAT cells a practical cell source. Second, DFAT cells have multi–lineage differentiation capacity regardless of donors' age and show low immunogenicity after transplantation.1 Last but not least, the generation of DFAT cells is through a traditional ceiling culture method, which avoids the potential problems associated with cell reprograming using virus vectors.
Although DFAT cell research makes great progress in the past few years, there are many obstacles researchers need overcome to make the next big move. In this review, we summarize the current main challenges in DFAT cell research including cell culture purity, phenotypic properties, and dedifferentiation mechanisms.
Cell culture purity of DFAT cells
The most common approach used to derive DFAT cells from adipose tissue is a ceiling culture method based on the buoyancy properties of mature lipid–filled adipocytes. There are mainly 2 types of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is more often used to isolate stem cells. Yagi et al. first reported the method of deriving DFAT cells from the subcutaneous WAT from mice.10 Briefly, adipose tissues were minced and then underwent enzymatic digestion with collagenase type II. After centrifugation, the resulting pellet containing the SVF was cultured as ASCs and the floating adipocytes were collected, washed, and transferred to an inverted cell culture flask filled with cell culture medium. The cells floated in the media and adhered to the ceiling of the flask. After the cells were firmly attached and fibroblast–like cells were observed, the flask was re–inverted to culture the attached cells as DFAT cells (Fig. 1A). Following this procedure, we also observed the reported cell morphology changes indicating the dedifferentiation process of adipocytes (Fig. 2). Within 7 d of ceiling culture, the adipocytes attached to the top surface of the flask began releasing lipid droplets and gradually changed cell morphology to fibroblast–like cells. Adipocyte derived fibroblast–like cells showed a high proliferation rate similar to what have been reported after long–term culture in vitro.11 Jumabay et al. reported another method for isolation of DFAT cells without using ceiling culture (Fig. 1B).12 The harvested adipocytes isolated from tissue were incubated floating on culture medium for 24 hours and then cells were transferred to a new dish with 70µm insert filter. DFAT cells derived from the adipocytes were allowed to sink through the filter to the bottom of the dish and collected after 5 d. DFAT cells isolated through the filter have been reported to express significantly increased pluripotency markers.
Figure 1.
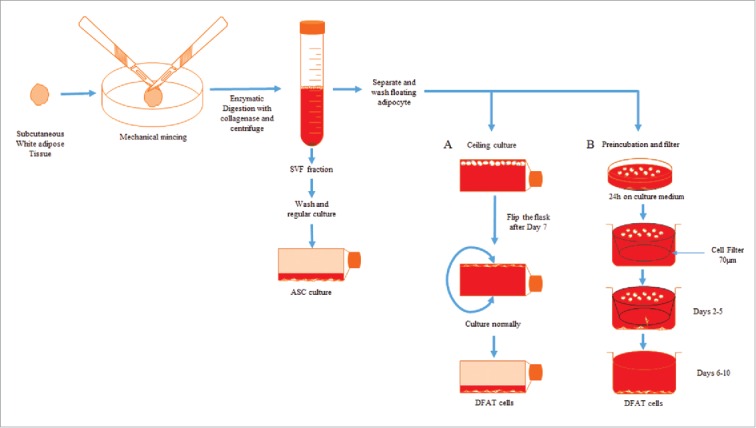
Schematic for isolation of DFAT and ASCs from adipose tissue. (A) Ceiling culture method and (B) Preincubation and filter method.
Figure 2.
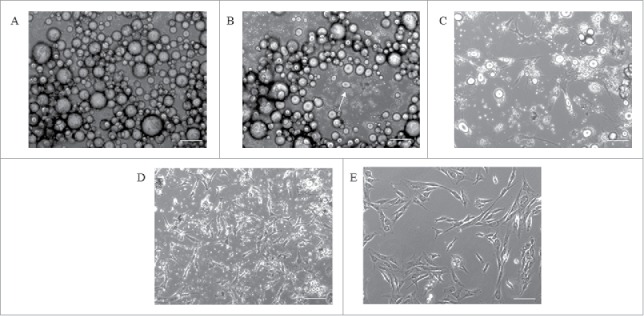
Cell morphology during DFAT cell production using the ceiling culture method. The morphology of porcine adipocytes in ceiling culture on day 1 (A), day 3 (B) and day 7 (C). The flask is flipped at day 7 and culture normally for 5∼7 d to obtain primary DFAT cells (D). The morphology of Passage 2 DFAT cells (E). Scale bar 100µm.
Cell purity is one of the main issues with current DFAT cells isolation methods. Adipocyte is not the only cell type present in adipose tissue. Other cell types in adipose tissue include stem cells, preadipocytes, macrophages, neutrophils, lymphocytes, and endothelial cells.13 During the DFAT cell isolation process, a small number of other cell types (e.g. preadipocytes, fibroblasts, stem cells) with undistinguished morphology but similar buoyancy might be co–isolated and attached together with mature adipocytes, resulting in the derived DFAT cells contaminated by other cell types. Tholpady et al. observed the contamination of fibroblast like cells within 48 hours of ceiling culture along with mature adipocytes.14 The concern of possible preadipocytes or stromal fraction cells contamination during mature adipocyte isolation has been raised by several research groups.15,16
The purity of mature adipocytes isolated from adipose tissue is a very critical parameter for the uniform production of DFAT cells. It takes approximately 5-7 d to dedifferentiate adipocytes into DFAT cells depending on animal species. Therefore, a small amount of contaminated cells initially co–isolated with the adipocytes could significantly proliferate and cause inhomogeneity of the produced DFAT cells. A few types of cell purity testing have been employed including checking cell morphology using microscopy or stain isolated cells using mature adipocyte specific markers such as perilipin and Nile red.3,17 The results from the purity test have shown some presence of non–adipocyte cells. To improve cell purity, several approaches have been explored including methods using differential plating and cloning (Fig. 3). Differential plating exploits the difference of time needed for mature adipocyte and other cell types to attach to the flask for separating the contaminating cells. After 1–2 d of ceiling culture, mature adipocytes will be floating in the medium but non–lipid containing cells will attach to the bottom surface. In early differential plating, floating mature adipocytes in the medium are transferred to the new flask leaving the attached contaminating cells behind. Mature adipocytes adhere to the top surface after 3–4 d of ceiling culture. In late differential plating, the cells attached to the ceiling are trypsinized and centrifuged after 3–4 d of ceiling culture, to eliminate the contaminating cells culturing along with mature adipocytes by the buoyant nature of adipocytes. For cloning procedure, after 5 d of ceiling culture, the vessel is inverted and non–lipid containing cells growing along with mature adipocytes are marked and scraped off from the flask by pipette under a microscope to purify the population.18
Figure 3.
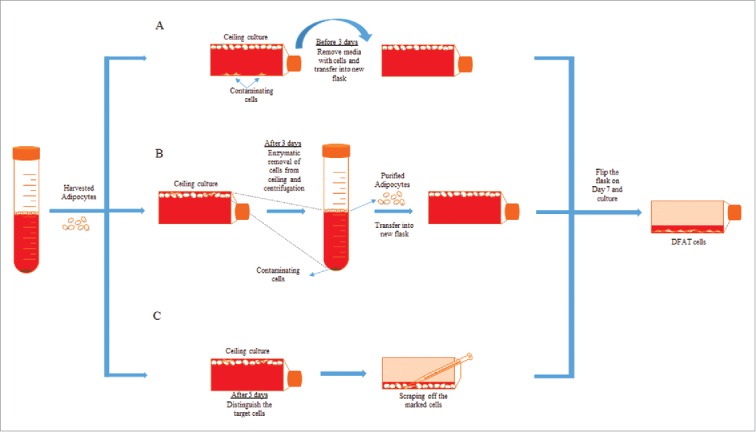
Purification methods of mature adipocytes. After the separation of mature adipocytes and SVF fraction from fat tissue, mature adipocytes can be purified by early differential plating (A), late differential plating (B) or cloning technique (C).
Even with the improved methods pure adipocytes still cannot be guaranteed for the downstream DFAT cell production. The resulted heterogeneous population of DFAT cells will cause inconsistent results even under the same experimental conditions. Therefore, specific cell sorting to obtain high purity adipocytes is needed to overcome the cell purity issue of DFAT cell production. Fluorescence–activated cell sorting (FACS) and Magnetic–activated cell sorting (MACS) are the two most powerful techniques in separating the desired cell type from a mixed population. These two techniques have been routinely used for the isolation of many cell types including stem cells. For example, CD34–positive mononuclear blood cells (MBCD34+) have been isolated from human peripheral blood using MACS.19 Neural crest stem cells (NCSCs) from mammalian fetal peripheral nerve have been isolated using P75 and P0 cell markers by FACS.20 However, to the best of our knowledge, neither FACS nor MACS has been applied to separate pure adipocytes for DFAT cell production.
Phenotypic properties of DFAT cells
The pluripotency of DFAT cells has been approved by specific stem cell markers expression and multilineage differentiation. DFAT cells have shown the loss of adipocyte markers during the ceiling culture and the expression of various stem cell markers after dedifferentiation. Several studies have reported that genes related to lipid metabolism are abundantly expressed in mature adipocytes (i.e. LPL, LEP, GLUT4, and PPARG) but not expressed in DFAT cells.1,3 DFAT cells in the early passages have shown the expression of embryonic stem cell markers such as Oct4, sox2, cMyc, Klf 4, and nanog.21 In addition, DFAT cells have been shown to have higher telomerase activity than ASCs.12
DFAT cells have multilineage differentiation ability and have been reported to differentiate into multiple lineages like osteogenic,21 chondrogenic,22 adipogenic,23 myogenic,24 neurogenic,25 and angiogenic 5 lineages. In vitro osteogenic differentiation of DFAT cells has been reported to be induced by osteogenic differentiation medium (ODM), which consisted of DMEM supplemented with 10 % FBS, antibiotics, 10 mM β–glycerophosphate, 10 μg/ml ascorbic acid, and 10 μM all–trans retinoic acid (first 3 days only).26 The mineralization of the cells has been confirmed by increased alkaline phosphatase activity and Alizarin Red staining. In vivo experiments also proved the formation of bone in a rat calvarial bone defect model after the implantation of DFAT cells using a poly (lactic–co–glycolic acid) / hyaluronic acid (PLGA/HA) scaffold.26 Briefly, PLGA/HA scaffold was seeded with 1×106 rat DFAT cells and cultured using normal growth medium for 3 d. Then, the osteo–induced cells were produced by replacing normal culture media with ODM for 6 d before implantation of the cell seeded scaffold in the center of parietal bone defect. After 8 weeks, the defect closure by new bone in PLGA/HA with DFAT cells was observed to be significantly higher than control group by histology and histometric analysis. Jumabay et al. reported the differentiation of rat DFAT cells into cardiomyocytes induced by 1% methylcellulose in Iscove's modified Dulbecco's medium supplemented with 1% bovine serum albumin, 15% FBS, 2–mercaptoethanol (0.1 mM), l–glutamine (2 mM), recombinant human insulin (10 μg/ml), human transferrin (200 μg/ml), recombinant murine interleukin 3 (IL–3; 10 ng/ml), recombinant human IL–6 (10 ng/ml), and recombinant mouse stem cell factor (50 ng/ml).2 The morphological changes and cardiac markers like Nkx2.5, troponin–T, and sarcomeric actin were confirmed by immune staining. Rat DFAT cells have been used to repair infracted cardiac tissue induced by left coronary artery ligation in Sprague–Dawley rats.2 Three hours after ligation, 106 DFAT cells were injected in 5 different ischemic sites. After 8 weeks, engraftment of the cells and neovascularization in the scar region were observed by immunohistological analysis. Yamada et al. showed locomotor functional recovery by remyelination and glial scar reduction by DFAT cells after spinal cord injury in mice.25 Spinal cord injury was induced at the Th10 level in mice by using an Infinite Horizon Impactor. On the 8th day post injury, 105 DFAT cells isolated from mice were injected at Th10 level. After 36 d post injury, locomotor function was significantly improved by Basso mouse scale (BMS) score in mice with injected DFAT cells. Immuno–histological studies revealed expression of neurotrophic factors like brain–derived neurotrophic factor (BDNF), glial–derived neurotrophic factor (GDNF), and reduction of scar by DFAT cell transplantation.
One of the great challenges in DFAT cell studies is to identify the unique phenotypic profile of DFAT cells. DFAT cells and ASCs, derived from same source, have very similar expression marker profile: positive for CD13, CD29, CD44, CD90, CD105, HLA–A, B, C, and negative for CD56.1,27 The differences of cell marker expression between the DFAT cells and ASCs are shown in Table 1. As shown in the table, several studies have reported the expression of αSMA higher in DFAT than ASCs.1,28 The expressions of other surface markers have been reported to vary in different studies, which does not help clearly distinguish between these two cell types from the same source. Also, human DFAT cells have been reported to have the similar surface marker profile as bone marrow–derived Mesenchymal Stem Cells (MSCs), which are both positive for CD90, CD105, CD73, CD44, and CD29, and negative for CE34, CD117, CD133, CD271, CD45, HLA–DR, and CD14.17 To distinguish the DFAT cells from all the other cell types, defined cell surface marker expression profile needs to be further established.
Table 1.
Comparison of cell surface markers in DFAT cells and ASCs. + : positive expression and – : negative expression.
Dedifferentiation mechanism of DFAT cells
Two phenomena have been reported during the dedifferentiation process pointing toward different cell sources that give rise to the generated DFAT cells. Yagi et al. along with several other groups have demonstrated that mature adipocytes in ceiling culture loses the lipids and converts into the DFAT cells.10 Mature adipocytes were observed under light microscope to break into small lipid droplets, loose their round contour and fibroblast–like morphology is obtained after 10 d.14,29 Some other studies showed that during dedifferentiation process, mature adipocytes divide asymmetrically and give rise to another adipocyte as well as a non–adipocyte daughter cell that further produce the DFAT cells. Matsumoto et al. showed that DNA synthesis in the mature adipocyte is induced by the ceiling culture by BrdU incorporation method.1 Approximately 50% of the adherent adipocyte cells in ceiling culture showed BrdU incorporation and 40% exhibited a fibroblast–like morphology by day 7 of culture, indicating adipocytes gain capacity for DNA synthesis. Also, time–lapse video images of cultured adipocytes pre–labeled with Hoechst 33342 showed that the adipocytes with a single nucleus divided asymmetrically and produced fibroblast–like cells.23 Both the phenomena have proven to produce the multipotent and highly proliferative DFAT cells.
Dedifferentiation mechanism of DFAT cells has not been revealed. In spite of having limited potential to regenerate, terminally differentiated mammalian cells have been shown to dedifferentiate under defined circumstances.30 Dedifferentiation of mature cells can be a driven by external stimuli and/or gene regulation. In vitro culturing of adult human cartilage chondrocytes (HAC) in monolayer leads to their dedifferentiation and cells regain proliferation and multipotent differentiation ability.31 Culturing 1∼2 × 104 cells/cm2 HAC in monolayer in vitro with culture medium containing high–glucose DMEM, 2 mM l–glutamine, 50 μg/ml gentamycin, and 10% FBS for 4 d leads to cell morphology change and dedifferentiation. Dedifferentiated HAC express several embryonic stem cell markers such as SSEA–3, SSEA–4, TRA1–60, and TRA1–81 and show alkaline phosphatase activity. Dedifferentiated HAC cultures showed multilineage potential for chondrogenic, osteogenic, and adipogenic lineages demonstrated by lineage specific histochemical and immunofluorescence staining. Following nerve injury, a differentiated myelinating Schwann cell can dedifferentiate by activation of Ras/Raf/ERK signaling and regain the potential to proliferate.32 Induced expression of oncogenic Ras with retroviral vector in early–passage Schwann cells showed that Ras expression induces Schwann cell dedifferentiation via the ERK signaling pathway. Raf/ERK signaling was shown to dedifferentiate Schwann cells that had been induced to undergo myelination in response to axonal signals. Mouse myotubes, treated with an extract derived from the regenerating limbs of newts, were shown to undergo dedifferentiation.33 The cells showed the ability to reenter the cell cycle, exhibit a reduction in muscle differentiation proteins like MyoD, myogenin, and troponin T, and cleave to form smaller myotubes or mononucleated cells. The main factor for conversion of mature adipocytes into DFAT cells in ceiling culture is considered to be hypoxia, which was also shown in the dedifferentiation process of chondrocytes and smooth muscle cells.34,35 However, more studies needed to be done to establish the factor/s driving dedifferentiation process in mature adipocytes. Understanding the process of dedifferentiation, will give the insight of regeneration mechanism in adult differentiated cells.
Future directions
DFAT cells, being isolated from the abundant adipose tissue with pluripotent potential, can be great source for stem cells and tissue engineering. There are still many areas of improvement needed before the translation of DFAT cells in clinical applications. The purity of cells used for the isolation process is a major concern; contaminating cells can dramatically affect the downstream studies and application of DFAT cells. Cell sorting techniques like FACS or MACS should be implemented to obtain nearly 100% pure mature adipocyte population to solve the DFAT cell purity issue. The distinct cell surface marker profile needs to be further established for DFAT cells to distinguish them from other stem cells. The factors (e.g., stress, hypoxia) and culture conditions that trigger the dedifferentiation of fat cells need to be thoroughly investigated to understand the underlying mechanisms.
ABBREVIATIONS
- ASCs
Adipose derived Stem Cells
- BDNF
Brain–Derived Neurotrophic Factor
- BAT
Brown Adipose Tissue
- DFAT cells
Dedifferentiated Fat cells
- ECs
Endothelial Cells
- FACS
Fluorescence–Activated Cell Sorting
- GDNF
Glial–Derived Neurotrophic Factor
- HAC
Human Articular Chondrocytes
- IL
Interleukin
- MACS
Magnetic–Activated Cell Sorting
- MSCs
Mesenchymal Stem Cells
- NCSCs
Neural Crest Stem Cells
- ODM
Osteogenic Differentiation Medium
- PLGA/HA
Poly (Lactic–co–Glycolic Acid) / Hyaluronic Acid
- SCI
Spinal Cord Injury
- SVF
Stromal–Vascular Fraction
- WAT
White Adipose Tissue
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- [1].Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al.. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 2008; 215:210-22; PMID:18064604; http://dx.doi.org/ 10.1002/jcp.21304 [DOI] [PubMed] [Google Scholar]
- [2].Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, Mitsumata M, Saito S, Hirayama A, Mugishima H, et al.. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol 2009; 47:565-75; PMID:19686758; http://dx.doi.org/ 10.1016/j.yjmcc.2009.08.004 [DOI] [PubMed] [Google Scholar]
- [3].Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res 2008; 332:435-46; PMID:18386066; http://dx.doi.org/ 10.1007/s00441-008-0593-9 [DOI] [PubMed] [Google Scholar]
- [4].Kishimoto N, Momota Y, Hashimoto Y, Tatsumi S, Ando K, Omasa T, Kotani J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin Oral Investig 2014; 18:1893-901; PMID:24362590; http://dx.doi.org/ 10.1007/s00784-013-1166-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, Roos KP, Yao Y, Boström KI. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J Mol Cell Cardiol 2012; 53:790-800; PMID:22999861; http://dx.doi.org/ 10.1016/j.yjmcc.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ohta Y, Takenaga M, Tokura Y, Hamaguchi A, Matsumoto T, Kano K, Mugishima H, Okano H, Igarashi R. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant 2008; 17:877-86; PMID:19069631; http://dx.doi.org/ 10.3727/096368908786576516 [DOI] [PubMed] [Google Scholar]
- [7].Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 2002; 290:763-9; PMID:11785965; http://dx.doi.org/ 10.1006/bbrc.2001.6270 [DOI] [PubMed] [Google Scholar]
- [8].Bacou F, el Andalousi RB, Daussin P-A, Micallef JP, Levin JM, Chammas M, Casteilla L, Reyne Y, Nouguès J. Transplantation of adipose tissue-derived stromal cells increases mass and functional capacity of damaged skeletal muscle. Cell Transplant 2004; 13:103-11; PMID:15129756; http://dx.doi.org/ 10.3727/000000004773301771 [DOI] [PubMed] [Google Scholar]
- [9].Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells. J Cell Sci 2006; 119:2945-52; PMID:16825428; http://dx.doi.org/ 10.1242/jcs.03029 [DOI] [PubMed] [Google Scholar]
- [10].Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun 2004; 321:967-74; PMID:15358122; http://dx.doi.org/ 10.1016/j.bbrc.2004.07.055 [DOI] [PubMed] [Google Scholar]
- [11].Peng X, Song T, Hu X, Zhou Y, Wei H, Peng J, Jiang S. Phenotypic and Functional Properties of Porcine Dedifferentiated Fat Cells during the Long-Term Culture In Vitro. BioMed Res Int 2015; 2015:673651; PMID:26090433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jumabay M, Abdmaulen R, Ly A, Cubberly MR, Shahmirian LJ, Heydarkhan-Hagvall S, Dumesic DA, Yao Y, Boström KI. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl Med 2014; 3:161-71; PMID:24396033; http://dx.doi.org/ 10.5966/sctm.2013-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano GMC, Fabbri A, Caprio M. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem 2010; 110:564-72; PMID:20512917; http://dx.doi.org/ 10.1002/jcb.22598 [DOI] [PubMed] [Google Scholar]
- [14].Tholpady SS, Aojanepong C, Llull R, Jeong J-H, Mason AC, Futrell JW, Ogle RC, Katz AJ. The cellular plasticity of human adipocytes. Ann Plast Surg 2005; 54:651-6; PMID:15900154; http://dx.doi.org/ 10.1097/01.sap.0000158065.12174.40 [DOI] [PubMed] [Google Scholar]
- [15].Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012; 53:227-46; PMID:22140268; http://dx.doi.org/ 10.1194/jlr.R021089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fernyhough ME, Vierck JL, Dodson MV. Assessing a non-traditional view of adipogenesis: adipocyte dedifferentiation–mountains or molehills? Cells Tissues Organs 2006; 182:226-8; PMID:16914923; http://dx.doi.org/ 10.1159/000093970 [DOI] [PubMed] [Google Scholar]
- [17].Poloni A, Maurizi G, Leoni P, Serrani F, Mancini S, Frontini A, Zingaretti MC, Siquini W, Sarzani R, Cinti S. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells Dayt Ohio 2012; 30:965-74; http://dx.doi.org/ 10.1002/stem.1067 [DOI] [PubMed] [Google Scholar]
- [18].Fernyhough ME, Vierck JL, Hausman GJ, Mir PS, Okine EK, Dodson MV. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology 2004; 46:163-72; PMID:19003270; http://dx.doi.org/ 10.1007/s10616-005-2602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Handgretinger R, Lang P, Schumm M, Taylor G, Neu S, Koscielnak E, Niethammer D, Klingebiel T. Isolation and transplantation of autologous peripheral CD34+ progenitor cells highly purified by magnetic-activated cell sorting. Bone Marrow Transplant 1998; 21:987-93; PMID:9632271; http://dx.doi.org/ 10.1038/sj.bmt.1701228 [DOI] [PubMed] [Google Scholar]
- [20].Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999; 96:737-49; PMID:10089888; http://dx.doi.org/ 10.1016/S0092-8674(00)80583-8 [DOI] [PubMed] [Google Scholar]
- [21].Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct 2008; 33:211-22; PMID:19088398; http://dx.doi.org/ 10.1247/csf.08038 [DOI] [PubMed] [Google Scholar]
- [22].Kikuta S, Tanaka N, Kazama T, Kazama M, Kano K, Ryu J, Tokuhashi Y, Matsumoto T. Osteogenic effects of dedifferentiated fat cell transplantation in rabbit models of bone defect and ovariectomy-induced osteoporosis. Tissue Eng Part A 2013; 19:1792-802; PMID:23566022; http://dx.doi.org/ 10.1089/ten.tea.2012.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nobusue H, Kano K. Establishment and characteristics of porcine preadipocyte cell lines derived from mature adipocytes. J Cell Biochem 2010; 109:542-52; PMID:20013788 [DOI] [PubMed] [Google Scholar]
- [24].Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun 2008; 377:780-5; PMID:18938140; http://dx.doi.org/ 10.1016/j.bbrc.2008.10.046 [DOI] [PubMed] [Google Scholar]
- [25].Yamada H, Ito D, Oki Y, Kitagawa M, Matsumoto T, Watari T, Kano K. Transplantation of mature adipocyte-derived dedifferentiated fat cells promotes locomotor functional recovery by remyelination and glial scar reduction after spinal cord injury in mice. Biochem Biophys Res Commun 2014; 454:341-6; PMID:25451251; http://dx.doi.org/ 10.1016/j.bbrc.2014.10.082 [DOI] [PubMed] [Google Scholar]
- [26].Shirakata Y, Nakamura T, Shinohara Y, Taniyama K, Sakoda K, Yoshimoto T, Noguchi K. An exploratory study on the efficacy of rat dedifferentiated fat cells (rDFATs) with a poly lactic-co-glycolic acid/hydroxylapatite (PLGA/HA) composite for bone formation in a rat calvarial defect model. J Mater Sci Mater Med 2014; 25:899-908; PMID:24363067; http://dx.doi.org/ 10.1007/s10856-013-5124-x [DOI] [PubMed] [Google Scholar]
- [27].Shen J, Sugawara A, Yamashita J, Ogura H, Sato S. Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci 2011; 3:117-24; PMID:21789960; http://dx.doi.org/ 10.4248/IJOS11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kono S, Kazama T, Kano K, Harada K, Uechi M, Matsumoto T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet J Lond Engl 1997 2014; 199:88-96 [DOI] [PubMed] [Google Scholar]
- [29].Song N, Kou L, Lu XW, Sugawara A, Shimizu Y, Wu MK, Du L, Wang H, Sato S, Shen JF. The perivascular phenotype and behaviors of dedifferentiated cells derived from human mature adipocytes. Biochem Biophys Res Commun 2015; 457:479-84; PMID:25603054; http://dx.doi.org/ 10.1016/j.bbrc.2015.01.033 [DOI] [PubMed] [Google Scholar]
- [30].Cai S, Fu X, Sheng Z. Dedifferentiation: A New Approach in Stem Cell Research. BioScience 2007; 57:655; http://dx.doi.org/ 10.1641/B570805 [DOI] [Google Scholar]
- [31].De la Fuente R, Abad JL, García-Castro J, Fernández-Miguel G, Petriz J, Rubio D, Vicario-Abejón C, Guillén P, González MA, Bernad A. Dedifferentiated adult articular chondrocytes: a population of human multipotent primitive cells. Exp Cell Res 2004; 297:313-28; PMID:15212937; http://dx.doi.org/ 10.1016/j.yexcr.2004.02.026 [DOI] [PubMed] [Google Scholar]
- [32].Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J 2004; 23:3061-71; PMID:15241478; http://dx.doi.org/ 10.1038/sj.emboj.7600309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci U S A 2001; 98:13699-704; PMID:11717431; http://dx.doi.org/ 10.1073/pnas.221297398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aitken KJ, Tolg C, Panchal T, Leslie B, Yu J, Elkelini M, Sabha N, Tse DJ, Lorenzo AJ, Hassouna M, et al.. Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle. Am J Pathol 2010; 176:304-19; PMID:20019183; http://dx.doi.org/ 10.2353/ajpath.2010.080834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lafont JE. Lack of oxygen in articular cartilage: consequences for chondrocyte biology. Int J Exp Pathol 2010; 91:99-106; PMID:20384821; http://dx.doi.org/ 10.1111/j.1365-2613.2010.00707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells Dayt Ohio 2007; 25:818-27; http://dx.doi.org/ 10.1634/stemcells.2006-0589 [DOI] [PubMed] [Google Scholar]
- [37].Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells Dayt Ohio 2005; 23:412-23; http://dx.doi.org/ 10.1634/stemcells.2004-0021 [DOI] [PubMed] [Google Scholar]
- [38].Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001; 189:54-63; PMID:11573204; http://dx.doi.org/ 10.1002/jcp.1138 [DOI] [PubMed] [Google Scholar]


