Abstract
Mesenchymal stem cells (MSCs) have been established after isolation from various tissue sources, including bone marrow and synovial fluid. Recently, synovial-fluid-derived MSCs were reported to have multi-lineage differentiation potential and immunomodulatory features, which indicates that these cells can be used for tissue engineering and systemic treatments. This study presents a protocol for simple and non-invasive isolation of MSCs derived from the bone marrow and synovial fluid of minipigs to analyze cell surface markers for cell phenotyping and in vitro culturing. Using sexually mature six-month-old minipigs, bone marrow was extracted from the iliac crest bone using a bone marrow extractor, and the synovial fluid was aspirated from the femorotibial joint. Procedures for the collection of samples from both sources were non-invasive. The protocols for effective isolation of MSCs from harvested cell sources and for creating in vitro culture conditions to expand stable MSCs from minipigs and the application of systemic autologous treatments are provided. For cell phenotyping, the cell surface markers of both cells were analyzed using flow cytometry. In the results, the MSCs were isolated from the synovial fluid of the minipigs and showed that synovial-fluid-derived MSCs have a similar morphology and cell phenotype to bone-marrow-derived MSCs. Therefore, non-invasively obtained synovial fluid is a valuable source of MSCs.
Keywords: Medicine, Issue 113, Mesenchymal stem cell, minipig, synovial fluid, bone marrow, cell isolation, cell culture, cellular phenotype, cell surface marker, flow cytometry, stem cell biology
Introduction
Multipotent mesenchymal stem cells (MSCs) can be classified into mesenchymal cell lineages, and MSCs have been established and isolated from various tissue sources, such as from bone marrow, umbilical cords, placentas, adipose tissue, dermal skin, skeletal muscle, hair follicles, synovial membranes, and teeth1-5. Currently, attention has been given to synovial-derived MSCs because these cells may help treat joint diseases, such as bone fraction, osteoarthritis, and rheumatoid arthritis (RA), and due to the regenerative potential of MSCs in damaged cartilage or bone, they may also help treat immune modulation or autoimmune diseases6-8. The majority of research of MSCs derived from synovial sources have employed MSCs from the synovial membrane rather than from synovial fluid2,9; thus, the biological understanding of synovial-fluid-derived MSCs is limited.
Synovial fluid is an easily accessible source of MSCs, which can be aspirated during the diagnoses or treatments of patients and during diagnostic confirmations of various arthritis conditions that do not require invasive procedures, such as osteoarthritis or RA. The population of synovial-fluid-derived MSCs in the synovial cavity is significantly increased in arthritic patients compared to non-arthritic patients10-12. Therefore, synovial fluid is an excellent source candidate for MSCs, particularly for autologous stem cell therapy in patients with inflamed or injured joints. Additionally, synovial-fluid-derived MSCs have a chondrogenic capacity, immunomodulation abilities, and a high rate of proliferation. This study established protocols for bone marrow extraction and synovial fluid aspiration from minipigs using non-invasive methods and for the in vitro expansion of isolated cells and analysis of cell phenotypes.
Protocol
Animal experiments were authorized by the Animal Center for Biomedical Experimentation at Gyeongsang National University.
1. Preparations for the Animal Procedure
- Prepare the adult female minipigs for the non-invasive collection of MSCs from bone marrow and synovial fluid. Perform the clinical examination of the minipigs one day prior to anesthesia and sample collection.
- Provide clinically normal animals for physical examinations, which include body temperature, respiratory rate, and heart rate, and for the observation of the pigs' behavior and hematological statuses, using complete blood count tests. Note: Parameter intervals for clinically healthy minipigs are 11 - 29/min for the respiration rate, 68 - 98/min for the heart rate, and 37 - 38 °C for the body temperature.
Remove all edible bedding from the cage during the preoperative fasting period of 6 - 12 hr prior to the administration of anesthesia for young and adult minipigs, 3 hr of fasting should be observed for the neonates. Provide drinking water until the time of anesthesia.
2. Preparation of the Animals for Anesthesia
- Medicate the minipigs before the administration of anesthesia with acepromazine (0.1 mg/kg), medetomidine (0.03 mg/kg), and atropine (0.04 mg/kg).
- Inject all medications intramuscularly (IM) using a 21 G needle with a length of at least 30 mm for adult minipigs. Perform the IM injection 1-3 cm behind the ear into the lateral cervical muscle or into the thigh muscle. Note: Perform all the procedures without eliciting fear or stress in the animals or requiring rough handling to safely inject the premedication.
Place the minipigs in a dorsal-recumbent position.
- Fifteen minutes after the administration of the premedication, initiate anesthesia using 1.5% isoflurane administered through inhalation.
- Continue the inhalation of isoflurane (1.5%) through an endotracheal tube, followed by an increase in concentration to 2.5%, with oxygen (1.5 L/min) as the carrier gas.
Continuously monitor the heart rate, body temperature, and percutaneous blood oxygen saturation (SpO2) of the minipigs during anesthesia. Use a circulating water blanket at a temperature of 38 - 39 °C to maintain body temperature. Apply an ophthalmologic ointment to protect the eyes from drying out during anesthesia.
Follow Guedel's classification to assess the depth of anesthesia13.
3. Preparations for Cell Isolation and Cultivation
- To cultivate the MSCs derived from bone marrow and synovial fluid, prepare 500 ml of advanced Dulbecco's modified eagle medium (ADMEM) supplemented with 10% fetal bovine serum (FBS), 1% Glutamax, 10 ng/ml of basic fibroblast growth factor (bFGF), and 1.0% penicillin-streptomycin (10,000 IU and 10,000 µg/ml, respectively, Pen-Strep).
- Incubate the ADMEM at 38.5 °C in a humidified atmosphere of 5% CO2 within a CO2 incubator.
Prepare 500 ml of Dulbecco's phosphate buffer saline (DPBS) according to the manufacturer's instructions for cell isolation and washing.
4. Collecting Bone Marrow from the Minipigs
Select an area approximately 15 x 15 cm in size on the iliac crest (Figure 1), and remove the hair from this area using sterilized safety razors or electric clippers. Once the minipig is positioned, alternately scrub the procedure site three times with the following sterilization agents: povidone iodine and 70% ethanol. After the area is thoroughly dry, apply sterile drapes.
Pre-coat the 10 ml syringe with 100 U/ml of heparin by rinsing it with 3 - 4 ml of heparin before ejecting the anti-coagulating agent. Note: Perform this step to avoid coagulation in the syringe during bone marrow aspiration.
Extract a minimum of 5 ml of bone marrow from the iliac crest bone of the minipig under anesthesia, using a bone marrow extractor (3.0 × 100 mm) tightly attached to the heparin pre-coated 10 ml syringe.
5. Isolation of Cells Extracted from the Bone Marrow and In Vitro Cultivation
- Isolate the cells from the extracted bone marrow.
- Dilute the extracted bone marrow with an equal volume of DPBS in a 15 ml conical tube at room temperature.
- Add 3 ml of density centrifugation media (Ficoll-Paque) to the 15 ml conical tube.
- Dilute a 4 ml layer of the bone marrow with DPBS, which is placed on the density centrifugation media without mixing, and centrifuge it at 400 × g for 40 min at room temperature.
- Harvest the layer of mononuclear cells (MNCs) from the buffy coat between the two layers containing the plasma (top layer) and the density centrifugation media (bottom layer), and transfer the MNCs into a 15 ml conical tube.
- Dilute the transferred MNCs with 7 - 8 ml of DPBS and centrifuge them at 500 × g for 10 min at room temperature. Discard the supernatant and wash the cells twice with DPBS.
- Suspend the cell pellet in 2 ml of ADMEM and transfer it to a 35 mm tissue culture dish. Incubate the culture dish at 38.5 °C in a humidified atmosphere of 5% CO2.
- In vitro cultivation of cells isolated from bone marrow.
- After 48 hr, aspirate the medium with the cells that have not attached to the dish, and replace 2 ml of the ADMEM and incubate it at 38.5 °C in a humidified atmosphere of 5% CO2. Change the medium every third days.
- At 70 - 80% confluency, wash the cells with DPBS, dissociate them with 1 ml of 0.25% trypsin/EDTA, and incubate them at 37 °C for 3 - 5 min until the cells detach.
- Harvest the cells using 10 ml of ADMEM, transfer them into a 15 ml conical tube, and centrifuge them at 300 × g for 5 min at room temperature. Discard the supernatant.
- Suspend the harvested cells in 1 ml of fresh ADMEM and count them using a hemocytometer.
- Place the cells in a 5 - 7.5 x 105/25T flask containing 4 ml of ADMEM and incubate them at 38.5 °C in a humidified atmosphere of 5% CO2. Change the medium every third day.
6. Collection of Synovial Fluid from the Minipigs
Similar to the procedure for bone marrow aspiration, select an appropriate area of approximately 15 x 10 cm (length/width) over the femoral joint, and remove the hair using sterilized safety razors or electric clippers, follow by sanitization procedures (step 4.1).
- Insert a 3 ml syringe with a 23 G needle into the joint after the palpation landmarks (patella and tibia crest).
- Apply gentle suction to the syringes for the aspiration of synovial fluid in the joint. The approximate volume of synovial fluid collected from a joint is 0.5 - 1 ml, but this varies according to the size of the minipig or the joint. Flush through DPBS in the synovial cavity if the collected volume of synovial fluid is too small.
- Immediately withdraw the syringe to avoid contamination with blood.
7. Cell Isolation from the Collected Synovial Fluid and In Vitro Cultivation
- Isolate the cells from the aspirated synovial fluid.
- Dilute the aspirated synovial fluid with an equal volume of DPBS in a 15 ml conical tube at room temperature.
- To remove debris, filter the diluted synovial fluid using a 40 µm nylon cell strainer.
- Add 10 ml of DPBS to the tube containing the filtered synovial fluid and centrifuge it at 400 × g for 10 min at room temperature. Discard the supernatant and wash the cells twice with DPBS.
- Suspend the cell pellet in 2 ml of ADMEM, place the cells on a 2 - 3 x 105 cells/35 mm tissue culture dish with 2 ml of ADMEM, and incubate them at 38.5 °C in a humidified atmosphere of 5% CO2.
Repeat step 5.2 for the in vitro cultivation of isolated synovial fluid-derived MSCs.
8. Post-procedure Care of the Minipigs
If needed, close the skin at the bone marrow extractor injection site with a single suture using a skin stapler, after the bone marrow collection.
After the procedure is completed, administer an intramuscular injection of enrofloxacin (5 mg/kg) and meloxicam (0.2 mg/kg) to the minipigs once a day for 3 days.
After the procedure is completed, disinfect the bone marrow and synovial fluid collection site with 0.1% povidone iodine once a day for 3 days. Carefully observe daily to monitor for signs of complications (e.g., swelling, pus, or redness).
9. Cell Phenotyping Using Flow Cytometry
When the MSCs are 70 - 80% confluent at passages 3 - 5, wash the cells with DPBS and treat them with 1 ml of 0.25% (v/v) Trypsin/EDTA. Then, incubate them at 37 °C for 3 - 5 min until the cells are detached.
Harvest the cells using 10 ml of DPBS, transfer them into a 15 ml conical tube, and centrifuge them at 300 × g for 5 min at room temperature. Discard the supernatant solution.
- To fix the cells, suspend the cell pellet in 10 ml of a 3.7% formaldehyde solution and keep them at 4 °C for one day before the analysis.
- One day after fixing, wash the cells with DPBS twice using centrifugation at 300 × g for 5 min each time. Discard the supernatant solution.
- Suspend the cell pellet in 4 ml of DPBS, which is supplemented with 1% (w/v) bovine serum albumin (BSA), and incubate the pellet for 1 hr at 4 °C to block non-specific Fc-mediated interactions.
- Distribute 500 µl aliquots of the cell suspension into 1.5 ml tubes.
- For non-conjugated antibodies (CD29 and Vimentin), wash the cells by centrifugation at 300 × g for 3 min. Discard the supernatant solution.
- Suspend the cell pellet in 500 µl of a 1:100 dilution of the primary antibody and incubate it for 1 hr at 4 °C.
- Wash the cells twice with 1% bovine serum albumin by centrifugation at 300 × g for 5 min each time. Discard the supernatant solution.
- Suspend the cell pellet in 500 µl of a 1:100 dilution of the fluorescein isothiocyanate (FITC)-conjugated secondary antibody and incubate it for 1 hr in the dark at 4 °C.
- For the FITC-conjugated antibodies (Isotype, CD34, CD44 and CD45), wash the cells by centrifugation at 300 × g for 3 min. Discard the supernatant solution.
- Suspend the cell pellet in 500 µl of the 1:100 FITC-conjugated antibody and incubate it for 1 hr in the dark at 4 °C.
- For both staining conditions, wash the cells twice with DPBS by centrifugation at 300 × g for 5 min each time. Discard the supernatant solution.
- Suspend the cells in 500 µl of DPBS, transfer them into a 5 ml round-bottom tube, and analyze the results using a flow cytometer8.
Representative Results
Establishment of MSCs Derived from the Bone Marrow and Synovial Fluid of Minipigs
MSCs were successfully isolated from the bone marrow and articular synovial joints of the minipigs and expanded in vitro (Figure 2). Syringe aspiration of synovial fluid is simple, and it is possible to obtain sufficient adherent cells during in vitro cultivation of the primary cells. The morphology of synovial-fluid-derived MSCs is similar to that of bone-marrow-derived MSCs, and both MSCs have a fibroblastic spindle shape and are homogeneously adherent. They were observed to have a positive expression for alkaline phosphatase (AP) staining, after the third subculture was complete.
Evaluation of the Cell Phenotype of MSCs Derived from the Bone Marrow and Synovial Fluid of Minipigs
Assays of the cell surface markers were performed to create a phenotype of the bone-marrow and synovial-fluid-derived MSCs of minipigs. Both types of MSCs expressed significant amounts of positive cell surface markers, such as CD29, CD44, and Vimentin (≥96 - 99% of the positive cells), whereas the expressions of CD34 and CD45 were negative and low (≤2% of the positive cells) (Figure 3). These results were identical to those obtained from distinctive MSCs, including cells isolated from synovial fluid, and there were no significant differences in the cell surface markers among these MSCs. The cells isolated from bone marrow and synovial fluid have been confirmed as MSCs in our previous study8. Both bone marrow- and synovial fluid-derived MSCs expressed stem cell transcriptional factors (Oct3/4, Nanog, and Sox2); moreover, an in vitro differentiation ability has been confirmed not only into the mesenchymal lineage (osteocytes, adipocytes, and chondrocytes), but also neural cells by cytochemical staining and detections of cell-specific gene expression8.
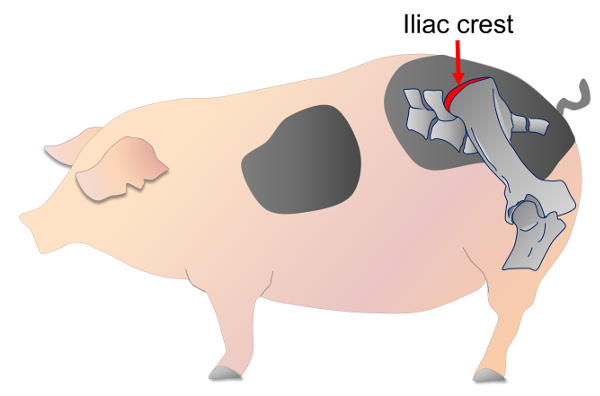 Figure 1. Location of the iliac crest of the minipig. The red arrow represents the site on the iliac crest used for the bone marrow collection. Please click here to view a larger version of this figure.
Figure 1. Location of the iliac crest of the minipig. The red arrow represents the site on the iliac crest used for the bone marrow collection. Please click here to view a larger version of this figure.
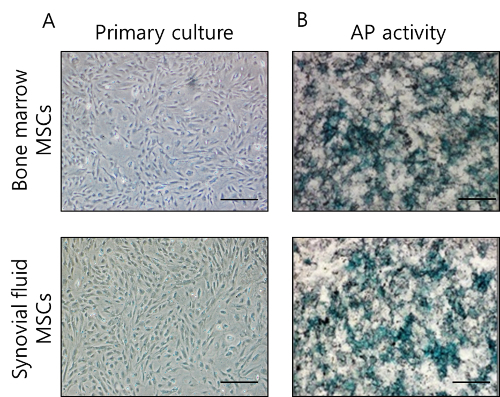 Figure 2. MSCs derived from the bone marrow and synovial fluid of minipigs. (A) The homogeneous morphology of primary cultures of MSCs revealed a fibroblastic shape. (B) Staining with mixture of 5-bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium (BCIP/NBT) revealed AP activity in the MSCs at passage 3. Scale bar: 100 µm Please click here to view a larger version of this figure.
Figure 2. MSCs derived from the bone marrow and synovial fluid of minipigs. (A) The homogeneous morphology of primary cultures of MSCs revealed a fibroblastic shape. (B) Staining with mixture of 5-bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium (BCIP/NBT) revealed AP activity in the MSCs at passage 3. Scale bar: 100 µm Please click here to view a larger version of this figure.
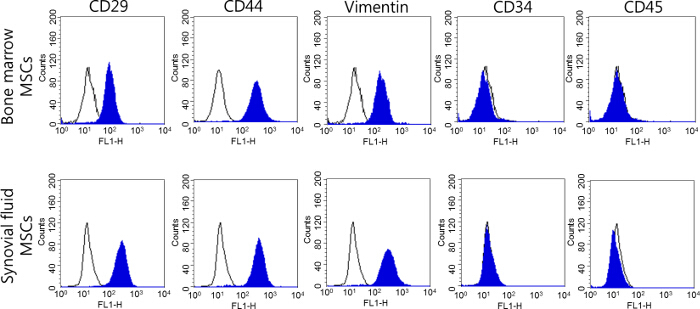 Figure 3. Analysis of the cell surface markers of MSCs derived from the bone marrow and synovial fluid of minipigs at passage 3. The cell surface markers of MSCs were identified using a flow cytometry analysis, and MSCs were identified as being positive for CD29, CD44, and Vimentin and negative for CD34 and CD45. Please click here to view a larger version of this figure.
Figure 3. Analysis of the cell surface markers of MSCs derived from the bone marrow and synovial fluid of minipigs at passage 3. The cell surface markers of MSCs were identified using a flow cytometry analysis, and MSCs were identified as being positive for CD29, CD44, and Vimentin and negative for CD34 and CD45. Please click here to view a larger version of this figure.
Discussion
Minipigs were used to establish MSCs isolation from bone marrow and synovial fluid. To eliminate various physiological conditions, such as age, gender, and disease, minipigs from isogenic background donors were chosen to accurately evaluate the cell source dependent characterization. Pigs are known to be anatomically, physiologically, and genetically similar to humans, and in particular, minipigs can produce size-matched organs; therefore, they can be used as a substitute donor species for xenotransplantation14,15. Regarding the recently suggested immunomodulatory property of MSCs, indoleamine 2, 3-dioxygenase (IDO) and nitric oxide synthase (NOS) were used with MSCs to mediate immunosuppression, which is species-specific, although MSCs from pigs and humans have the same IDO9. Thus, a pig model as a MSCs donor might provide important insights of the immune responses of MSCs for human therapies. Furthermore, the research of the porcine stem cells plays an important role in the biological understanding of in vitro processes and their various preclinical applications in humans16,17. Bone marrow and synovial fluid can be non-invasively harvested from minipigs. The entire procedure, from anesthesia to sample collection, reduced the stress level of systemic or local organs; therefore, minipigs can be used as recipients for autologous transplantation. In this experiment, MSCs were isolated from the bone marrow and synovial fluid of minipigs, based on the confirmation of synovial-fluid-derived MSCs with immunosuppression capacities derived from an RA mouse model in a previous study8
Collection of Bone Marrow from the Minipigs
In pigs, adult progenitor cells derived from bone marrow are multipotent and have induced neovascularization and cardiomyocyte regeneration. The age of a donor is associated with increased fat content and decreased proliferation and differentiation of the bone marrow; therefore, bone marrow is not the best source of MSCs in aged animals. The iliac crest in minipigs is the preferred site for bone-marrow-derived MSC isolation because it is broad and is a low-risk procedure site for sciatic nerve damage. In the case of aged or overweight minipigs, fat-enriched thick muscles may be present on the iliac crest. Under such circumstances, a stabbing incision on the skin is necessary and the bone marrow biopsy needle must be inserted properly, which can be difficult to apply at the iliac crest. Failure to find the correct biopsy site may lead to failed bone marrow aspirations because the needle becomes blocked by the bone or may not be able to reach the bone marrow inside the iliac crest. Therefore, the biopsy needle must be checked properly, and knowledge of the thickness of soft tissue and the cortex of the bone of the iliac crest is required before beginning sample collections.
Aspiration of Synovial Fluid from the Joints of Minipigs
Synovial fluid exists in the cavity of synovial joints, and the volume of synovial fluid is usually increased in inflamed joints. Synovial-fluid-derived MSCs are taken from preferred candidate sites for cartilage regeneration, both in vivo and in vitro. Additionally, they have been shown to elicit immunomodulatory properties in RA8. Synovial fluid is not only an easily accessible source but also has valuable mesenchymal properties. Limitations of using synovial fluid as a source for MSCs are the different volumes found in the joints based on the varying sizes and pathological conditions of the joints and the difficulty in using a 3 ml syringe for synovial fluid aspiration from the joints. Therefore, the size of the syringe should be 5 - 10 ml with high pressure. In addition, flushing of the synovial fluid with 1 - 2 ml of DPBS from a syringe is preferable. By using this method, a large number of cells can be obtained, even from small spaces in the joints.
Characterizations of the Bone-marrow- and Synovial-fluid-derived MSCs of Minipigs
In humans, the morphologies of synovial-fluid- and synovial-membrane-derived MSCs from osteoarthritis patients are similar but narrower than the morphology of bone-marrow-derived MSCs12. However, in in vitro cultures, the morphologies of both synovial-fluid-derived MSCs and bone-marrow-derived MSCs were observed to be similar in this experiment. In the researchers' previous study, the proliferation ability of synovial-fluid-derived MSCs was high, compared to that of bone-marrow-derived MSCs8.
MSCs are established from various tissue sources in adult donors; therefore, non-invasively obtained tissue-source-derived MSCs are valuable for autologous stem cell therapy. The cell source is dependent on the characterizations and capacities of the disease or purpose of the stem cell therapy. In pigs, adult progenitor cells derived from bone marrow are multipotent and have induced neovascularization and cardiomyocyte regeneration18,19. MSCs have been mainly isolated from bone marrow, but also from the periosteum, skeletal muscles, synovium, umbilical cords, and dental tissues, although obtaining cells from these tissues is not easy and can require additional surgical procedures for the patients. However, the volume of synovial fluid usually increases in the joints of osteoarthritis or RA patients, and biopsies of these increased synovial fluids are used to diagnose and treat patients. The number of MSCs also increases in the synovial fluid of patients with degenerated cartilage and osteoarthritis20.
In this study, the cell phenotypes of bone-marrow- and synovial-fluid-derived MSCs were evaluated, and both MSCs presented similar levels of expression for cell surface markers. Therefore, cells isolated from synovial fluid and confirmed as MSCs have similar phenotypes to bone-marrow-derived MSCs in minipigs. The researchers' previous research evaluated other cell surface markers, such as CD90 and the major histocompatible and complex class II (MHC II), and confirmed a capacity for multi-lineage differentiation into the osteocytes, adipocytes, chondrocytes, and neurons of synovial-fluid-derived MSCs from minipigs8. In humans, the cell surface antigen profile of synovial-fluid-derived MSCs is similar to that of synovial-membrane-2,21 and bone-marrow-derived MSCs. However, CD34 and CD45 expressions were not detected in MSCs derived from synovial fluid in temporomandibular joint disorder patients. Thus, the microenvironment in the joint may affect the cell phenotype of MSCs derived from synovial fluid. In this experiment, the cells were obtained from the bone marrow and synovial fluid of minipigs using non-invasive methods. Both types of in vitro cultured cells were confirmed as MSCs, and they had similar cell phenotypes and shared compatible mesenchymal characteristics. However, synovial-fluid-derived MSCs showed a greater potential for autologous stem cell therapy for not only cartilage and bone regeneration but also for immunosuppression of osteoarthritis and RA.
Disclosures
The authors declare that they have no conflict of interest.
Acknowledgments
We gratefully acknowledge the financial support provided by the Next-Generation BioGreen 21 Program (No. PJ007969), Rural Development Administration, and the National Research Foundation (grant no. NRF-2015R1D1A1A01056639) of the Republic of Korea.
References
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- De Coppi P, et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177(1):369–376. doi: 10.1016/j.juro.2006.09.103. [DOI] [PubMed] [Google Scholar]
- Arufe MC, De la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111(4):834–845. doi: 10.1002/jcb.22768. [DOI] [PubMed] [Google Scholar]
- Patil R, et al. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp Cell Res. 2013;320(1):92–107. doi: 10.1016/j.yexcr.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Hatsushika D, et al. Intra articular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354–1359. doi: 10.1002/jor.22370. [DOI] [PubMed] [Google Scholar]
- Hagmann S, et al. The influence of bone marrow- and synovium-derived mesenchymal stromal cells from osteoarthritis patients on regulatory T cells in co-culture. ClinExpImmunol. 2013;73(3):454–462. doi: 10.1111/cei.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, et al. Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp Cell Res. 2015;333(2):273–288. doi: 10.1016/j.yexcr.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Su J, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58(6):1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- Morito T, et al. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 2008;47(8):1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- Sekiya I, et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2011;30(6):943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- John T, Lerche P. Anesthesia and analgesia for veterinary technicians. St. Louis, Missouri: Mosby Elsevier press; 2011. [Google Scholar]
- Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog. Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- Millard AL, Mueller NJ. Critical issues related to porcine xenograft exposure to human viruses: Lessons from allotransplantation. CurrOpin Organ Transplant. 2010;15(2):230–235. doi: 10.1097/MOT.0b013e328336b8f9. [DOI] [PubMed] [Google Scholar]
- Ringe J, et al. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002;307(3):321–327. doi: 10.1007/s00441-002-0525-z. [DOI] [PubMed] [Google Scholar]
- Petersen B, Carnwath JW, Niemann H. The perspectives for porcine-to-human xenografts. Comp Immunol Micro biol Infect Dis. 2009;32(2):91–105. doi: 10.1016/j.cimid.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Zeng L, et al. Multipotent adult progenitor cells from swine bone marrow. Stem Cells. 2006;24(11):2355–2366. doi: 10.1634/stemcells.2005-0551. [DOI] [PubMed] [Google Scholar]
- Zeng L, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115(14):1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- Sekiya I, et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30(6):943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54(3):843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]


