Abstract
This study was designed to evaluate the protective effect of the Bacillus subtilis strain against complications related to heat stress. Thirty-two Sprague-Dawley rats were used in this study. Animals were orally treated twice a day for two days with B. subtilis BSB3 strain or PBS. The next day after the last treatment, each group was divided and two experimental groups (one treated with PBS and one treated with B. subtilis) were placed at 45 oC for 25 min. Two control groups stayed for 25 min at room temperature. All rats were euthanized and different parameters were analyzed in all groups. Adverse effects of heat stress are registered by the decrease of villi height and total mucosal thickness in the intestinal epithelium; translocation of bacteria from the lumen; increased vesiculation of erythrocytes and elevation of the lipopolysaccharides (LPS) level in the blood. The protective efficacy of treatment is evaluated by prevention of these side effects. The protocol was set up for the oral treatment of rats with bacteria for prevention of heat stress complications, but this protocol can be modified and used for other routes of administration and for analysis of different compounds.
Keywords: Immunology, Issue 113, Bacillus subtilis, heat stress, gut microbiota, probiotics, erythrocyte vesiculation, translocation of bacteria, lipopolysaccharides
Introduction
Different stressors affect human and animal health. Temperature is one of the most stressful factors, causing chronic, acute and even lethal illnesses1. Changes in intestinal morphology and a loss of gut barrier integrity after heat stress were documented in many cases2,3. This protective barrier is responsible for defense against translocation of gut bacteria and their toxins, in particular lipopolysaccharides (LPS), from the lumen to the internal circulation4,5. Stability of the gut microbiota significantly influences intestinal barrier function, the immune response of the host, and tolerance to stress conditions6. Thus, modulation of the intestinal microbiota can provide a novel approach for prevention of stress-related adverse effects.
Beneficial bacteria have been used as a promising strategy to modify the gut microbiota for successful management of various clinical conditions, such as diarrhea, inflammatory bowel disease, atopic dermatitis, metabolic disorders7-10. Probiotic effects of beneficial bacteria include production of essential metabolites to support intestinal health, stimulation of the immune system, promotion of lactose tolerance, restoration of epithelial dysfunction. Probiotics were effective in prevention of the stress-related complications in vitro and in animal models11,12.
Researchers have given more attention to Bacillus bacteria as probiotics because these bacteria support intestinal homeostasis13 and have beneficial effects on the host14. Our previous data demonstrated high efficacy of Bacillus probiotics against pathogens in vitro15,16 and in clinical trials17,18. Here, we aim to evaluate the preventive effect of B. subtilis BSB3 strain against complications after heat stress.
Protocol
All experimental procedures were approved by IACUC of Auburn University, Auburn, Alabama.
1. Preparation of Culture Media, Dishes and Bacterial Culture
Inoculate 10 ml of nutrient broth in a flask with a single colony of Bacillus subtilis BSB3 culture, grown overnight on a nutrient agar plate.
Make 500 ml of sporulation medium19 (per liter: Bacto Nutrient Broth, 8 g; 10% (w/v) KCl, 10 ml; 1.2% (w/v) MgSO4 x 7H2O, 10 ml; agar, 15 g) and autoclave at 121 oC for 20 min. Allow to cool to 50 oC and add the following sterile solutions:1 M Ca(NO3)2, 1 ml; 0.01 M MnCl2, 1 ml; 1 mM FeSO4, 1 ml.
Cool prepared medium to 40 oC for 20 min at room temperature and pour 25 ml into each of 20 sterile Petri dishes in the sterile cabinet. Let the medium solidify overnight.
Spread 0.5 ml of overnight culture of Bacillus subtilis BSB3 onto the surface of the medium in the Petri dishes. Incubate culture at 37 oC for 5 days until 90% sporulation. Reveal phase-bright spores by high resolution microscopy.
Harvest bacteria by adding 1 ml of sterile phosphate buffered saline (PBS) to the plate and scraping bacteria from a surface using a sterile cell spreader. Store the suspension in the refrigerator until needed.
Confirm viability of bacteria by plating 0.1 ml of the appropriate dilution (10-5 to 10-6) of a bacterial suspension on sporulation medium plates followed by incubation for 18 - 24 hr at 37 oC.
Count bacterial colonies on the plates using a colony counter and calculate the titer of bacteria in the tested suspension. Prepare bacterial suspension with the final concentration 1 x 108 CFU/ml in PBS.
2. Animals
Use 32 male Sprague–Dawley rats (250 - 300 g). Maintain animals under specific pathogen-free conditions with free access to standard pellet chow and water. Establish a mean temperature (21 ± 1 oC), humidity (50 ± 5%) and a 12 hr light-dark cycle.
3. Experimental Design
Start treatment of animals by oral gavage11, after 2 days of acclimatization. Use syringe with the oral gavage needle. Treat 16 rats with B. subtilis BSB3 suspension (1 ml per rat) twice a day for two days (B. subtilis group). Treat 16 rats with PBS (1 ml per rat) twice a day for two days (PBS group) (Figure 1).
After treatment, subdivide each group (8 rats per group): Group 1- control (PBS/no stress), Group 2- B. subtilis (B. subtilis /no stress), Group 3- stress (PBS/heat stress) and Group 4- B. subtilis + stress (B. subtilis/heat stress).
Measure the rectal temperature of each rat using an electronic digital thermometer. Keep rats of groups 1 and 2 at room temperature for 25 min. Place animals of groups 3 and 4 in a climate chamber at 45 oC and relative humidity 55% for 25 min.
Afterwards, measure the rectal temperature of each rat in all groups using an electronic digital thermometer. Place all animals at room temperature for 4 hr.
Anesthetize rats with isoflurane (2 - 4%) in a sealable anesthesia jar and observe until the rats are deeply anesthetized (absence of response to toe pinch). Euthanize rats by rapid decapitation with a guillotine.
Collect trunk blood from each rat20 (15 - 16 ml) with the apyrogenic pipettes into apyrogenic tubes to obtain serum and immediately take samples of blood (7 µl from each rat) with a pipette for microscopic examination.
Put tubes with the blood in a refrigerator for at least 30 min to allow clotting. Centrifuge tubes at 20 oC, 7,000 x g for 10 min and quickly remove serum with a pipette. Aliquot serum obtained from each rat into 50 µl volumes and store at -20 oC until assay.
4. Surgical Procedure
Cut/trim all fur from the abdomen and thorax of the underside of the carcass with electric shears. Place the carcass in a sterile cabinet and treat the shaved surface of the carcass with 70% alcohol.
Use a sterile scalpel to create a midline incision of the abdomen. Remove liver, mesenteric lymph nodes, spleen and small intestine from each rat using sterile tweezers.
5. Analysis of Bacterial Translocation
Place each sample of liver, mesenteric lymph nodes and spleen into pre-weighed sterile tubes and weigh. Calculate the weight of the sample as a difference between the weight of a tube with a sample and the weight of an empty tube.
Add sterile PBS to the tube to obtain a 1:10 dilution (w/v) of the sample and homogenize each sample with a sterile glass tissue homogenizer to obtain a homogeneous suspension21.
Make serial dilutions (10-1 to 10-4) of each sample in sterile PBS. Plate 0.1 ml of all dilutions of each tissue onto surface of MacConkey's and 5% blood agar and Brucella blood agar with hemin (0.005 g/L) and vitamin K1 (0.01 g/L) plates.
Incubate MacConkey's and 5% blood agar plates aerobically and Brucella blood agar plates under anaerobic conditions at 37 oC22.
Count colonies of aerobic bacteria after 24 hr, and colonies of anaerobic bacteria after 48 hr of incubation using a colony counter. Express the results as the number of colony-forming units (CFU) in a gram of tissue.
6. Histological Analysis
Remove small intestine samples from each rat by the surgical procedure (steps 4.1 - 4.2). Fix samples in Bouin’s Fixative, embed in paraffin, prepare 6 µm sections and stain sections with hematoxylin and eosin using standard procedures23.
Use a high-resolution microscope system for measuring height of intestinal villi and total mucosal thickness in each sample (magnification 100X)24. Analyze 4 samples from each rat and make at least twenty measurements in each sample. Express results in micrometers.
7. Cytokine Assay
Use commercial rat ELISA kits for IL-1β; IL-6; TNF-α; INF-γ, and IL-10 to measure levels of cytokines in serum according to manufacturer’s protocol.
Generate standard curves for each set of samples assayed using standards for the relevant kit. Define the level of cytokines in each sample by comparison of experimental data with the appropriate standard curve.
8. Lipopolysaccharide Assay
Analyze level of LPS in serum using a rat LPS ELISA kit according to manufacturer’s protocol. Estimate concentration of LPS in samples by comparison of the obtained values with the values of the standard curve.
9. High Resolution Light Microscopy of Blood
Use the microscope system described previously25,26. Use a vibration isolation platform as a base for the microscope system and video camera and computer25 to record the live images. Calibrate test images using a Richardson slide as previously described27.
Place 7 µl of freshly drawn blood from each rat on a glass slide, coverslip, photograph and record ten image frames of 72 × 53.3 µm2 in each sample (magnification 1,700X).
Measure the concentration of vesicles using software which provides high-resolution direct-view optical images in real time. Examine a minimum of 20 image frames for each experimental condition28.
10. Statistics
Express all values as mean and standard deviation. Perform statistical analysis and graph plotting. Analyze the results with the two sample t-test, set the significance level at 0.05 to define statistical significance.
Representative Results
The mean body temperature of animals before and immediately after heat stress was 36.7 ± 0.07 oC and 40.3 ± 0.17 oC, respectively (P < 0.05). Exposure of rats to heat (group 3) resulted in significant inhibition of villi height and total mucosal thickness (Figures 2, 3). Oral administration of BSB3 strain before stress protected intestine from the harmful effect of heat.
Translocation of bacteria from the gut was determined by bacteriological analysis of mesenteric lymph nodes (MLN), liver and spleen of each rat. Bacterial cultures in a concentration of 1.7 x 103 ± 4.6 x 102 CFU/g tissue (Figure 4) were isolated only from MLN and liver of rats pre-treated with PBS and exposed to heat (group 3). All tested samples from control rats (groups 1, 2) and from heat-stressed animals received a BSB3 strain (group 4) were sterile. No bacteria were isolated from spleen samples.
No change of IL-1β; IL-6, TNF-α, INF-Γ cytokines levels were registered in heat-stressed rats. In the serum of animals treated with PBS before heat exposure significant elevation of IL-10 and LPS levels was found (group 3) - (Figures 5, 6). Pre-treatment with B. subtilis BSB3 (group 4) prevented the rise of IL-10 and LPS in serum of heat-stressed animals.
A significant increase of free vesicles concentration from (1.4 ± 0.2) × 106 to (3.8 ± 0.3) × 106 vesicles µl-1 (p < 0.001) was found in the blood of rats that received PBS before heat stress (Figures 7, 8). In a group of animals pretreated with BSB3 no change in the number of free vesicles was found after heat exposure (Figure 7).
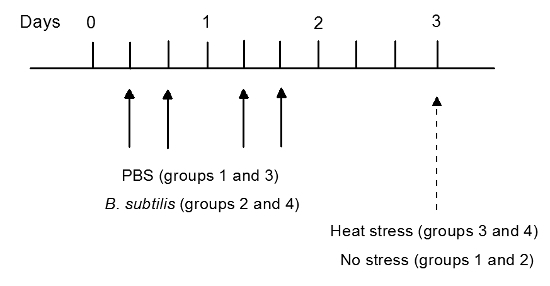 Figure 1. Study Design. Two groups of rats (16 rats in each group) were treated by oral gavage with B. subtilis BSB3 (B. subtilis group) or with PBS (PBS group) twice a day. On day 3 each group was subdivided by 8 rats in each group. Rats from group 3 and 4 were placed at 45 oC for 25 min. Control animals (groups 1 and 2) were kept at room temperature. Please click here to view a larger version of this figure.
Figure 1. Study Design. Two groups of rats (16 rats in each group) were treated by oral gavage with B. subtilis BSB3 (B. subtilis group) or with PBS (PBS group) twice a day. On day 3 each group was subdivided by 8 rats in each group. Rats from group 3 and 4 were placed at 45 oC for 25 min. Control animals (groups 1 and 2) were kept at room temperature. Please click here to view a larger version of this figure.
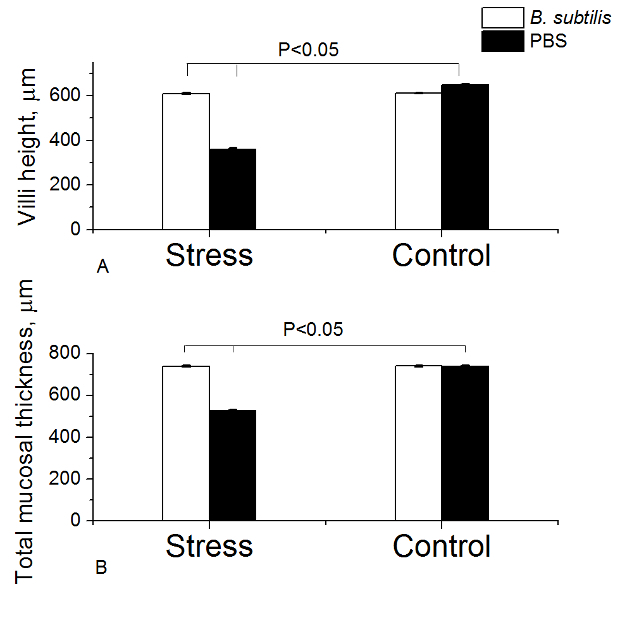 Figure 2. Measurement of Intestinal Villi Height (A) and Mucosal Thickness (B) in Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Twenty measurements of each parameter in each sample were taken. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
Figure 2. Measurement of Intestinal Villi Height (A) and Mucosal Thickness (B) in Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Twenty measurements of each parameter in each sample were taken. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
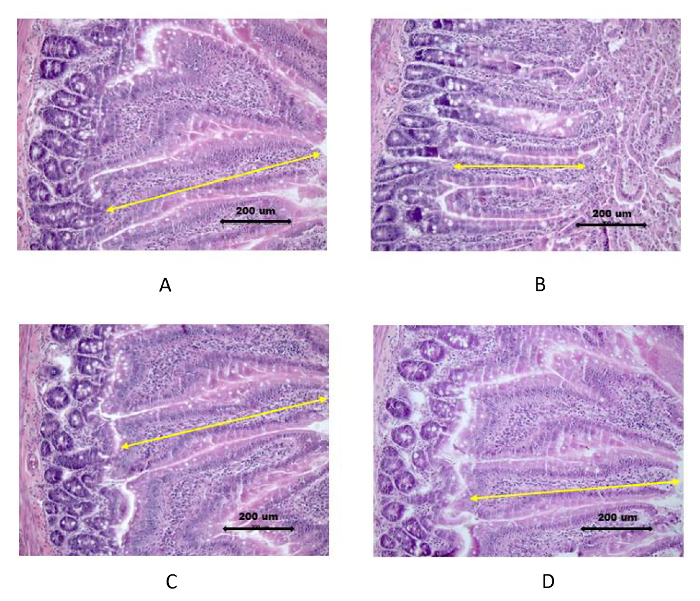 Figure 3. Histological Images of Intestinal Mucosa Stained with Hematoxylin and Eosin. Rats were pre-treated with PBS or B. subtilis BSB3 and exposed to 45 oC (Stress) or to room temperature (Control). A. PBS/no stress; B. PBS/heat stress; C.
B. subtilis/no stress; D. B. subtilis/heat stress. Villi height is indicated by arrows. Bar 200 µm. Please click here to view a larger version of this figure.
Figure 3. Histological Images of Intestinal Mucosa Stained with Hematoxylin and Eosin. Rats were pre-treated with PBS or B. subtilis BSB3 and exposed to 45 oC (Stress) or to room temperature (Control). A. PBS/no stress; B. PBS/heat stress; C.
B. subtilis/no stress; D. B. subtilis/heat stress. Villi height is indicated by arrows. Bar 200 µm. Please click here to view a larger version of this figure.
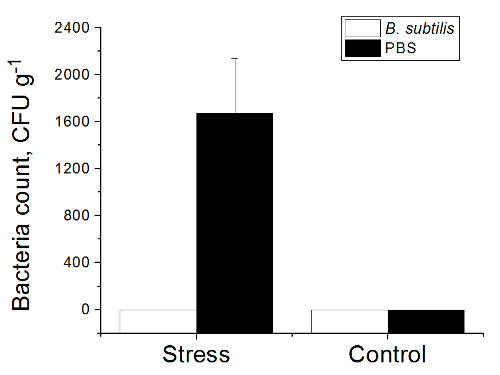 Figure 4. Total Number of Bacteria in Tissues of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Mesenteric lymph nodes, liver, and spleen were aseptically removed from each rat, homogenized and plated onto agar media to recover aerobic and anaerobic bacteria. Total count of translocated bacteria (aerobic and anaerobic) is presented as colony-forming units (CFU) per gram of tissue. Values are presented as the mean and the standard deviation. Please click here to view a larger version of this figure.
Figure 4. Total Number of Bacteria in Tissues of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Mesenteric lymph nodes, liver, and spleen were aseptically removed from each rat, homogenized and plated onto agar media to recover aerobic and anaerobic bacteria. Total count of translocated bacteria (aerobic and anaerobic) is presented as colony-forming units (CFU) per gram of tissue. Values are presented as the mean and the standard deviation. Please click here to view a larger version of this figure.
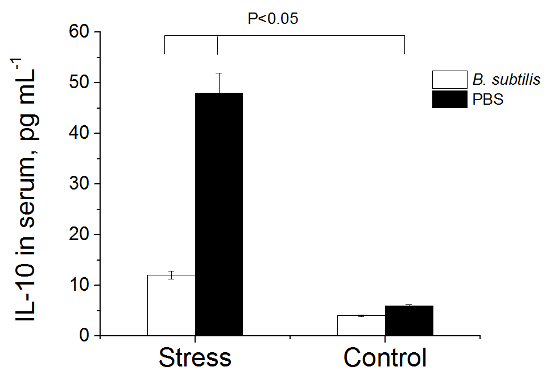 Figure 5. Level of IL-10 in Serum of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Blood serum was obtained from each rat and analyzed with a commercial rat ELISA kit. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
Figure 5. Level of IL-10 in Serum of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Blood serum was obtained from each rat and analyzed with a commercial rat ELISA kit. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
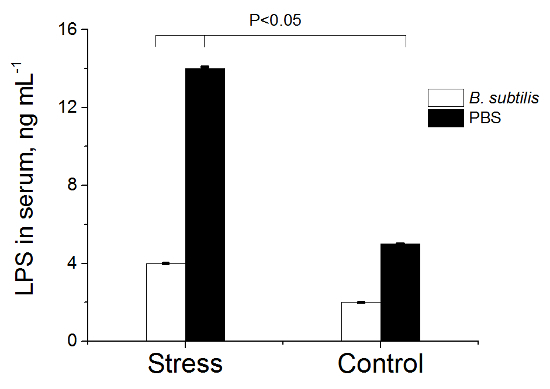 Figure 6. LPS Concentration in Serum of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Blood serum was obtained from each rat and analyzed with a commercial rat ELISA kit. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
Figure 6. LPS Concentration in Serum of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). Each group consisted of 8 rats. Blood serum was obtained from each rat and analyzed with a commercial rat ELISA kit. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
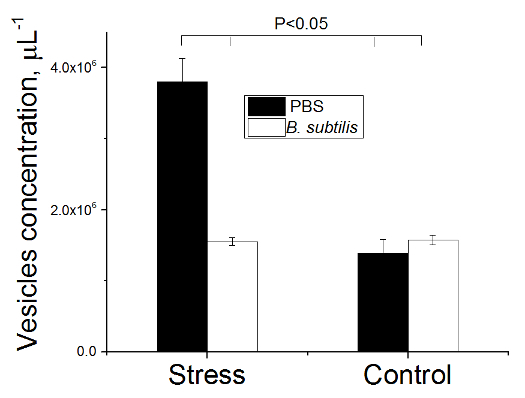 Figure 7. Number of Vesicles in Blood of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). A small droplet (7 µl) of freshly drawn blood was placed on a glass slide, coverslipped, photographed and recorded ten image frames in each sample (magnification 1,700X). Concentration of vesicles was measured using software which provides high-resolution direct-view optical images in real time. Twenty image frames were examined for each experimental condition. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
Figure 7. Number of Vesicles in Blood of Rats. Rats pre-treated with PBS or B. subtilis BSB3 were exposed to 45 oC (Stress) or to room temperature (Control). A small droplet (7 µl) of freshly drawn blood was placed on a glass slide, coverslipped, photographed and recorded ten image frames in each sample (magnification 1,700X). Concentration of vesicles was measured using software which provides high-resolution direct-view optical images in real time. Twenty image frames were examined for each experimental condition. Values are presented as the mean and the standard deviation. The results were analyzed with the two sample t-test at significance level 0.05 to define statistical significance. Please click here to view a larger version of this figure.
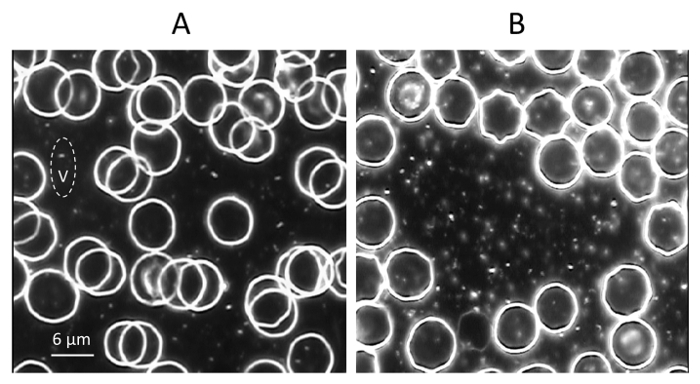 Figure 8. Increase of the Vesicles Concentration in Blood After Heat Stress. Video image of blood of rats pretreated with PBS before exposure to room temperature (A) or to 45 oC (B). A small droplet (7 µl) of freshly drawn blood was placed on a glass slide, coverslipped, photographed and recorded ten image frames in each sample (magnification 1,700X). The vesicle is indicated as V. Bar 6 µm. Please click here to view a larger version of this figure.
Figure 8. Increase of the Vesicles Concentration in Blood After Heat Stress. Video image of blood of rats pretreated with PBS before exposure to room temperature (A) or to 45 oC (B). A small droplet (7 µl) of freshly drawn blood was placed on a glass slide, coverslipped, photographed and recorded ten image frames in each sample (magnification 1,700X). The vesicle is indicated as V. Bar 6 µm. Please click here to view a larger version of this figure.
Discussion
Exposure to high temperature results in serious health conditions29. Prevention and early detection of complications related to heat stress are of vital importance30.The presented protocol can be used to evaluate the efficacy of various approaches for the prevention of heat stress adverse effects.
Critical steps within this protocol include: the heat treatment procedure (45 oC, 25 min); the usage of apyrogenic materials during collection and processing of blood to prevent contamination with enterotoxins; keeping serum aliquots at -20 oC to avoid repeated freezing and thawing; and following sterile procedure during analysis of bacterial translocation. The protocol was set up for the oral treatment of rats with bacteria for prevention of heat stress side effects. For other routes of administration and other compounds, this protocol can be modified. For example, the time of treatment before heat stress can be extended. Oral administration can be changed to rectal administration. Bacillus subtilis bacteria were used for prevention of heat stress complications by oral treatment of animals before exposure to elevated temperature. In order to cure complications of heat stress, this protocol can be modified by the use of bacteria for the treatment of animals after exposure to heat.
It was shown that the elevation of body temperature caused an increase of erythrocyte vesiculation, and this was completely prevented by pre-treatment with B. subtilis bacteria. Thus, the increase of the vesicles concentration in the blood during exposure to heat may indicate the level of heat stress and provides diagnostic evidence for the stability of erythrocytes and their resistance to heat. It is also a very useful and rapid test for the evaluation the efficacy of preventive treatment against heat stress complications. The induction of heat shock proteins as a marker of heat stress was previously discussed30, but this approach is time-consuming and cannot be used for real-time diagnosis of response to heat stress.
The presented approach for prevention of heat stress adverse effects is simple, cost effective and does not require additional equipment or special facilities. Future application of this work will help to understand the mechanisms of protection against heat stress complications and characterize the new procedures for this protection. This method is of significance for road workers, military personnel, athletes - groups of people that are at risk for heat stroke during intense outdoor physical activity29. The proposed approach allows one to examine the level of heat stress and to evaluate various methods to protect the health of people working in extreme conditions.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by the Auburn University fund FOP 101002-139294-2050.
References
- Crandall CG, Gonzalez-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol. 2010;199:407–423. doi: 10.1111/j.1748-1716.2010.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc. Sport Sci. Rev. 2004;32:185–190. doi: 10.1097/00003677-200410000-00011. [DOI] [PubMed] [Google Scholar]
- Yu J, et al. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2010;156:119–128. doi: 10.1016/j.cbpa.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Moseley PL, Gisolfi CV. New Frontiers in Thermoregulation and Exercise. Sports Med. 1993;16:163–167. doi: 10.2165/00007256-199316030-00001. [DOI] [PubMed] [Google Scholar]
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009;87:101–108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Berg A, Muller HM, Rathmann S, Deibert P. The gastrointestinal system - An essential target organ of the athlete's health and physical performance. Exerc. Immunol. Rev. 1999;5:78–95. [PubMed] [Google Scholar]
- Khan MW, et al. Microbes, intestinal inflammation and probiotics. Expert Rev Gastroent. 2012;6:81–94. doi: 10.1586/egh.11.94. [DOI] [PubMed] [Google Scholar]
- Quigley EMM. Prebiotics and Probiotics: Their Role in the Management of Gastrointestinal Disorders in Adults. Nutr Clin Pract. 2012;27:195–200. doi: 10.1177/0884533611423926. [DOI] [PubMed] [Google Scholar]
- Gore C, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2012;42:112–122. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- Gomes AC, Bueno AA, de Souza RGM, Mota JF. Gut microbiota, probiotics and diabetes. Nutr. J. 2014;13 doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J. Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol. Motil. 2009;21:585–593. doi: 10.1111/j.1365-2982.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- Fujiya M, et al. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal Homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299–308. doi: 10.1016/j.chom.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Pinchuk IV, et al. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Ch. 2001;45:3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokulova IB, Kirik DL, Pinchuk IV. Probiotics against Campylobacter pathogens. J. Travel Med. 1997;4:167–170. doi: 10.1111/j.1708-8305.1997.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Gracheva NM, et al. The efficacy of the new bacterial preparation biosporin in treating acute intestinal infections. Zh Mikrobiol Epidemiol Immunobiol. 1996. pp. 75–77. [PubMed]
- Horosheva TV, Vodyanoy V, Sorokulova I. Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: a randomized, double-blind, placebo-controlled clinical trial. JMM Case Report. 2014;1 [Google Scholar]
- Sorokulova IB, Krumnow AA, Pathirana S, Mandell AJ, Vodyanoy V. Novel Methods for Storage Stability and Release of Bacillus Spores. Biotechnol. Prog. 2008;24:1147–1153. doi: 10.1002/btpr.22. [DOI] [PubMed] [Google Scholar]
- Vogel HG, Vogel WH. Vogel HG, Vogel WH, editors. Drug Discovery and Evaluation: Pharmacological Assays. eds H.G. Vogel & W.H. Vogel. 1997. pp. 658–659.
- Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J. Neuroimmunol. 2006;171:29–37. doi: 10.1016/j.jneuroim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J. Appl. Physiol. 2006;100:1400–1409. doi: 10.1152/japplphysiol.01040.2005. [DOI] [PubMed] [Google Scholar]
- Ribeiro SR, et al. Weight loss and morphometric study of intestinal mucosa in rats after massive intestinal resection. Influence of a glutamine-enriched diet. Rev. Hosp. Clìn. Fac. Med. S. Paulo. 2004;59:349–356. doi: 10.1590/s0041-87812004000600007. [DOI] [PubMed] [Google Scholar]
- Vainrub A, Pustovyy O, Vodyanoy V. Resolution of 90 nm (lambda/5) in an optical transmission microscope with an annular condenser. Opt Lett. 2006;31:2855–2857. doi: 10.1364/ol.31.002855. [DOI] [PubMed] [Google Scholar]
- Moore T, Globa L, Pustovyy O, Vodyanoy V, Sorokulova I. Oral administration of Bacillus subtilis strain BSB3 can prevent heat stress-related adverse effects in rats. J Appl Microbiol. 2014;117:1463–1471. doi: 10.1111/jam.12606. [DOI] [PubMed] [Google Scholar]
- Richardson TM. Test slides: Diatoms to divisions-What are you looking at. Proc Roy Microsc Soc. 1988;22:3–9. [Google Scholar]
- Moore T, Sorokulova I, Pustovyy O, Globa L, Vodyanoy V. Microscopic evaluation of vesicles shed by rat erythrocytes at elevated temperatures. J Therm Biol. 2013;38:487–492. doi: 10.1002/jemt.22280. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Chawla A, Tatu U. Heat Shock Protein 70 as a Biomarker of Heat Stress in a Simulated Hot Cockpit. Aviat Space Env Med. 2007;74(7):711–716. [PubMed] [Google Scholar]


