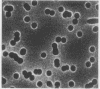
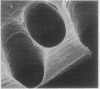
Abstract
Erythrocyte deformability was formerly measured by its contribution to whole blood viscosity. It is now more commonly measured by filtration of erythrocytes through, or aspiration into, pores of 3-5 microns diameter and by the measurement of shear induced erythrocyte elongation using laser diffractometry. Recent improvements in the technology for erythrocyte filtration have included the removal of acute phase reactants from test erythrocyte suspensions, ultrasonic cleaning and reuse of filter membranes, awareness of the importance of mean cell volume as a determinant of flow through 3 microns diameter pores, and the ability to detect subpopulations of less deformable erythrocytes. Measurements of erythrocyte elongation by laser diffractometry, using the Ektacytometer, are also influenced by cell size and need to be corrected for mean cell volume. These advances have greatly improved the sensitivity and specificity of rheological methods for measuring the deformability of erythrocytes and for investigating the mode of action of rheologically active drugs.
Full text
PDF
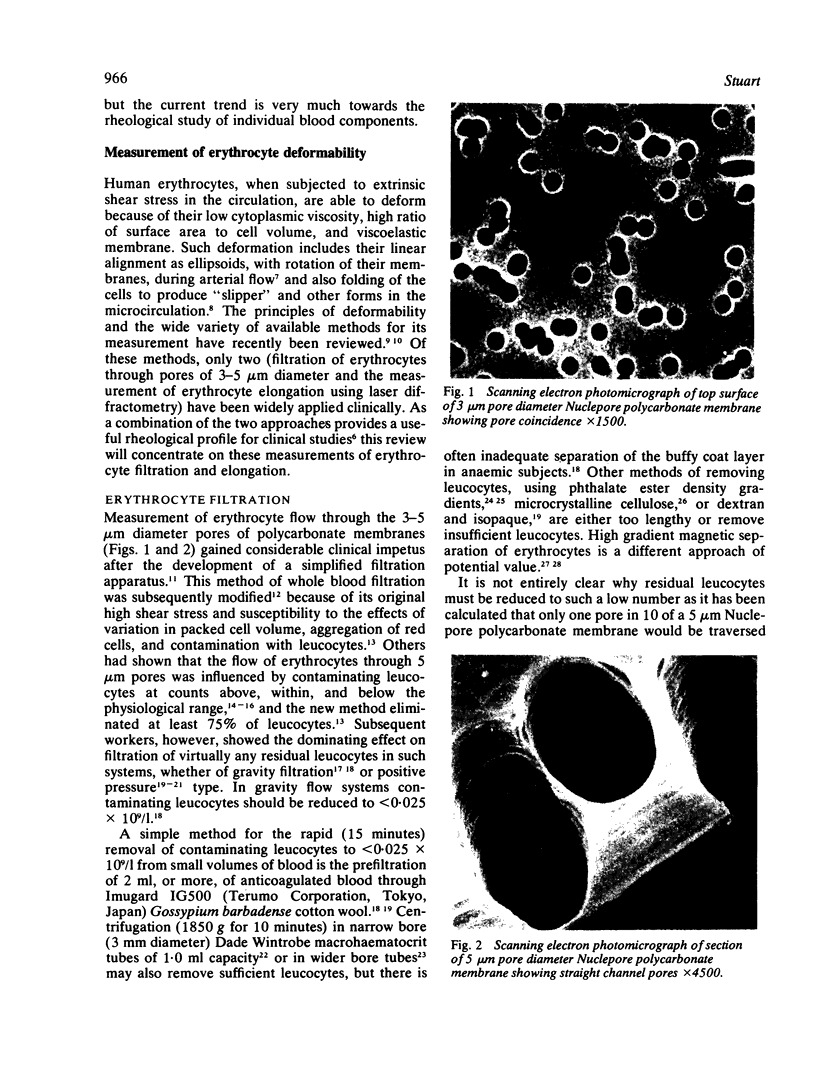

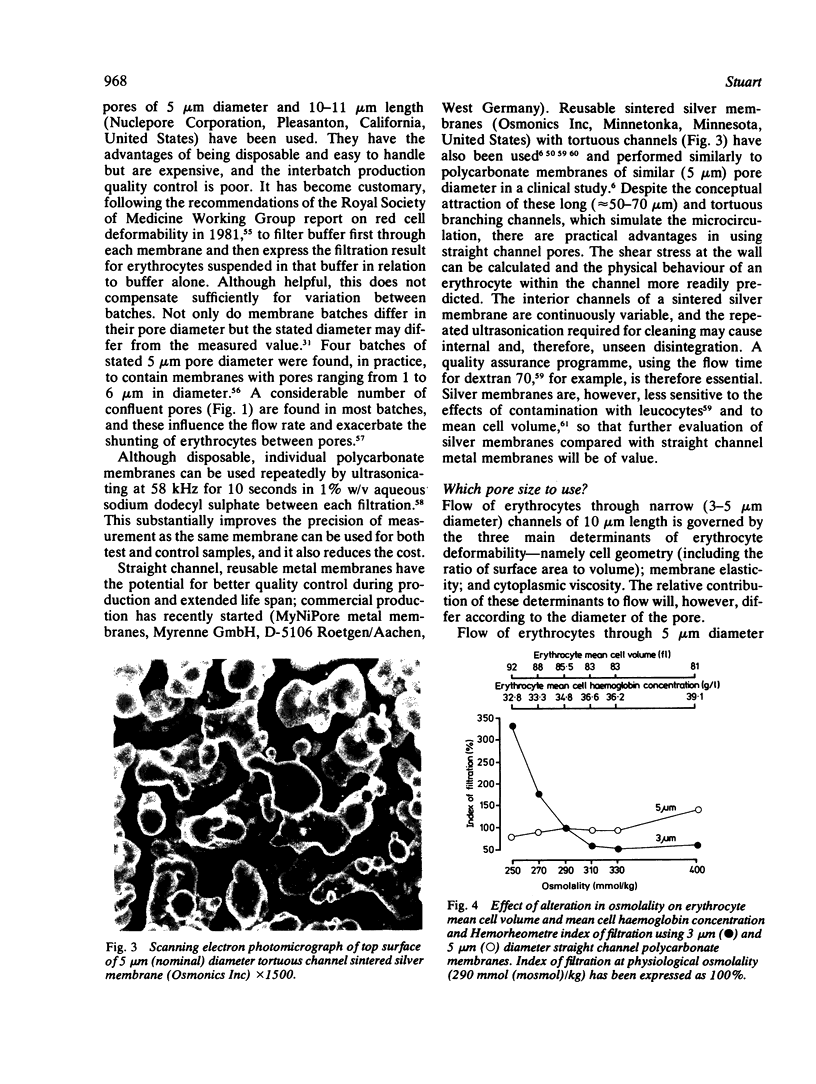

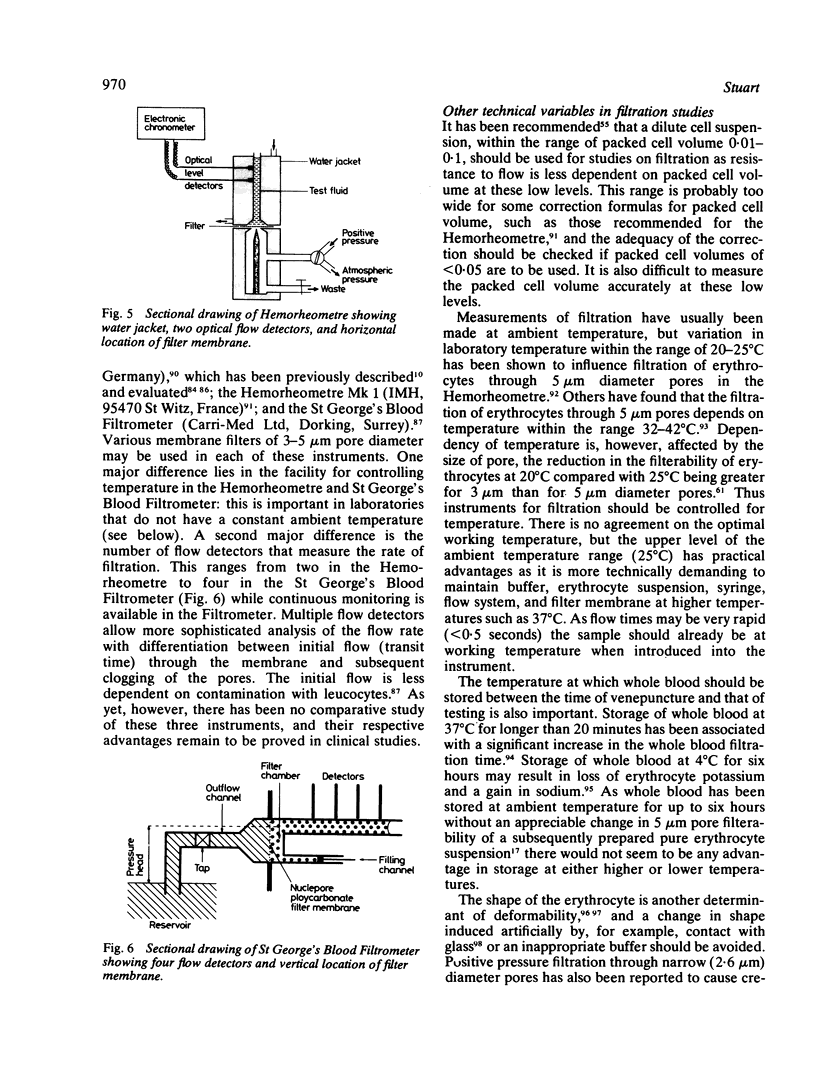
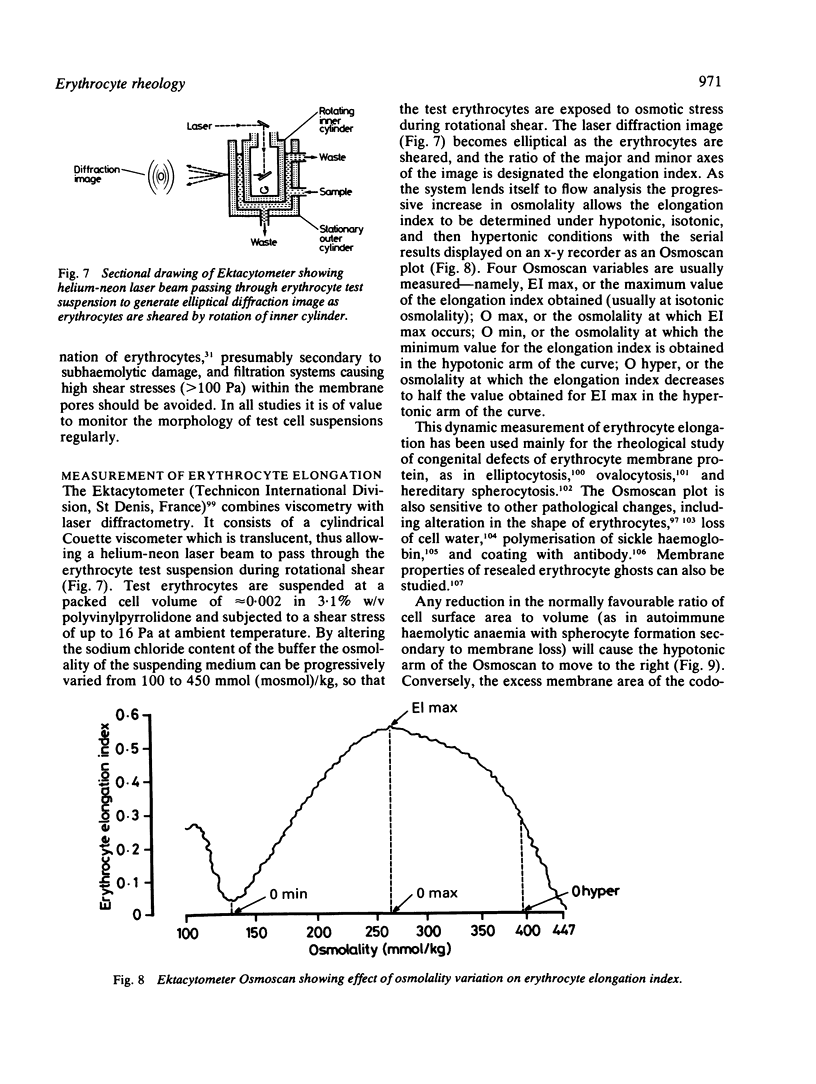
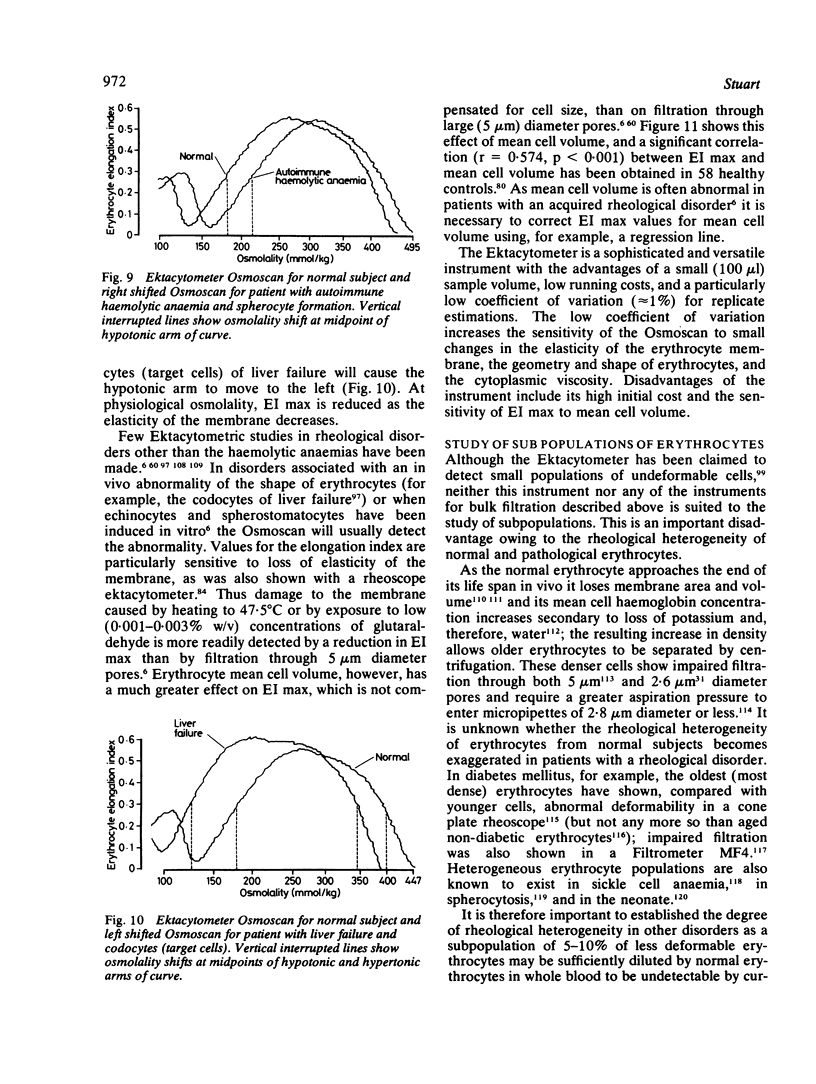
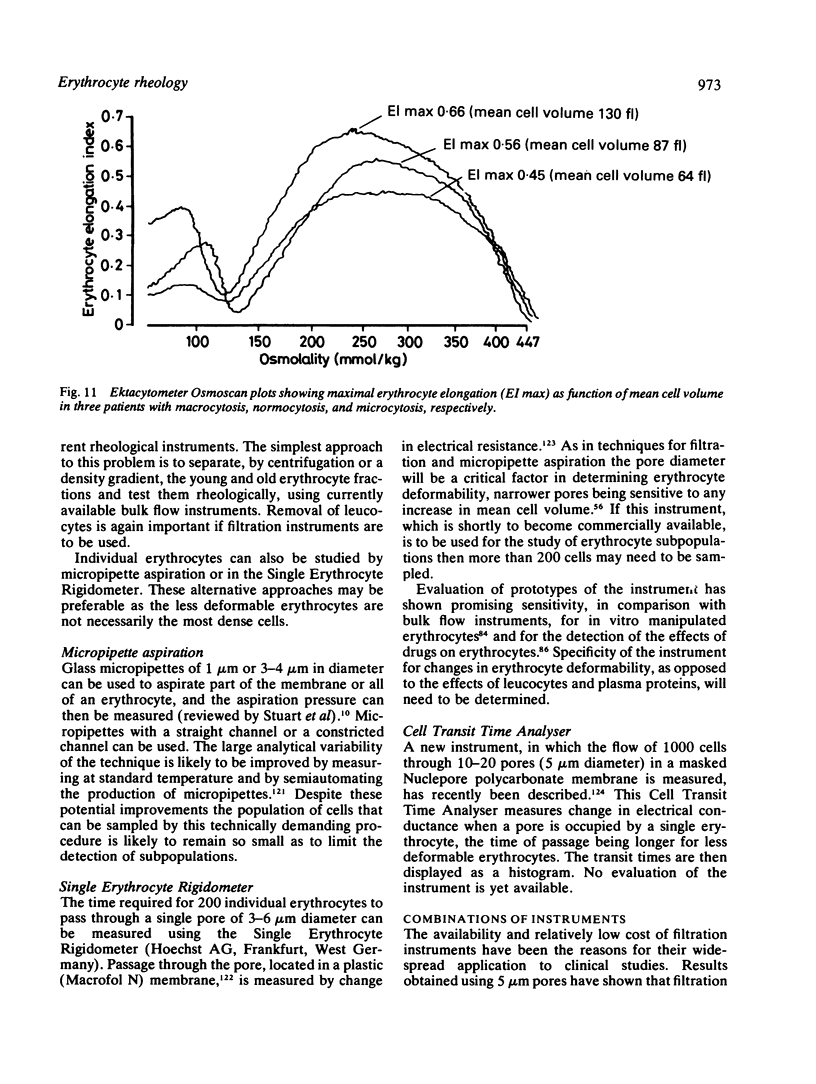




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarts P. A., Bolhuis P. A., Sakariassen K. S., Heethaar R. M., Sixma J. J. Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood. 1983 Jul;62(1):214–217. [PubMed] [Google Scholar]
- Aarts P. A., Heethaar R. M., Sixma J. J. Red blood cell deformability influences platelets--vessel wall interaction in flowing blood. Blood. 1984 Dec;64(6):1228–1233. [PubMed] [Google Scholar]
- Alderman M. J., Ridge A., Morley A. A., Ryall R. G., Walsh J. A. Effect of total leucocyte count on whole blood filterability in patients with peripheral vascular disease. J Clin Pathol. 1981 Feb;34(2):163–166. doi: 10.1136/jcp.34.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundel P. A., Torr N. C., Mackay J. D. Application of a low-pressure differential filtration technique to suspensions of red blood cells. Med Biol Eng Comput. 1984 Nov;22(6):516–520. doi: 10.1007/BF02443864. [DOI] [PubMed] [Google Scholar]
- Baar S. A convenient and reproducible filtration technique for the determination of erythrocyte flexibility. Br J Haematol. 1976 Sep;34(1):69–78. doi: 10.1111/j.1365-2141.1976.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Ballas S. K., Mohandas N., Clark M. R., Shohet S. B. Rheological properties of antibody-coated red cells. Transfusion. 1984 Mar-Apr;24(2):124–129. doi: 10.1046/j.1537-2995.1984.24284173342.x. [DOI] [PubMed] [Google Scholar]
- Ballas S. K., Saidi P., Constantino M. Reduced erythrocytic deformability in megaloblastic anemia. Am J Clin Pathol. 1976 Dec;66(6):953–957. doi: 10.1093/ajcp/66.6.953. [DOI] [PubMed] [Google Scholar]
- Bareford D., Lucas G. S., Caldwell N. M., Stone P. C., Baar S., Stuart J. Erythrocyte deformability in peripheral occlusive arterial disease. J Clin Pathol. 1985 Feb;38(2):135–139. doi: 10.1136/jcp.38.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. M., Parker A. C., Maddy A. H. Abnormal pattern of erythrocyte ageing in hereditary spherocytosis as shown by Percoll density gradient centrifugation. Clin Chim Acta. 1984 Sep 15;142(1):91–102. doi: 10.1016/0009-8981(84)90104-9. [DOI] [PubMed] [Google Scholar]
- Bessis M., Feo C., Jones E., Nossal M. Adaptation of the ektacytometer to automated continuous pO2 changes: determination of erythrocyte deformability in sickling disorders. Cytometry. 1983 Jan;3(4):296–299. doi: 10.1002/cyto.990030412. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Born G. V. Fluid-mechanical and biochemical interactions in haemostasis. Br Med Bull. 1977 Sep;33(3):193–197. doi: 10.1093/oxfordjournals.bmb.a071435. [DOI] [PubMed] [Google Scholar]
- Brown C. H., 3rd, Leverett L. B., Lewis C. W., Alfrey C. P., Jr, Hellums J. D. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. J Lab Clin Med. 1975 Sep;86(3):462–471. [PubMed] [Google Scholar]
- Buchan P. C. Evaluation and modification of whole blood filtration in the measurement of erythrocyte deformability in pregnancy and the newborn. Br J Haematol. 1980 May;45(1):97–105. doi: 10.1111/j.1365-2141.1980.tb03815.x. [DOI] [PubMed] [Google Scholar]
- Bull B., Feo C., Bessis M. Behavior of elliptocytes under shear stress in the rheoscope and ektacytometer. Cytometry. 1983 Jan;3(4):300–304. doi: 10.1002/cyto.990030413. [DOI] [PubMed] [Google Scholar]
- Chan M. T., Catry E., Weill D., Marcel G. A., George C. Assessment of erythrocyte deformability by constant flow filtration technique: analysis of factors influencing the initial pressure. Biorheology Suppl. 1984;1:267–270. doi: 10.3233/bir-1984-23s146. [DOI] [PubMed] [Google Scholar]
- Chien S., Schmalzer E. A., Lee M. M., Impelluso T., Skalak R. Role of white blood cells in filtration of blood cell suspensions. Biorheology. 1983;20(1):11–27. doi: 10.3233/bir-1983-20102. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- Cohen N. S., Ekholm J. E., Luthra M. G., Hanahan D. J. Biochemical characterization of density-separated human erythrocytes. Biochim Biophys Acta. 1976 Jan 21;419(2):229–242. doi: 10.1016/0005-2736(76)90349-7. [DOI] [PubMed] [Google Scholar]
- Colley C. M., Fleck A., Goode A. W., Muller B. R., Myers M. A. Early time course of the acute phase protein response in man. J Clin Pathol. 1983 Feb;36(2):203–207. doi: 10.1136/jcp.36.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dodds A. J., Boyd M. J., Allen J., Bennett E. D., Flute P. T., Dormandy J. A. Changes in red cell deformability and other haemorrheological variables after myocardial infarction. Br Heart J. 1980 Nov;44(5):508–511. doi: 10.1136/hrt.44.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds A. J., O'Reilly M. J., Yates C. J., Cotton L. T., Flute P. T., Dormandy J. A. Haemorrheological response to plasma exchange in Raynaud's syndrome. Br Med J. 1979 Nov 10;2(6199):1186–1187. doi: 10.1136/bmj.2.6199.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy J. A., Ernst E. Whole blood filtration. J Clin Pathol. 1981 Aug;34(8):938–939. doi: 10.1136/jcp.34.8.938-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., La Celle P. L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood. 1975 Jan;45(1):29–43. [PubMed] [Google Scholar]
- Fiocco R., Quattrocchi D., Neirotti M., Petracchini V., Pernigotti L. M., Porta M. Effects of temperature on red cell filtration. Panminerva Med. 1984 Jul-Sep;26(3):193–195. [PubMed] [Google Scholar]
- Fischer T. M., Stöhr-Lissen M., Schmid-Schönbein H. The red cell as a fluid droplet: tank tread-like motion of the human erythrocyte membrane in shear flow. Science. 1978 Nov 24;202(4370):894–896. doi: 10.1126/science.715448. [DOI] [PubMed] [Google Scholar]
- Gaehtgens P., Dührssen C., Albrecht K. H. Motion, deformation, and interaction of blood cells and plasma during flow through narrow capillary tubes. Blood Cells. 1980;6(4):799–817. [PubMed] [Google Scholar]
- Goebel K. M., Lanser K. G. Biorheological and metabolic dysfunctions of density-fractionated erythrocytes in diabetics with peripheral vascular disease. Biomed Biochim Acta. 1983;42(11-12):S102–S106. [PubMed] [Google Scholar]
- Gregersen M. I., Bryant C. A., Hammerle W. E., Usami S., Chien S. Flow Characteristics of Human Erythrocytes through Polycarbonate Sieves. Science. 1967 Aug 18;157(3790):825–827. doi: 10.1126/science.157.3790.825. [DOI] [PubMed] [Google Scholar]
- Groner W., Mohandas N., Bessis M. New optical technique for measuring erythrocyte deformability with the ektacytometer. Clin Chem. 1980 Sep;26(10):1435–1442. [PubMed] [Google Scholar]
- Hanss M. Erythrocyte filtrability measurement by the initial flow rate method. Biorheology. 1983;20(2):199–211. doi: 10.3233/bir-1983-20209. [DOI] [PubMed] [Google Scholar]
- Hanss M., Koutsouris D. Thermal transitions of red blood cell deformability. Correlation with membrane rheological properties. Biochim Biophys Acta. 1984 Jan 25;769(2):461–470. doi: 10.1016/0005-2736(84)90331-6. [DOI] [PubMed] [Google Scholar]
- Heath B. P., Mohandas N., Wyatt J. L., Shohet S. B. Deformability of isolated red blood cell membranes. Biochim Biophys Acta. 1982 Oct 7;691(2):211–219. doi: 10.1016/0005-2736(82)90409-6. [DOI] [PubMed] [Google Scholar]
- Heinemann G., Schievelbein H., Eber S. Effect of cigarette smoking on white blood cells and erythrocyte enzymes. Arch Environ Health. 1982 Sep-Oct;37(5):261–265. doi: 10.1080/00039896.1982.10667576. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L., CASTLE W. B. Red cell filtration and the pathogenesis of certain hemolytic anemias. Blood. 1961 Aug;18:133–148. [PubMed] [Google Scholar]
- Jones J. G., Holland B. M., Humphrys J., Wardrop C. A. The flow of blood cell suspensions through 3 microns and 5 microns Nuclepore membranes: a comparison of kinetic analysis with scanning electron microscopic examinations. Br J Haematol. 1985 Mar;59(3):541–546. doi: 10.1111/j.1365-2141.1985.tb07341.x. [DOI] [PubMed] [Google Scholar]
- Juhan I., Vague P., Buonocore M., Moulin J. P., Jouve R., Vialettes B. Abnormalities of erythrocyte deformability and platelet aggregation in insulin-dependent diabetics corrected by insulin in vivo and in vitro. Lancet. 1982 Mar 6;1(8271):535–537. doi: 10.1016/s0140-6736(82)92045-1. [DOI] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Windisch P., Baez S., Nagel R. L. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983 Jul;72(1):22–31. doi: 10.1172/JCI110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter H., Dauer U., Teitel P., Schmid-Schönbein H., Trapp R. The single erythrocyte rigidometer (SER) as a reference for RBC deformability. Biorheology. 1982;19(6):737–753. doi: 10.3233/bir-1982-19610. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Koyama T. Effects of Na+ and K+ on protein adsorption on red blood cell surface. Am J Physiol. 1984 Nov;247(5 Pt 2):H748–H753. doi: 10.1152/ajpheart.1984.247.5.H748. [DOI] [PubMed] [Google Scholar]
- Kovacs I. B., O'Grady J. Prostacyclin increases filterability of normal and rigidified human red blood cells in vitro. Agents Actions. 1984 Feb;14(2):306–310. doi: 10.1007/BF01966658. [DOI] [PubMed] [Google Scholar]
- Kucera W., Meier W., Lerche D., Paulitschke M. pH-Wert- und osmolaritätsbedingte Veränderungen deformierbarkeitsbestimmender Faktoren für die Filtrierbarkeit menschlicher Erythrozyten. Biomed Biochim Acta. 1984;43(3):337–348. [PubMed] [Google Scholar]
- LOVELOCK J. E. The physical instability of human red blood cells. Biochem J. 1955 Aug;60(4):692–696. doi: 10.1042/bj0600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L. Evaluation of a simplified filtration technique for the routine measurement of erythrocyte deformability. Scand J Clin Lab Invest Suppl. 1981;156:35–40. doi: 10.3109/00365518109097427. [DOI] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L. The measurement of erythrocyte deformability using micropore membranes. A sensitive technique with clinical applications. J Lab Clin Med. 1979 Jul;94(1):133–143. [PubMed] [Google Scholar]
- Legge D. G., Shortman K. The effect of pH on the volume, density and shape of erythrocytes and thymic lymphocytes. Br J Haematol. 1968 Mar;14(3):323–335. doi: 10.1111/j.1365-2141.1968.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Linderkamp O., Wu P. Y., Meiselman H. J. Deformability of density separated red blood cells in normal newborn infants and adults. Pediatr Res. 1982 Nov;16(11):964–968. doi: 10.1203/00006450-198211000-00013. [DOI] [PubMed] [Google Scholar]
- Lingard P. S. Capillary pore rheology of erythrocytes. II. A method for the preparation of leucocyte-poor erythrocyte suspensions suitable for capillary pore rheometry. Microvasc Res. 1974 Sep;8(2):181–191. doi: 10.1016/0026-2862(74)90093-4. [DOI] [PubMed] [Google Scholar]
- Lingard P. S. Capillary pore rheology of erythrocytes. III. On the interpretation of human erythrocyte behaviour in narrow capillary pores. Microvasc Res. 1977 Jan;13(1):29–58. doi: 10.1016/0026-2862(77)90114-5. [DOI] [PubMed] [Google Scholar]
- Lingard P. S. Capillary pore rheology of erythrocytes. IV. Effect of pore diameter and haematocrit. Microvasc Res. 1977 Jan;13(1):59–77. doi: 10.1016/0026-2862(77)90115-7. [DOI] [PubMed] [Google Scholar]
- Maeda H., Morinaga T., Mori I., Nishi K. Further characterization of the effects of alpha-1-acid glycoprotein on the passage of human erythrocytes through micropores. Cell Struct Funct. 1984 Sep;9(3):279–290. doi: 10.1247/csf.9.279. [DOI] [PubMed] [Google Scholar]
- Meade T. W., Chakrabarti R., Haines A. P., North W. R., Stirling Y. Characteristics affecting fibrinolytic activity and plasma fibrinogen concentrations. Br Med J. 1979 Jan 20;1(6157):153–156. doi: 10.1136/bmj.1.6157.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade T. W., North W. R., Chakrabarti R., Stirling Y., Haines A. P., Thompson S. G., Brozovié M. Haemostatic function and cardiovascular death: early results of a prospective study. Lancet. 1980 May 17;1(8177):1050–1054. doi: 10.1016/s0140-6736(80)91498-1. [DOI] [PubMed] [Google Scholar]
- Meiselman H. J. Rheology of shape-transformed human red cells. Biorheology. 1978;15(3-4):225–237. doi: 10.3233/bir-1978-153-410. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Lie-Injo L. E., Friedman M., Mak J. W. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984 Jun;63(6):1385–1392. [PubMed] [Google Scholar]
- Myers M. A., Fleck A., Sampson B., Colley C. M., Bent J., Hall G. Early plasma protein and mineral changes after surgery: a two stage process. J Clin Pathol. 1984 Aug;37(8):862–866. doi: 10.1136/jcp.37.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash G. B., Wyard S. J. Changes in surface area and volume measured by micropipette aspiration for erythrocytes ageing in vivo. Biorheology. 1980;17(5-6):479–484. [PubMed] [Google Scholar]
- Norton J. M., Barker N. D., Rand P. W. Effect of cell geometry, internal viscosity, and pH on erythrocyte filterability. Proc Soc Exp Biol Med. 1981 Mar;166(3):449–456. doi: 10.3181/00379727-166-41089. [DOI] [PubMed] [Google Scholar]
- Paul F., Melville D., Roath S. The morphology and viability of blood components processed by high-gradient magnetic separation. Clin Lab Haematol. 1985;7(1):43–53. doi: 10.1111/j.1365-2257.1985.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Paul F., Roath S., Melville D. Differential blood cell separation using a high gradient magnetic field. Br J Haematol. 1978 Feb;38(2):273–280. doi: 10.1111/j.1365-2141.1978.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Reid H. L., Barnes A. J., Lock P. J., Dormandy J. A., Dormandy T. L. A simple method for measuring erythrocyte deformability. J Clin Pathol. 1976 Sep;29(9):855–858. doi: 10.1136/jcp.29.9.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers R. C., Sutera S. P., Joist J. H. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 1984 Dec;64(6):1200–1206. [PubMed] [Google Scholar]
- Reinhart W. H., Usami S., Schmalzer E. A., Lee M. M., Chien S. Evaluation of red blood cell filterability test: influences of pore size, hematocrit level, and flow rate. J Lab Clin Med. 1984 Oct;104(4):501–516. [PubMed] [Google Scholar]
- Reizenstein P. The haematological stress syndrome. Br J Haematol. 1979 Nov;43(3):329–334. doi: 10.1111/j.1365-2141.1979.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Rem J., Brandt M. R., Kehlet H. Prevention of postoperative lymphopenia and granulocytosis by epidural analgesia. Lancet. 1980 Feb 9;1(8163):283–284. doi: 10.1016/s0140-6736(80)90780-1. [DOI] [PubMed] [Google Scholar]
- Roggenkamp H. G., Jung F., Kiesewetter H. Ein Gerät zur elektrischen Messung der Verformbarkeit von Erythrozyten. Biomed Tech (Berl) 1983 May;28(5):100–104. doi: 10.1515/bmte.1983.28.5.100. [DOI] [PubMed] [Google Scholar]
- Roggenkamp H. G., Kiesewetter H., Spohr R., Dauer U., Busch L. C. Production of single pore membranes for the measurement of red blood cell deformability. Biomed Tech (Berl) 1981 Jul-Aug;26(7-8):167–169. doi: 10.1515/bmte.1981.26.7-8.167. [DOI] [PubMed] [Google Scholar]
- Rossi G., Fiocco R., Neirotti M., Petracchini V., Porta M. Red cell filtration and aging of the samples. Panminerva Med. 1984 Jul-Sep;26(3):191–192. [PubMed] [Google Scholar]
- Schmid-Schöenbein H., Wells R. Fluid drop-like transition of erythrocytes under shear. Science. 1969 Jul 18;165(3890):288–291. doi: 10.1126/science.165.3890.288. [DOI] [PubMed] [Google Scholar]
- Schröer R., Muth K. Filtrability of whole blood and erythrocyte suspensions under the influence of several anticoagulants. Ric Clin Lab. 1981;11 (Suppl 1):109–116. [PubMed] [Google Scholar]
- Seiffge D. Dependency of red blood cell passage time on pore geometry in the single-pore erythrocyte rigidometer (SER). Biorheology Suppl. 1984;1:245–247. doi: 10.3233/bir-1984-23s142. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Mohandas N., Shohet S. B. Variability in erythrocyte deformability among various mammals. Am J Physiol. 1979 May;236(5):H725–H730. doi: 10.1152/ajpheart.1979.236.5.H725. [DOI] [PubMed] [Google Scholar]
- Stoltz J. F., Duvivier C., Malher E. Erythrometer: a new device for measuring erythrocyte filterability and plasma viscosity. Biorheology Suppl. 1984;1:255–259. doi: 10.3233/bir-1984-23s144. [DOI] [PubMed] [Google Scholar]
- Stuart J., George A. J., Davies A. J., Aukland A., Hurlow R. A. Haematological stress syndrome in atherosclerosis. J Clin Pathol. 1981 May;34(5):464–467. doi: 10.1136/jcp.34.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J., Kenny M. W. Blood rheology. J Clin Pathol. 1980 May;33(5):417–429. doi: 10.1136/jcp.33.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J., Kenny M. W., Inglis T. C. Erythrocyte filterability in normal pregnancy and pre-eclampsia. Br J Haematol. 1983 Feb;53(2):353–355. doi: 10.1111/j.1365-2141.1983.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Stuart J. The acute-phase reaction and haematological stress syndrome in vascular disease. Int J Microcirc Clin Exp. 1984;3(2):115–129. [PubMed] [Google Scholar]
- Tatsumi N., Tsuda I., Maeda H., Tomioka A. A non-aspiration method for erythrocyte filterability measurement. Med Lab Sci. 1984 Jan;41(1):68–70. [PubMed] [Google Scholar]
- Thompson C. B., Galli R. L., Melaragno A. J., Valeri C. R. A method for the separation of erythrocytes on the basis of size using counterflow centrifugation. Am J Hematol. 1984 Aug;17(2):177–183. doi: 10.1002/ajh.2830170209. [DOI] [PubMed] [Google Scholar]
- Tillmann W., Levin C., Prindull G., Schröter W. Rheological properties of young and aged human erythrocytes. Klin Wochenschr. 1980 Jun 2;58(11):569–574. doi: 10.1007/BF01477168. [DOI] [PubMed] [Google Scholar]
- Usami S., Chien S., Bertles J. F. Deformability of sickle cells as studied by microsieving. J Lab Clin Med. 1975 Aug;86(2):274–279. [PubMed] [Google Scholar]
- Weissberg P. L., West M. J., Woods K. L. An improved method for measuring intracellular electrolytes in erythrocytes and the effects of cold storage. Clin Chim Acta. 1983 Mar 28;129(1):85–89. doi: 10.1016/0009-8981(83)90154-7. [DOI] [PubMed] [Google Scholar]
- White J. G., Burris S. M., Tukey D., Smith C., 2nd, Clawson C. C. Micropipette aspiration of human platelets: influence of microtubules and actin filaments on deformability. Blood. 1984 Jul;64(1):210–214. [PubMed] [Google Scholar]
- Whittington R. B., Harkness J. Whole-blood viscosity, as determined by plasma viscosity, haematocrit, and shear. Biorheology. 1982;19(1/2):175–184. doi: 10.3233/bir-1982-191-220. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L., Svärdsudd K., Korsan-Bengtsen K., Larsson B., Welin L., Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984 Aug 23;311(8):501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Gardner R. A., Boylan C. W., Carroll G. L., Chang K., Marvel J. S., Gonen B., Kilo C., Tran-Son-Tay R., Sutera S. P. Microrheologic investigation of erythrocyte deformability in diabetes mellitus. Blood. 1985 Feb;65(2):283–288. [PubMed] [Google Scholar]
- Williamson J. R., Kilo C., Sutera S. P., Gardner R. A., Boylan C. W., Chang K. C. Shear induced deformation of red cells: decreased elongation and membrane rotation in diabetes mellitus. Horm Metab Res Suppl. 1981;11:103–104. [PubMed] [Google Scholar]
- Yip R., Mohandas N., Clark M. R., Jain S., Shohet S. B., Dallman P. R. Red cell membrane stiffness in iron deficiency. Blood. 1983 Jul;62(1):99–106. [PubMed] [Google Scholar]