Abstract
Understanding the role of factors that regulate intestinal epithelial homeostasis and response to injury and regeneration is important. The current literature describes several different methodological approaches to obtain images of intestinal tissues for data validation. In this paper, we delineate a common protocol relating to the derivation and processing of mouse intestinal tissues. Proper fixation of intestinal tissues and Swiss-roll techniques that enhance intestinal epithelial morphology are discussed. Postresection processing and reorientation of embedded intestinal tissues are critical in obtaining paraffin-embedded blocks that display intact intestinal structural features after sectioning. The Swiss-rolling technique helps in histological assessment of the complete intestinal or colonic sections examined. An ability to differentiate intestinal structural features can be vital in quantitative measurements of intestinal inflammation and tumorigenesis along the entire length. Finally, paraffin-embedded sections are ideal for robust processing using both immunohistochemical and immunofluorescent detection methods. Nonfluorescent immunohistochemical sections provide a vibrant image of the tissue detailing different cellular structural features but do not provide flexibility for intracellular co-localization experiments. Multiple fluorescent channels can be appropriately utilized with immunofluorescent detection for co-localization experiments, lending support to mechanistic studies.
Keywords: Basic Protocol, Issue 113, In vivo mouse model, Swiss roll, Intestinal epithelial cells, Stem cells, Immunohistochemistry, Immunofluorescence
Introduction
The mammalian intestinal epithelium comprises a single layer of columnar cells. In the small intestine, the proliferative cells are confined to the crypts while differentiated cells occupy the villus region. However, because there are no villi in the large bowel, the proliferative cells are localized to the bottom of the crypts and differentiated cells occupy the upper region of the crypts. The intestinal epithelium undergoes rapid replenishment (about 3 - 5 days) that is driven by continuous division of the proliferative cells within the crypts. The proliferative cells of the crypts are not a homogeneous population and are further subdivided into stem cells and transit-amplifying (TA) cells1. The stem cells reside at the bottom of the crypt, within the first 4 - 5 cells from the very bottom2. The current model supports the existence of two types of stem cells: crypt base columnar (CBC) stem cells and reserve quiescent stem cells. The CBC stem cells are actively proliferating and are marked by Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5)3, Olfactomedin 4 (Olfm4)4 and Achaete scute-like 2 (Ascl2)5. On the other hand, reserve quiescent stem cells are labeled by B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1)6, mouse telomerase reverse transcriptase (mTert)7, HOP Homeobox (Hopx)8, Doublecortin-Like And CAM Kinase-Like 1 (Dclk1)9, and Leucine-Rich Repeats And Immunoglobulin-Like Domains 1 (Lrig1)10. The actively proliferating stem cells give rise to TA cells then undergo further differentiation into absorptive cells (enterocytes) and secretory cells (enteroendocrine, goblet, Paneth, and Tuft cells). Continuous cell division in the proliferative zone results in upward movement of epithelial cells along the crypt-villus axis until they reach top of the villi, where they undergo apoptosis and are sloughed off from the surface of the epithelium. The different types of intestinal epithelial cells are marked by the expression of distinct proteins (e.g., intestinal goblet cells can be recognized by staining with antibody against Muc2 and Paneth cells with antibody against lysozyme). We study the role of Krüppel-like factors (KLFs) in the homeostasis and pathobiology of the intestinal epithelium11-13. The results presented here supporting the feasibility of a modified Swiss-rolling technique are based on previous studies of the role of Krüppel-like factor 5 (KLF5) in the maintenance of the actively proliferating intestinal epithelial stem cells14. KLF5 is a zinc-finger transcription factor that is highly expressed in the active intestinal stem and TA cells12. Previous studies demonstrated that KLF5 is co-expressed with Ki-67, a known proliferative marker in the intestinal crypts.
The gastrointestinal tract is not a structurally or functionally homogeneous tissue. The small intestine is divided into duodenum, jejunum, and ileum and the large intestine into cecum and colon, with the latter further divided into proximal, middle, and distal portions. Each of these sections has unique histological features and plays distinct roles15. As such, the effects of insults and the degree of the response of the intestinal epithelium may depend on the region of studied tissue16. Additionally, various mice strains demonstrate diversity of the response at the histological level based on the type of insult used in the studies16. Thus, befitting tissue preparation is necessary to permit appropriate histological and molecular analysis of the intestinal tissues. As such, the Swiss-roll technique grants analysis of the complete length of the intestinal epithelium at one time and thus ascertains well-informed conclusions based on comprehensive information.
The Swiss-roll technique was first mentioned by Magnus17, and described in detail by Moolenbeck and Ruitenberg and Park et al. as a method for preparing tissues and performing histological analyses of the rodent intestine18,19, respectively. The protocol delineated in this publication presents an improved version of the original method that permits for timely and reliable tissue preparation for diagnostic purposes. This modified technique allows for efficient collections and preparation of the intestinal epithelium for universally used techniques, such as immunohistochemistry, immunofluorescence, as well as in situ hybridization (fluorescent and chromogenic20). Furthermore, the modified tissue specimen preparation method utilizes readily available and relatively inexpensive reagents while offering a method of rapid tissue fixation and allows for recovery of protein, DNA, and RNA for additional evaluation. Taken together, this technique is excellent for comprehensive assessment of histopathological, pathological, and molecular features of the intestinal epithelium.
Protocol
1. Mice
- All studies involving mice were approved by the Stony Brook University Institutional Animal Care and Use Committee (IACUC). The mice were maintained on a 12:12 hr light-dark cycle.
- Commercially obtain C57BL/6 mice. Obtain C57BL/6 mice carrying Klf5 alleles flanked by loxP sites (Klf5fl/fl). These mice were previously described21 and graciously provided by Dr. Ryozo Nagai.
Purchase C57BL/6 mice carrying the inducible Cre recombinase gene under regulation of the Lgr5 promoter (Lgr5-EGFP/CreERT2 mice).
To establish Lgr5-EGFP/CreERT2/Klf5fl/fl mice, cross Klf5fl/fl mice with Lgr5-EGFP/CreERT2 mice and then backcross the progeny toKlf5fl/flmice.
Treat mice with tamoxifen as per the protocol previously described22. Inject 8-week-old experimental and control mice intraperitoneally (I.P.) with 1 mg of tamoxifen (10 mg/ml), dissolved in sterile corn oil, for 5 consecutive days.
On day 14 after first tamoxifen injection, sacrifice mice using CO2 asphyxiation by placing them in a chamber connected to CO2 gas source. Allow the gas to flow into the chamber until the mice are unconscious and all movement ceases. To ensure euthanasia perform cervical dislocation.
2. Tissue Preparation and Swiss Rolling
Using dissection forceps and scissors, cut an incision in the skin of the abdominal side. With a pair of forceps, hold the skin at the incision and pull gently away from the abdominal muscle tissue. Use a pair of scissors to cut the abdominal skin and expose the abdominal muscles.
Hold and pull up the peritoneum with the forceps. Make sure not to be holding and pulling at the intestines. Carefully make an incision in the peritoneal tissue and continue cutting away the skin to expose the abdominal muscles. Carefully make an incision in the abdominal muscles and continue cutting them away to expose the intestines.
Identify the colon and trace to the distal end where it joins the rectum/anus. With a pair of scissors, cut as close to the anal opening as possible.
Identify the stomach and trace where it joins with the small intestine. With a pair of scissors, cut free the intestine about 1 cm away from the stomach. Transfer the entire length of the freed small intestine and colon to a clean Petri dish containing phosphate-buffered saline (PBS).
With one hand or a pair of forceps, hold the distal end of the colon and use the other hand to gently unravel the entire length of the colon from any mesenteric connective and/or fat tissue.
Identify the cecum (small pouch between the small and large intestine) and cut where the cecum and the colon join (the proximal end of the colon) to free the colon.
With one hand or a pair of forceps, hold the small intestine and use the other hand to gently unravel the entire length of the small intestine from any mesenteric connective and/or fat tissue. Cut away the cecum and discard if there is no need for it. Note: The process can be stopped here by incubating the dissected out intestines in modified Bouin's fixative (50% ethanol/5% acetic acid in dH2O) overnight and continue the procedure the following day.
Handling one segment of the intestine (small intestine or colon), fill a 10-ml syringe with modified Bouin's fixative and attach a gavage needle to it. Insert the needle about half a centimeter in the anterior opening of the intestinal segment.
Hold the gavage needle inside the intestinal segment by applying firm pressure with an instrument on the intestinal segment. With the other hand holding the syringe, apply gentle but consistent pressure to flush the contents of the intestinal segment using modified Bouin's fixative. This step allows simultaneous cleaning of the intestinal segment and immediate fixation. Use a Petri dish to collect the flow-through waste. Fixation can be observed by the colon color turning opaque. Note: Make sure not to apply too much pressure during flushing or the intestinal segment might burst open.
- Using scissors, cut open the intestinal segment longitudinally along the mesenteric line. Hold it with a pair of forceps and rinse it briefly in a Petri dish containing PBS.
- Cut the small intestine into 3 equal segments: proximal, mid, and distal. The proximal segment is the one immediately following the stomach, and the distal segment is the one immediately before the colon. The proximal segment is equivalent to the duodenum, the mid to the jejunum, and the distal to the ileum.
- For each segment mark the proximal and the distal end. The proximal end is the one that was originally pointing toward the stomach, and the distal pointed toward the colon.
- Use the top of a Petri dish to place the cleaned and opened intestinal segment. Place the intestinal segment with the luminal side facing upward.
- For the colon, identify the luminal side by the variegations/ridges running across its width and are present at the proximal end. The mid and distal regions have some variegations that run longitudinally. For the small intestine, identify the luminal side by the rough-appearing surface, which marks the villi. Note: For the small intestine, there are no external macroscopic anatomical demarcation of proximal, middle and distal regions. However these regions can be can identified in sections under the microscope. The villi are longest in the proximal region and become gradually shorter distally where they are the shortest in length. Microscopically, the colonic crypts are shortest in the proximal region which can also be identified by the presence of ridges. The colonic crypts are tallest in the midsection and shorter in the distal region yet still taller than in the proximal region.
- Proceed with Swiss rolling the colon:
- Keep the colon flat open and pull it with forceps from its proximal end toward the edge of the Petri dish.
- Keep luminal side facing up and hold the proximal end with the forceps with one hand and with the other hand hold a toothpick.
- Wrap the edge of the proximal end around the toothpick using the forceps and slightly pinch the wrapped edge against the toothpick to hold it in place.
- Gently and slowly start rolling the toothpick with fingers to roll the colon around the toothpick to form a Swiss roll.
- Once the entire colon length has been rolled up, use a pair of forceps to carefully slide the colon Swiss roll off the toothpick and into a tissue-processing/-embedding cassette. Place the cassette in 10% buffered formalin overnight at room temperature.
- Proceed with Swiss rolling the small intestine:
- Handling one segment at a time, keep the segment flat open with luminal side up and pull it with forceps from its proximal end toward the edge of the Petri dish.
- Proceed with Swiss rolling as described for the colon.
- Proceed with processing formalin-fixed tissues for paraffin embedding. Proceed as follows: NOTE: Use an automated processor (see materials and Equipment's for details).
- While tissue is in the cassette, rinse tissue with PBS until fixative is completely removed.
- Dehydrate tissue using ethanol in the following sequence: 50% ethanol for 10 min, 70% ethanol for 10 min, 80% ethanol for 10 min, 95% ethanol for 10 min, 100% ethanol for 10 min, 100% ethanol for 10 min, and 100% ethanol for 10 min.
- Exchange ethanol with xylene in the following sequence: 2:1 ethanol : xylene for 10 - 15 min, 1:1 ethanol : xylene for 10 - 15 min, 1:2 ethanol : xylene for 10 - 15 min, 100% xylene for 10 - 15 min, 100% xylene for 10 - 15 min, and 100% xylene for 10 - 15 min. CAUTION: Xylene is a health hazard, thus the workplace should be equipped with a local exhaust ventilation with a proper hood and the proper protective equipment should be wore by personnel.
- Exchange xylene with paraffin. Perform the following steps in a vacuum oven set for 54 - 58 °C: 2:1 xylene : paraffin for 30 min, 1:1 xylene : paraffin for 30 min, 1:2 xylene : paraffin for 30 min, 100% paraffin for 1 - 2 hr, and 100% paraffin for 1 - 2 hr or overnight. Note: Do not let the paraffin exceed 60 °C for prolonged periods of time because this will degrade the paraffin polymers and make it hard and brittle.
- Embed in fresh new paraffin and orient tissue as desired before the paraffin hardens. For the Swiss rolls, following tissue paraffinization, it is important to reposition each roll on its side during paraffin embedding. This ensures that during cutting, sections of the entire length of each roll will be produced.
For staining, cut 5-μm thick sections using microtome. Collect the sections on charged slides and dry them (bake) in a 65 °C oven overnight; let cool to room temperature and subsequently used them for staining. Note: The baking time can be shortened to 1 - 2 hr depending on how freshly cut the slides are. If less than a month since sectioning, then it is advisable to bake overnight. If it has been more than a month, then 1 - 2 hr of baking is sufficient to save time. If running out of time, overnight backing can always be used.
3. Histological Analysis and Immunofluorescence Staining
Perform histological analysis and immunofluorescence staining as previously described23,24, with modifications. For enhanced green fluorescent protein (EGFP) immunofluorescence:
Bake the slides in a 65 °C oven for 1 hr to inactivate endogenous alkaline phosphatase activity and subsequently deparaffinize in xylene.
Incubate sections in a bath of 3% hydrogen peroxide in methanol to block endogenous tissue peroxidases; then rehydrate by incubation in a decreasing alcohol bath series (100%, 95%, 70%) followed by antigen retrieval in citrate buffer solution (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) at 125 °C for 10 min using a decloaking chamber.
Draw a circle around tissue sections using marking pen that provides hydrophobic barrier and incubate sections with blocking buffer (2.5% bovine serum albumin [BSA] in TBS-Tween) for 30 min at 37 °C and then with primary antibody against EGFP (dilution 1:500) at 4 °C overnight in a humidified chamber while shaking gently.
Wash sections and incubate with fluorescent-tagged secondary antibody at the appropriate concentration for 30 min at 37 °C.
- Wash slides after secondary antibody treatment and stain nuclei with Hoechst and mount with mounting media.
- For Klf5/EGFP/Ki-67 co-staining, process slides as described in steps 3.1 - 3.3 and perform Klf5 staining by incubating with Klf5 antibody (dilution 1:150) overnight.
- Apply rabbit AP polymer detection to slides as per protocol.
- Reveal Klf4 staining using chromogen immunohistochemical staining as per manufacturer's protocol.
- Perform antibody elution using the previously described protocol25. Incubate slides at 50 °C in Glycine-SDS (pH 2.0) solution with agitation for 1 hr.
- Wash slides thoroughly with distilled water and incubate with blocking buffer (2.5% BSA in TBS-Tween) for 30 min at 37 °C.
- Add Ki-67 (dilution 1:500) and EGFP (dilution 1:500) antibodies simultaneously and incubate at 37 °C for 1 hr.
- Perform fluorescent detection with appropriate secondary antibodies before staining with Hoechst (data not shown) and mount with mounting medium.
Observe histological morphology of the tissues on staining 5-μm sections with hematoxylin and eosin (H&E)26. For fluorescent images, use emission wavelengths of 535, 646, and 700 to visualize EGFP, Klf5, and Ki-67 staining, respectively.
Representative Results
The Swiss rolling technique in combination with immunohistochemical staining allows for comprehensive analysis of small or large intestinal tissue. The example of H&E staining of a large bowel of a C57BL/6 mouse (Figure 1) is an illustration of the feasibility and the effectiveness of this technique. As shown in Figure 1, the image is able to capture all portions of the colon: proximal, middle, and distal. Thus, it allows for comprehensive histological assessment. The Swiss rolling and the ability to capture the entire length of the small or large intestinal tissue is extremely helpful for heterogeneous gene expression and marker staining or a variable response of the intestinal tissue to the insult.
An example is the EGFP staining pattern in Lgr5-EGFP/CreERT2 mice. In this mouse model, expression of EGFP is driven by the Lgr5 promoter, which is active only in the actively proliferating intestinal crypts. Additionally, this mouse model is characterized by low (approximately 5 - 10%) penetrance of transgene expression. As shown in Figure 2, the EGFP expression pattern in the intestinal tissue is variable. Consequently, the capability to capture a large view of the tissue helps to identify the region of interest.
The technique described here is powerful especially with application of multifluorophore staining. Here, we show an example of trifluorophore staining of the intestinal tissue that was prepared using the Swiss-roll technique. The main aim of this study was to investigate the role of Klf5 in the maintenance of intestinal stem cells expressing Lgr5 marker. Therefore, we deleted Klf5 from the Lgr5-positive intestinal stem cells in Lgr5-EGFP/CreERT2 mice and collected intestinal tissues on day 14 after first tamoxifen injection. For immunohistological analysis, the tissues were prepared according to the protocol presented in this manuscript, and staining for EGFP (Lgr5 marker), Klf5, and Ki-67 was performed. In the control mice, denoted as Lgr5-EGFP/CreERT2, both EGFP-positive (marked with blue arrows) and EGFP-negative crypts (marked with white arrows) exhibited co-staining for Klf5 and Ki-67 at two examined time points, as shown in Figure 3D. In contrast, in Lgr5-EGFP/CreERT2/Klf5fl/fl small intestinal tissue, Klf5/Ki-67 co-staining was missing from the EGFP-positive CBC stem cells (marked by blue arrows) but present in the EGFP-negative crypts adjoining the green crypts (marked by white arrows), as shown in Figure 3H. This is an excellent example that the tissue preparation techniques presented here does not negatively influence staining quality.
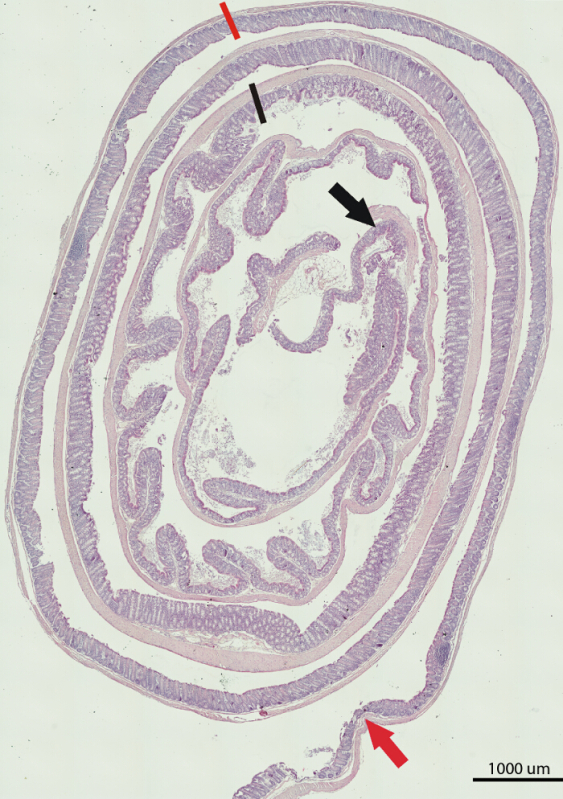 Figure 1. H&E Staining of a Large Bowel from a C57BL/6 Mouse. Shown is the composite image of the whole length of the large bowel. The portion between the black arrow and black line marks the proximal part, between the black and orange lines marks the middle, and between the orange line and orange arrow marks the distal part of the large bowel. Scale bar = 1,000 μm. Please click here to view a larger version of this figure.
Figure 1. H&E Staining of a Large Bowel from a C57BL/6 Mouse. Shown is the composite image of the whole length of the large bowel. The portion between the black arrow and black line marks the proximal part, between the black and orange lines marks the middle, and between the orange line and orange arrow marks the distal part of the large bowel. Scale bar = 1,000 μm. Please click here to view a larger version of this figure.
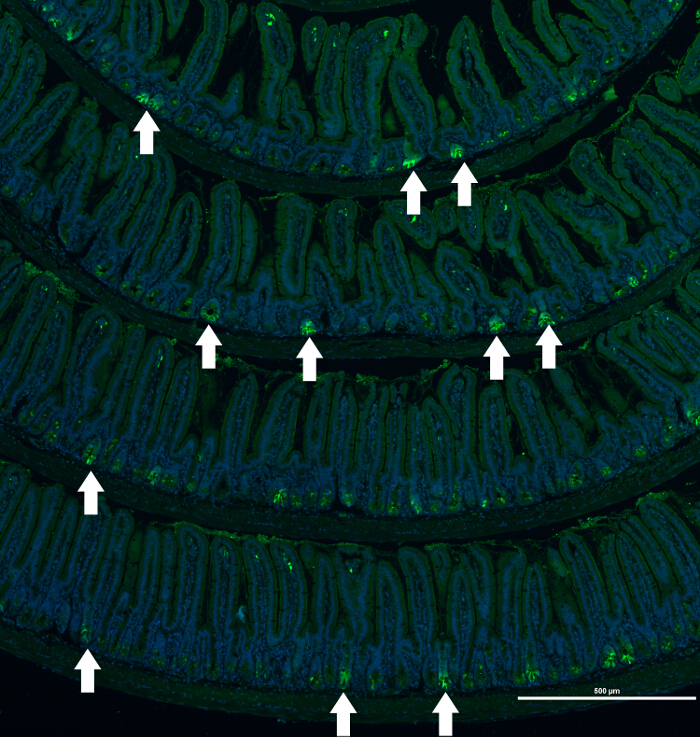 Figure 2. Immunofluorescence Staining of a Small Bowel of an Lgr5-EGFP/CreERT2 Mouse. Composite image of EGFP (labeling Lgr5-positive epithelial cells) staining of small intestine sections of an Lgr5-EGFP/CreERT2 mouse. Example of heterogeneous immunofluorescence staining of protein marker (EGFP) that labels Lgr5-postive epithelial cells in the crypts of the Lgr5-EGFP/CreERT2 mouse. Nuclei are visualized with Hoechst. White arrows mark crypts positive for EGFP expression. Scale bar = 500 μm. Please click here to view a larger version of this figure.
Figure 2. Immunofluorescence Staining of a Small Bowel of an Lgr5-EGFP/CreERT2 Mouse. Composite image of EGFP (labeling Lgr5-positive epithelial cells) staining of small intestine sections of an Lgr5-EGFP/CreERT2 mouse. Example of heterogeneous immunofluorescence staining of protein marker (EGFP) that labels Lgr5-postive epithelial cells in the crypts of the Lgr5-EGFP/CreERT2 mouse. Nuclei are visualized with Hoechst. White arrows mark crypts positive for EGFP expression. Scale bar = 500 μm. Please click here to view a larger version of this figure.
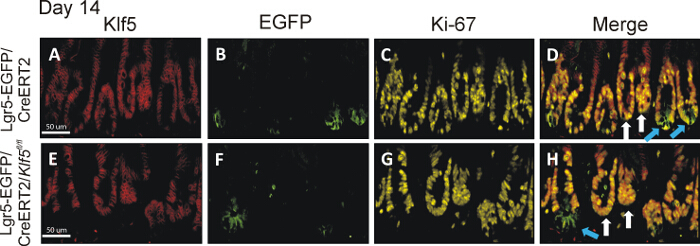 Figure 3. Klf5 Deletion in Lgr5-EGFP/CreERT2/Klf5fl/fl Mice Persists Long Term in Lgr5-EGFP-positive Crypts. The top and bottom set of images are representative small intestinal tissue from the Lgr5-EGFP/CreERT2 control and Lgr5-EGFP/CreERT2/Klf5fl/fl mice on day 14 day after first tamoxifen injection, respectively. Panels A-D are representative of staining from tissue collected from Lgr5-EGFP/CreERT2 control mice, while panels E-H show staining representative of Lgr5-EGFP/CreERT2/Klf5fl/fl mice. Panels A and E display Klf5 immunofluorescent staining in red; panels B and F show EGFP staining in green; panels C and G show Ki-67 staining in yellow; and panels D and H show merged images of Klf5, EGFP and Ki-67 stains. Blue arrows point to green crypts; white arrows point to nongreen crypts in the merged images. Lgr5-EGFP/CreERT2/Klf5fl/fl mice showed long-term loss of Klf5 only in the EGFP-labeled CBC cells14. Scale bar = 50 μm. Please click here to view a larger version of this figure.
Figure 3. Klf5 Deletion in Lgr5-EGFP/CreERT2/Klf5fl/fl Mice Persists Long Term in Lgr5-EGFP-positive Crypts. The top and bottom set of images are representative small intestinal tissue from the Lgr5-EGFP/CreERT2 control and Lgr5-EGFP/CreERT2/Klf5fl/fl mice on day 14 day after first tamoxifen injection, respectively. Panels A-D are representative of staining from tissue collected from Lgr5-EGFP/CreERT2 control mice, while panels E-H show staining representative of Lgr5-EGFP/CreERT2/Klf5fl/fl mice. Panels A and E display Klf5 immunofluorescent staining in red; panels B and F show EGFP staining in green; panels C and G show Ki-67 staining in yellow; and panels D and H show merged images of Klf5, EGFP and Ki-67 stains. Blue arrows point to green crypts; white arrows point to nongreen crypts in the merged images. Lgr5-EGFP/CreERT2/Klf5fl/fl mice showed long-term loss of Klf5 only in the EGFP-labeled CBC cells14. Scale bar = 50 μm. Please click here to view a larger version of this figure.
Discussion
The Swiss rolling technique is a powerful method for preparing intestinal tissue for histological and morphological assessment on a large scale. In contrast to the previously described Swiss-rolling technique, which was originally developed for preparation of frozen sections18,19, the procedure presented here allows prompt intestinal tissue preparation and fixation for formalin fixation and paraffin embedding (FFPE). Compared to frozen tissue, FFPE tissue has much longer shelf life and is the preferred type of tissue for histological analysis because of better tissue integrity. Critical parts of the Swiss-rolling protocol involve tissue flexibility for rolling and maintaining the Swiss-roll integrity and tissue quality for staining postfixation. The standard Swiss-roll technique for FFPE of intestinal tissue is typically laborious and time consuming (2 days)18,19. Additionally, this standard method usually yields intestinal tissue that is relatively stiff and not easy to form in a Swiss roll. This is because overnight incubation of the dissected intestinal tissue in 10% buffered formalin is required before tissue rolling. Other modifications of the technique for FFPE purposes have been also developed, but they have a major problem of the tendency of intestinal tissue to unroll and/or for the center of the roll to be distorted. This is because the tissue used in these techniques is fresh unfixed tissue, which is slippery from the mucus produced by the intestine. The new modified technique shown here overcomes these problems by using a modified form of Bouin's fixative. Original Bouin's fixative consists of a mixture of acetic acid, ethanol, picric acid and paraformaldehyde (or formalin). The two most hazardous components, picric acid and paraformaldehyde (or formalin), have been eliminated to allow much safer use of the fixative with an acetic acid/ethanol mix. Picric acid presents an explosion hazard, and paraformaldehyde (or formalin) is a cancer hazard. The significance of using the modified Bouin's fixative is that it (1) is less hazardous compared to its original formula, (2) is easy to prepare with relatively nonexpensive reagents that are readily available in most labs, and (3) allows for quick, almost instant, fixation of the tissue when used for flushing. To knowledge, there are no known limitations to using this modified technique. Additionally, the quick fixation with this mixture immensely reduces the slipperiness of the intestinal tissue, allowing much faster and easier tissue rolling. This fixative also allows for excellent preservation of tissue and cell integrity for histological and immunostaining analysis. Thus, in comparison to other methods of intestinal tissue preparation, this improved technique overcomes several technical issues and grants greatly enhanced speed and ease of use.
Also, this modified fixative is useful to use with other thick tissues that require quick fixation. Because of the nature of acetic acid and of ethanol, that have the capacity of quick penetration of thick tissue while at the same time undergoing fixation. We have used it successfully to quickly fix thick tissue such as liver, spleen and kidneys. For example, fixation of an adult mouse whole liver using the modified Bouin's fixative is usually achieved within 15 - 20 min. This can be easily judged by cutting a cross section into the liver and observing whether the deep regions of the liver had changed color from blood-red to gray. The change in color is indicative of fixation. This fixation is then followed by crosslinking using buffered formalin for 24 - 48 hr with no fear of altered cellular/tissue composition, as the tissue is already fixed at this point.
By comparison, the use traditional buffered-formalin fixation method alone on an adult mouse whole liver would require 24 - 48 hr to achieve deep-tissue fixation, with the risk of altered cellular composition of deep tissue. The quick fixation methods of thick tissues has the superior advantage of reducing to a minimum the alteration of cellular components in response to stress conditions, such as hypoxia, following removal of the organ/tissue from the body. We anticipate that the modified fixative can be used in animal perfusion as an even faster way of fixing thick tissue/organs is desired. Thus, in conclusion, this method and modified fixative provide multiple advantages over traditional methods used for intestinal tissue harvesting and fixation and the modified fixative can be used to quickly fix other thick tissues/organs.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We would like to thank Ainara Ruiz de Sabando for providing H&E images. This work was supported by grants from the National Institutes of Health (DK052230, DK093680 and CA172113) awarded to Dr. Vincent W. Yang.
References
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981;199:565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136(5):903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108(1):179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26(3):630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40(10):1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29(6):549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan MO, Ghaleb AM, Bialkowska AB, Yang VW. Kruppel-like factor 5 is essential for proliferation and survival of mouse intestinal epithelial stem cells. Stem Cell Res. 2015;14(1):10–19. doi: 10.1016/j.scr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg HB. Comparative anatomy, physiology, and mechanisms of disease production of the esophagus, stomach, and small intestine. Toxicol Pathol. 2014;42(1):54–66. doi: 10.1177/0192623313518113. [DOI] [PubMed] [Google Scholar]
- De Robertis M, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10(9) doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus HA. Observations on the presence of intestinal epithelium in the gastric mucosa. The Journal of Pathology and Bacteriology. 1937;44(2):389–398. [Google Scholar]
- Moolenbeek C, Ruitenberg EJ. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15(1):57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- Park CM, Reid PE, Walker DC, MacPherson BR. A simple, practical 'swiss roll' method of preparing tissues for paraffin or methacrylate embedding. J Microsc. 1987;145:115–120. doi: 10.1111/j.1365-2818.1987.tb01321.x. Pt 1. [DOI] [PubMed] [Google Scholar]
- Summersgill B, Clark J, Shipley J. Fluorescence and chromogenic in situ hybridization to detect genetic aberrations in formalin-fixed paraffin embedded material, including tissue microarrays. Nat Protoc. 2008;3(2):220–234. doi: 10.1038/nprot.2007.534. [DOI] [PubMed] [Google Scholar]
- Takeda N, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120(1):254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- McConnell BB, et al. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology. 2011;141(4):1302–1313. doi: 10.1053/j.gastro.2011.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan MO, et al. Kruppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations. Mol Cancer. 2010;9(63) doi: 10.1186/1476-4598-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirici D, et al. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem. 2009;57(6):567–575. doi: 10.1369/jhc.2009.953240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb prot4986. [DOI] [PubMed] [Google Scholar]


