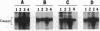Abstract
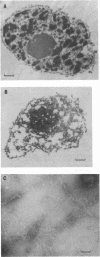
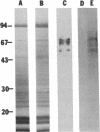
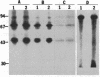
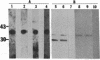
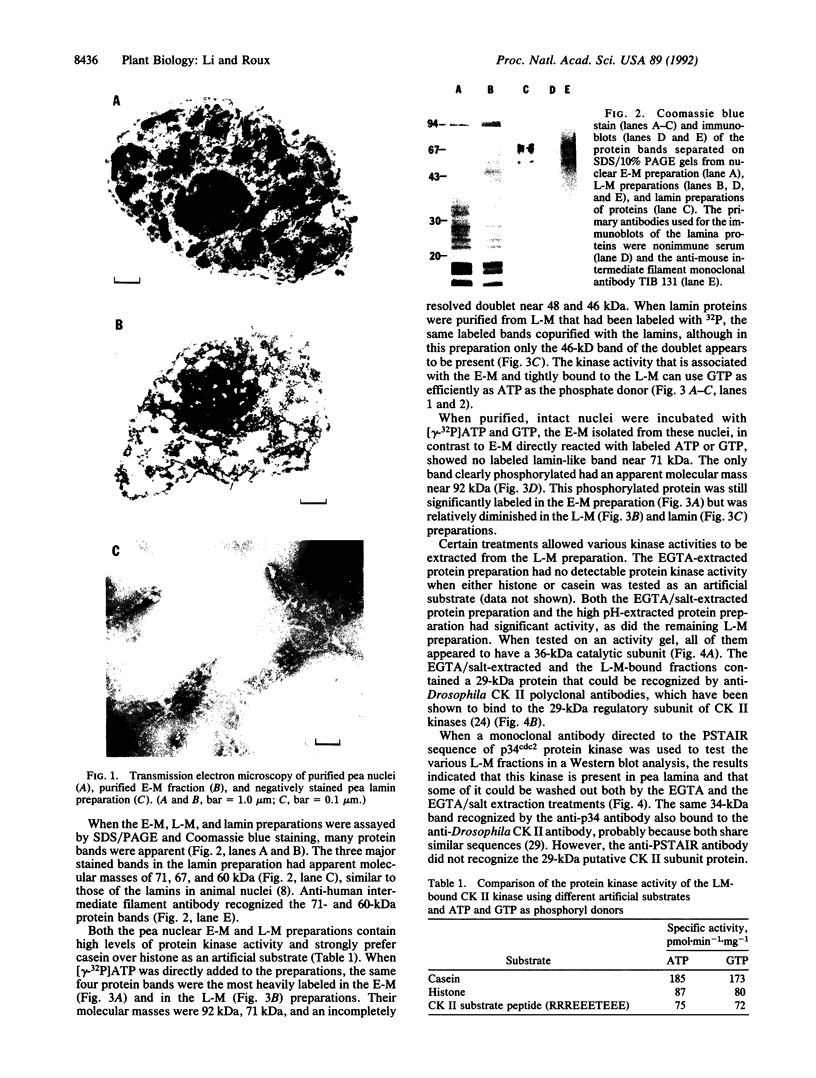
A casein kinase II (CK II)-like protein kinase was identified and partially isolated from a purified envelope-matrix fraction of pea (Pisum sativum L.) nuclei. When [gamma-32P]ATP was directly added to the envelope-matrix preparation, the three most heavily labeled protein bands had molecular masses near 71, 48, and 46 kDa. Protein kinases were removed from the preparation by sequential extraction with Triton X-100, EGTA, 0.3 M NaCl, and a pH 10.5 buffer, but an active kinase still remained bound to the remaining lamina-matrix fraction after these treatments. This kinase had properties resembling CK II kinases previously characterized from animal and plant sources: it preferred casein as an artificial substrate, could use GTP as efficiently as ATP as the phosphoryl donor, was stimulated by spermine, was calcium independent, and had a catalytic subunit of 36 kDa. Some animal and plant CK II kinases have regulatory subunits near 29 kDa, and a lamina-matrix-bound protein of this molecular mass was recognized on immunoblot by anti-Drosophila CK II polyclonal antibodies. Also found associated with the envelope-matrix fraction of pea nuclei were p34cdc2-like and Ca(2+)-dependent protein kinases, but their properties could not account for the protein kinase activity bound to the lamina. The 71-kDa substrate of the CK II-like kinase was lamin A-like, both in its molecular mass and in its cross-reactivity with anti-intermediate filament antibodies. Lamin phosphorylation is considered a crucial early step in the entry of cells into mitosis, so lamina-bound CK II kinases may be important control points for cellular proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chai L. S., Weinfeld H., Sandberg A. A. Ultrastructural changes in the nuclear envelope during mitosis of Chinese hamster cells: a proposed mechanism of nuclear envelope reformation. J Natl Cancer Inst. 1974 Oct;53(4):1033–1048. doi: 10.1093/jnci/53.4.1033. [DOI] [PubMed] [Google Scholar]
- Clawson G. A., Lackey A., Tokes Z. A. The 46-kDa nucleoside triphosphatase of rat liver nuclear scaffold represents the N-terminal portion of lamins A/C. Exp Cell Res. 1988 May;176(1):180–186. doi: 10.1016/0014-4827(88)90132-2. [DOI] [PubMed] [Google Scholar]
- Dahmus G. K., Glover C. V., Brutlag D. L., Dahmus M. E. Similarities in structure and function of calf thymus and Drosophila casein kinase II. J Biol Chem. 1984 Jul 25;259(14):9001–9006. [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Chen Y. R., Roux S. J. Phytochrome and calcium stimulation of protein phosphorylation in isolated pea nuclei. Biochem Biophys Res Commun. 1985 May 16;128(3):1403–1408. doi: 10.1016/0006-291x(85)91096-4. [DOI] [PubMed] [Google Scholar]
- Dessev G., Goldman R. Meiotic breakdown of nuclear envelope in oocytes of Spisula solidissima involves phosphorylation and release of nuclear lamin. Dev Biol. 1988 Dec;130(2):543–550. doi: 10.1016/0012-1606(88)90349-1. [DOI] [PubMed] [Google Scholar]
- Fields A. P., Pettit G. R., May W. S. Phosphorylation of lamin B at the nuclear membrane by activated protein kinase C. J Biol Chem. 1988 Jun 15;263(17):8253–8260. [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Hargreaves A. J., Goodbody K. C., Lloyd C. W. Reconstitution of intermediate filaments from a higher plant. Biochem J. 1989 Jul 15;261(2):679–682. doi: 10.1042/bj2610679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Gibson W., Shaper J. H. Characterization of the major polypeptides of the rat liver nuclear envelope. J Biol Chem. 1983 Feb 25;258(4):2710–2719. [PubMed] [Google Scholar]
- Klimczak L. J., Schindler U., Cashmore A. R. DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. Plant Cell. 1992 Jan;4(1):87–98. doi: 10.1105/tpc.4.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Maridor G., Nigg E. A. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992 Jan;116(1):43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li H., Dauwalder M., Roux S. J. Partial purification and characterization of a Ca(2+)-dependent protein kinase from pea nuclei. Plant Physiol. 1991;96:720–727. doi: 10.1104/pp.96.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Roux S. J. Purification and characterization of a casein kinase 2-type protein kinase from pea nuclei. Plant Physiol. 1992 Jun;99(2):686–692. doi: 10.1104/pp.99.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Brizuela L., Beach D., Eisenman R. N. A role for the p34cdc2 kinase and phosphatases in the regulation of phosphorylation and disassembly of lamin B2 during the cell cycle. EMBO J. 1991 Apr;10(4):865–875. doi: 10.1002/j.1460-2075.1991.tb08019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Marot J., Cayla X., Pouhle R., Belle R. Purification and characterization of a casein-kinase-II-type enzyme from Xenopus laevis ovary. Biological effects on the meiotic cell division of full-grown oocyte. Eur J Biochem. 1988 Jan 15;171(1-2):107–117. doi: 10.1111/j.1432-1033.1988.tb13765.x. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu Rev Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Nuclear function and organization: the potential of immunochemical approaches. Int Rev Cytol. 1988;110:27–92. doi: 10.1016/s0074-7696(08)61847-1. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Suprynowicz F. A., Gerace L. A fractionated cell-free system for analysis of prophase nuclear disassembly. J Cell Biol. 1986 Dec;103(6 Pt 1):2073–2081. doi: 10.1083/jcb.103.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Kuenzel E. A., Walsh K. A., Krebs E. G. Amino acid sequence of the beta subunit of bovine lung casein kinase II. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4851–4855. doi: 10.1073/pnas.84.14.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]