Abstract
Aim
Molybdenum cofactor deficiency (MoCD) and Sulfite oxidase deficiency (SOD) are rare autosomal recessive conditions of sulfur-containing amino acid metabolism with overlapping clinical features and emerging therapies. The clinical phenotype is indistinguishable and they can only be differentiated biochemically. MOCS1, MOCS2, MOCS3, and GPRN genes contribute to the synthesis of molybdenum cofactor, and SUOX gene encodes sulfite oxidase. The aim of this study was to elucidate the clinical, radiological, biochemical and molecular findings in patients with SOD and MoCD.
Methods
Detailed clinical and radiological assessment of 9 cases referred for neonatal encephalopathy with hypotonia, microcephaly, and epilepsy led to a consideration of disorders of sulfur-containing amino acid metabolism. The diagnosis of six with MoCD and three with SOD was confirmed by biochemical tests, targeted sequencing, and whole exome sequencing where suspicion of disease was lower.
Results
Novel SUOX mutations were detected in 3 SOD cases and a novel MOCS2 mutation in 1 MoCD case. Most patients presented in the first 3 months of life with intractable tonic–clonic seizures, axial hypotonia, limb hypertonia, exaggerated startle response, feeding difficulties, and progressive cystic encephalomalacia on brain imaging. A single patient with MoCD had hypertrophic cardiomyopathy, hitherto unreported with these diseases.
Interpretation
Our results emphasize that intractable neonatal seizures, spasticity, and feeding difficulties can be important early signs for these disorders. Progressive microcephaly, intellectual disability and specific brain imaging findings in the first year were additional diagnostic aids. These clinical cues can be used to minimize delays in diagnosis, especially since promising treatments are emerging for MoCD type A.
Keywords: Molybdenum cofactor deficiency, Sulfite oxidase deficiency, Microcephaly, Epilepsy, MOCS1 gene, MOCS2 gene, SUOX gene
1. Introduction
Molybdenum cofactor deficiency (MoCD, OMIM 252150) and isolated Sulphite oxidase deficiency (SOD, OMIM 272300) are rare recessive disorders of sulfur-containing amino acid metabolism with an overlapping clinical phenotype and severe neurodegeneration in newborns.1 Due to the large clinical overlap between MoCD and SOD, they can only be differentiated biochemically. Both disorders show elevated urinary sulfite levels and lead to an accumulation of S-sulphocysteine, as well as elevated levels of thiosulfate and taurine. MoCD differs from SOD in that elevations of urinary xanthine and hypoxanthine concentrations, and low serum uric acid concentrations are observed due to loss of function of xanthine dehydrogenase. So far, about 100 MoCD and 30 SOD patients have been reported in the literature with different ethnic backgrounds, mostly from Europe and North America.2 The prevalence of MoCD is not ascertained, but is considered to be in between one in 100,000–200,000 newborns worldwide.3 The aim of this study is to showcase the clinical and molecular findings in our cohort in order to expand shared knowledge in these ultra-rare disorders.
MoCD patients have a wide spectrum of clinical findings including facial dysmorphism, early refractory seizures, severe psychomotor retardation, failure to thrive, microcephaly, hypotonia, lens dislocation and renal stones.4–6 Neuroimaging by computer tomography (CT) scan shows diffuse edema in the neonatal period due to sulfite accumulation. Multicystic degeneration of the cerebral hemispheres develops as the disease progresses. Calcification in both thalami and/or basal ganglia may be detected.7 Magnetic resonance imaging (MRI) of the brain shows subcortical cystic changes, parenchymal loss, white matter gliosis, and cerebral atrophy which is indicated by enlargement of the ventricular and cortical sulci, interhemispheric fissure, and thinning of the corpus callosum.8
In MoCD, elevated urine sulfite and low plasma urate are detected, which is followed by evidence for high purine metabolites (xanthines and hypoxanthines) and high S-sulphocysteine, which are due to the reduced activity of xanthine oxidase and sulfite oxidase respectively, where molybdenum cofactor is required.9,10 Molybdenum cofactor synthesis requires the genes MOCS1, MOCS2, MOCS3 and GEPH.11 Recent studies suggested value in replacing cyclic pyranopterin monophosphate (cPMP), a biosynthetic precursor of the cofactor lacking in two-thirds of patients with MoCD Type A, making cPMP substitution the first promising therapy for patients with MoCD.3,12
SOD results in accumulation of sulfite and S-sulphocysteine, which can be detected in urine and plasma. Serum uric acid and urinary excretion of xanthine and hypoxanthine are normal in SOD.10,13 The clinical picture is very similar to MoCD with early onset seizures, intellectual disability, hypotonia in infancy followed by hypertonia, and brain atrophy. The gene for sulfite oxidase maps to chromosome 12q13.2 and mutations in SUOX gene leads to isolated sulfite oxidase deficiency.14 In the current study, we describe the clinical, biochemical and genetic findings of nine patients with disorders of sulfur-containing amino acid metabolism, upon genetic study, six had MoCD and three had SOD.
2. Material and methods
This study comprised 9 Egyptian patients diagnosed between 2012 and 2015 in the Neurogenetics clinic of National Research Centre and Cairo University Neurometabolic Clinic. In eight patients there was suspicion of disorders of sulfur-containing amino acid metabolism on the basis of typical clinical presentation. A single family with atypical presentation was identified by whole exome sequencing where suspicion was low. All patients were subjected to detailed family history, pedigree analysis and thorough clinical and neurological evaluation. Biochemical confirmation was with analysis of sulfur containing amino acids by Liquid Chromatography–Mass Spectrometry (LC–MS–HPLC–MS) that quantitatively estimated S-Sulphocysteine and Xanthine in urine and by the determination of the level of blood uric acid to differentiate between these disorders. Other investigations included complete eye evaluation, electroencephalogram (EEG), echocardiography, visual evoked potential (VEP), electroretinogram (ERG), urine analysis and abdominal ultrasound, and review of imaging findings.
Genomic DNA was extracted from peripheral blood lymphocytes of all patients and parents after informed consent according to the guidelines of the Ethical Committee of the National Research Centre (NRC). The coding regions of MOCS1, MOCS2, MOCS3, GPRN, and SUOX genes were amplified using specific primers designed by PRIMER3 software version 0.4.0. (Primers and PCR conditions are available upon request from the corresponding author.) Potentially causative mutations were further screened in a group of 100 unrelated Egyptian individuals to evaluate carrier frequency. Exome capture was performed using the SureSelect Human All Exome 50 Mb Kit (Agilent Technologies, Santa Clara, CA) with 150-bp paired-end read sequences generated on a HiSeq2000 (Illumina, San Diego, CA). Sequences were aligned to hg19 and variants identified through the GATK pipeline. Variations were annotated with in house software and the SeattleSeq server. Identified variants were checked against public databases dbSNP ver.144 (http://www.ncbi.nlm.nih.gov/SNP/), Exome Variant Server (http://evs.gs.washington.edu/EVS/), ExAC (http://exac.broadinstitute.org/), and the Greater Middle East Variant Server (http://igm.ucsd.edu/gme/).
3. Results
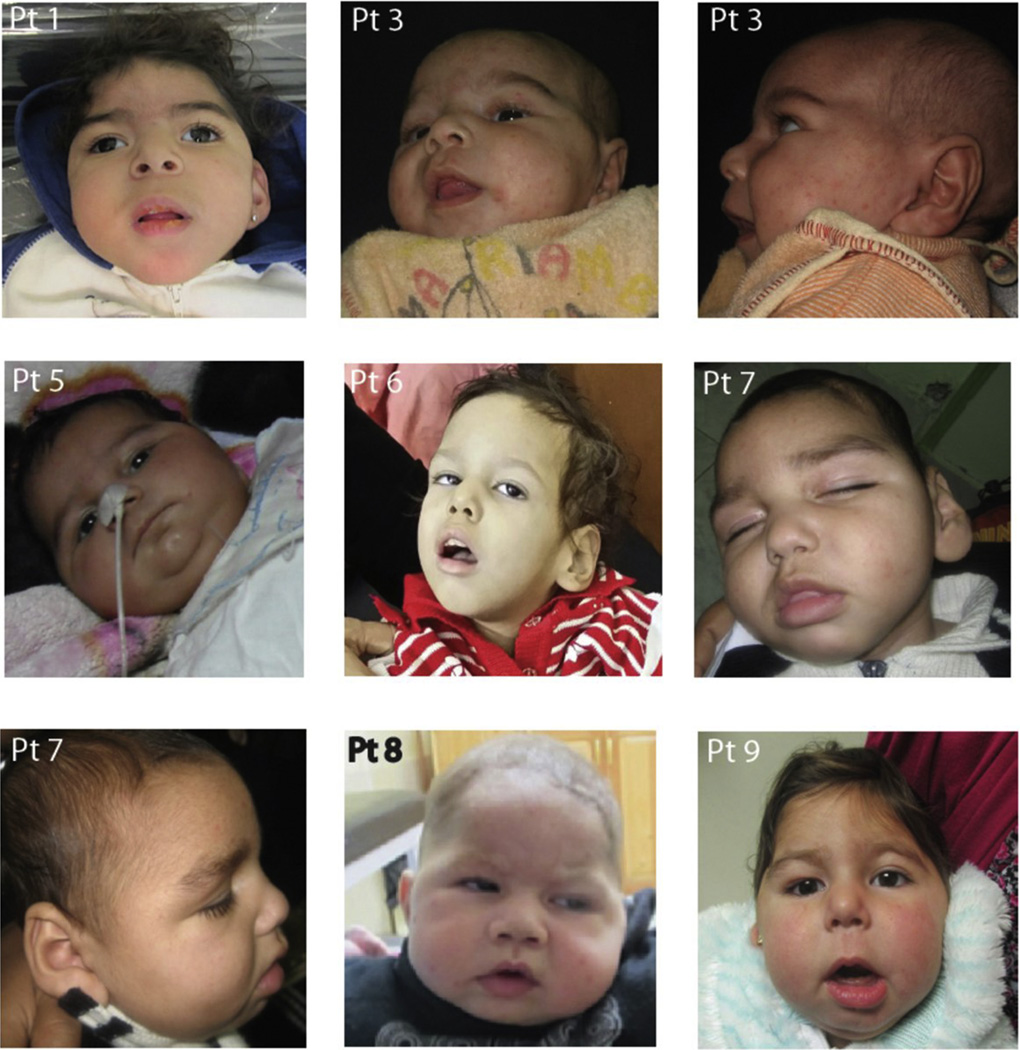
Clinical and biochemical testing identified 6 patients with MoCD and 3 with SOD (Table 1). The gender distribution of the cohort was 6 females (5 with MoCD, and 1 with SOD) and 3 males (1 with MoCD and 2 with SOD). The age at diagnosis ranged from 2 months to 10 months in eight cases. A single case (patient 6) was diagnosed at the age of 2 years with history of sudden deterioration after high fever at the age of 6 months that manifested with epilepsy, axial hypotonia and extrapyramidal manifestations. Positive family history of similar deceased siblings was found in 6 families; five were previously considered as possible neonatal encephalopathy but without notable perinatal hypoxic exposure. The patient with late presentation (Patient 6) also had a history of 3 similar siblings that died around the age of 3, which pointed to genetic etiology. All patients except patient 6 and 9 died between the ages of 1.5 and 2.5 years. Patient 6 died at 5.5 years of age and patient 9 was 2.5 years of age and alive at the time of writing. Five patients had the classic MoCD presentation with early onset of seizures within the neonatal period while in SOD presentation was from day 15 to day 50. All had severe developmental delay, microcephaly (−2.5 to −6 SD), failure to thrive, feeding difficulties, intractable seizures (generalized tonic clonic and multifocal myoclonic not responding to combined antiepileptic drug therapy), axial hypotonia and spastic quadriparesis. Severe extrapyramidal manifestations were detected in the late onset case. Excessive startle appearing as hyperekplexia was present in 5 cases, and only two had hypsarrhythmia on electroencephalogram. None had evidence of lens subluxation or eye anomalies. Mild facial dysmorphism (frontal bossing, depressed nasal bridge, anteverted nares, retrognathia, puffy checks and low-set ears) was present in 5 patients (Fig. 1). Renal stones were present in 3 out of 6 cases with MoCD and in none with SOD. Cardiomyopathy was found in one patient with MoCD on echocardiogram performed for tachycardia and murmur.
Table 1.
Clinical and radiological data of patients.
| Disease | Patient 1 MoCD |
Patient 2 MoCD |
Patient 3 MoCD |
Patient 4 MoCD |
Patient 5 MoCD |
Patient 6 MoCD |
Patient 7 SOD |
Patient 8 SOD |
Patient 9 SOD |
|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis | 8m | 2m | 2m | 5m | 7m | 2y | 10m | 4m | 2m |
| Sex | F | F | F | F | M | F | M | M | F |
| Consanguinity | + | + | + | + | + | + | + | + | + |
| Affected siblings | + | − | + | + | + | + | + | − | − |
| Severe developmental delay |
+ | + | + | + | + | + | + | + | + |
| Failure to thrive | + | + | − | − | + | + | + | + | + |
| Seizures | + | + | + | + | + | + | + | + | + |
| Onset age | D2 | D5 | D2 | D12 | D3 | 1 year | D50 | D15 | D40 |
| Response to AED | No | Fairly | No | No | No | No | No | No | No |
| Hyperekplexia | + | − | + | + | − | − | + | + | − |
| EEG | Focal | Focal | Focal | Hypsarrhythmia | Focal | Focal | Focal | Hypsarrhythmia | Focal |
| Head circumference (SD) |
−5 | −6 | −4 | −3.5 | −5 | −4 | −5.5 | −3 | −2.6 |
| Facial dysmorphism | + | − | + | + | − | + | + | + | − |
| Axial hypotonia | + | + | + | + | + | + | + | + | + |
| Spastic quadriparesis | + | + | + | + | + | + | + | + | + |
| Extrapyramidal | + | + | + | + | + | + | + | + | + |
| Lens dislocation | − | − | − | − | − | − | − | − | − |
| Renal stones | + | − | + | + | − | − | − | − | − |
| Cardiomyopathy | − | − | − | +a | − | − | − | − | − |
| Neuroimaging Calcifications |
Thalami/BG | −/− | −/− | −/− | Thalami | −/− | Thalami | Thalami | − |
| Subcortical cysts | + | + | + | + | + | − | + | + | + |
| Abnormal BG | + | + | + | + | − | + | + | Atrophy | − |
| Cerebral atrophy | + | + | + | + | + | + | + | + (Severe) | + |
| Wide IHF | + | + | + | + | + | + | + | + | − |
| Thinned CC | + | + | + | + | + | + | + | + | + |
| WM volume loss | + | + | + | + | − | + | + | + | + |
| CB/BS atrophy | +/− | +/+ | −/− | −/− | −/− | +/+ | +/− | +/+ | +/+ |
AED: antiepileptic drug, BG: basal ganglia, BS: brain stem, CB: cerebellar, CC: corpus callosum, CT: computerized tomography, D: day, EEG: electroencephalography, F: female, IHF: interhemispheric fissure, M: male, MoCD: molybdenum cofactor deficiency, MR: magnetic resonance imaging, SD: standard deviation, SOD: Sulphite oxidase deficiency, WM: white matter.
Echocardiogram revealed hypertrophic cardiomyopathy with severe left ventricular outlet (LVOT) obstruction with a gradient of 122 mmHg. Following beta blocker treatment it was improved at 3 and 4 years old to 50 mmHg and 12 mmHg, respectively.
Fig. 1.
Facial features of the patients showing frontal bossing, depressed nasal bridge, anteverted nares, retrognathia, long philtrum, full checks and low set ears. Patient number. Permission for the use of these pictures has been granted.
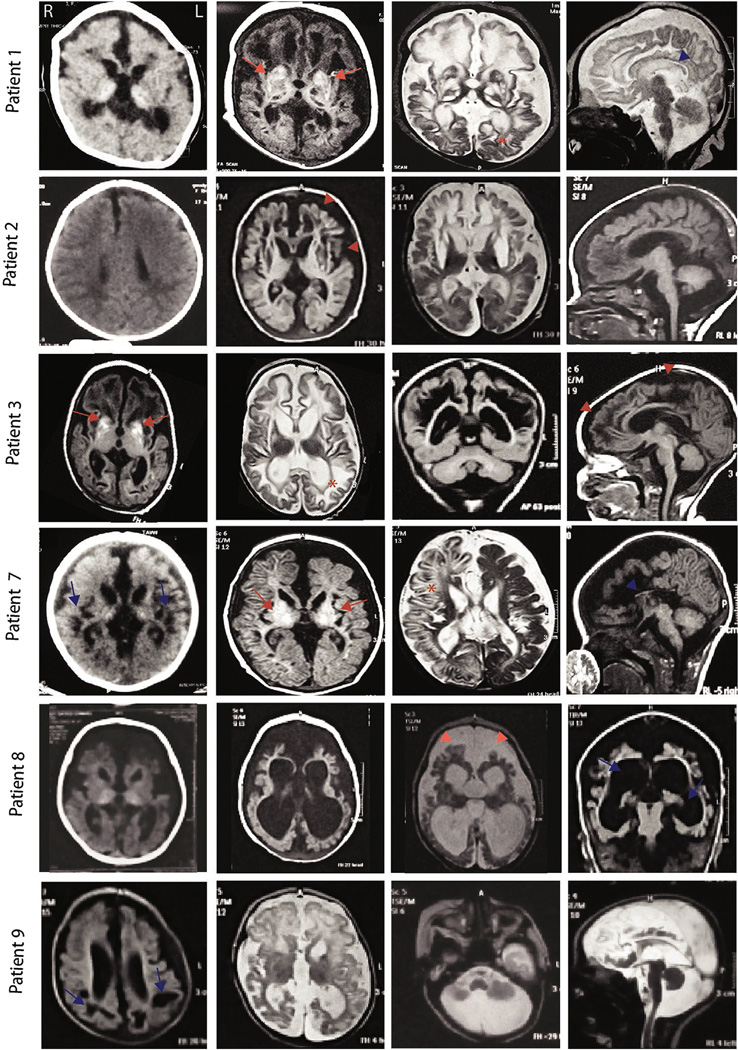
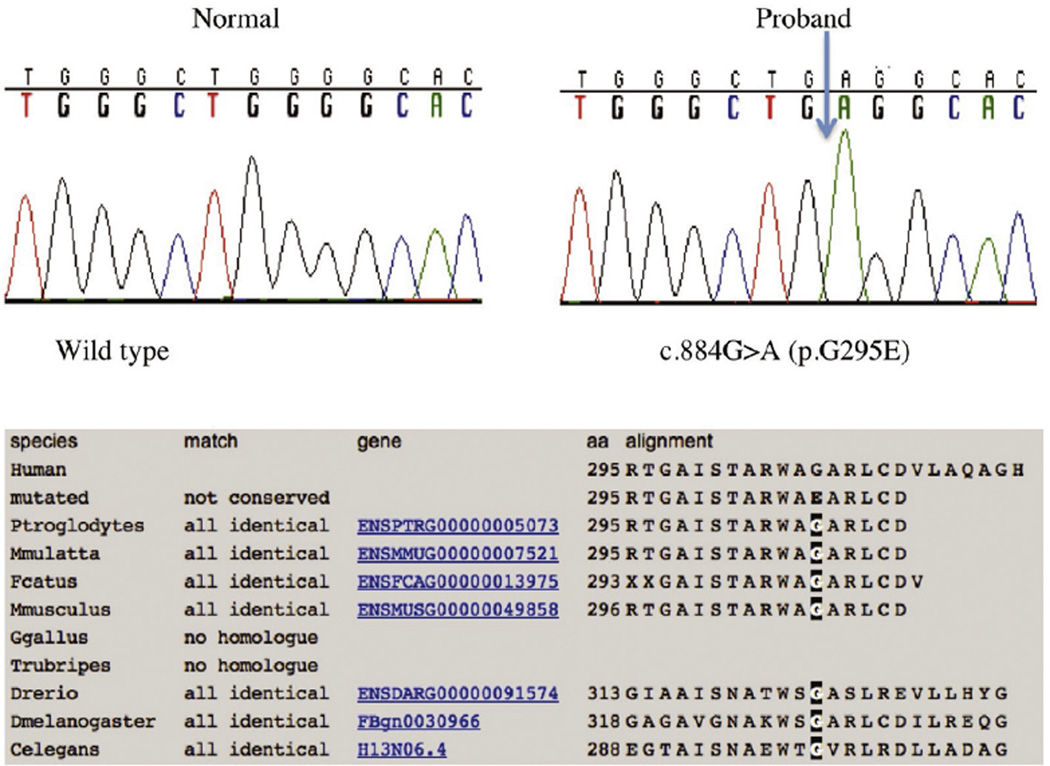
The brain CT and MRI showed calcification of thalami and/or basal ganglia in 3 patients (2 MoCD and one SOD). Subcortical cysts were found in 8 patients (5 MoCD and 3 SOD), and abnormal basal ganglia in 7 (5 MoCD and 2 SOD). Cerebral atrophy, thinning of the corpus callosum, white matter volume loss, and wide interhemispheric fissures were present in all patients (Figs. 2 and 3). Biochemical tests were performed for confirmation of the diagnosis; S-sulphocysteine concentration was high in all patients while xanthine was high and serum uric acid was low in MoCD but normal in the SOD patients (Table 2). Targeted genetic sequencing revealed 5 MoCD patients with known mutations in MOCS1 gene and 1 patient with a novel mutation in MOCS2 (Pathogenic, PVS1). There were 2 novel mutations (Pathogenic, PS3) identified in 3 patients with SOD, and a c.884G > Amutation was identified in 2 unrelated families (Table 3, and Fig. 4). Identified novel variants were checked and not found in public databases dbSNP ver.144, Exome Variant Server, ExAC or the Greater Middle East Variant Server. Potential pathogenicity was predicted using the software tools SIFT, PolyPhen-2 ver.2.2.2, and Mutation Assessor.
Fig. 2.
MR images of patients in our cohort. Note cerebral atrophy (red arrowheads), hyperintensity signal in basal ganglia (red arrows), subcortical cystic encephalomalacia (blue arrows), corpus callosum hypoplasia (blue arrowheads), and dysmyelination (asterisks). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
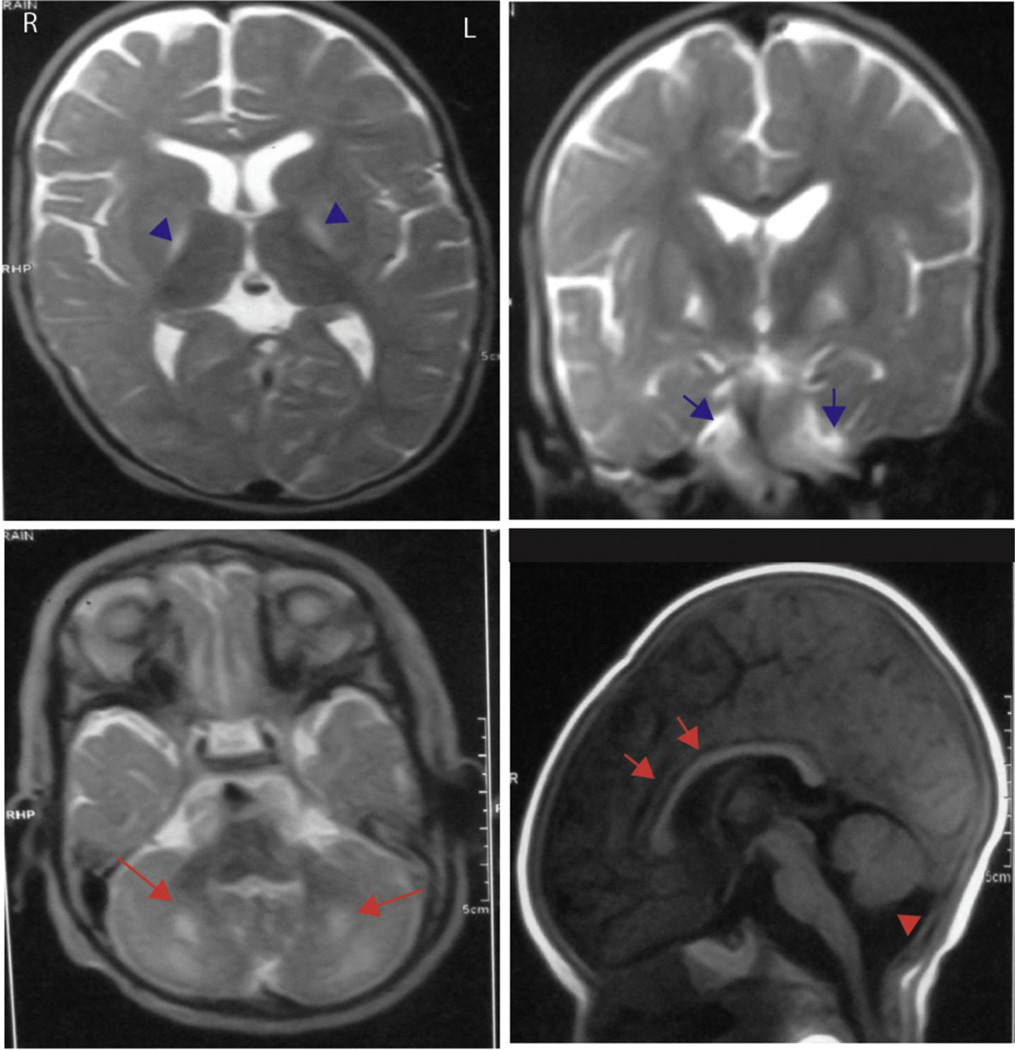
Fig. 3.
MRI brain for patient 6 with MOCD2 mutation. Note high signal of basal ganglia (blue arrowheads), high intensity of the white matter of cerebellum (blue arrows and bigger red arrows), thin corpus callosum (red arrows) and cerebellar atrophy (red arrowhead). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Biochemical data of the patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
|---|---|---|---|---|---|---|---|---|
| S-sulphocysteine/xanthinea | 442 (H)/1713 (H) | 540(H)/825 (H) | 450 (H)/1215(H) | 580 (H)/1368 (H) | 323 (H)/529 (H) | 920(H)/3 (N) | 980(H)/1.7 (N) | 1020(H)/1.6 (N) |
| Serum uric acidb | 0.6 (L) | 0.5 (L) | 0.5 (L) | 0.4 (L) | 3 (N) | 3.2 (N) | 2.5 (N) | 3 (N) |
S-sulphocysteine/xanthine (<10/<40 µmol/mmol creatinine) by LC–MS/MS method (Rashed et al., 2005)15.
Serum uric acid in children (2.0–5.5 mg/dL).
Table 3.
Molecular results of the patients.
| Patient | Disease | Gene | Type | Kind | Exon | Mutation | Reference |
|---|---|---|---|---|---|---|---|
| 1 | MoCD | MOCS1 | Homozygous | Nonsense | 2 | c.253C > T (p.Q85*) | Reiss et al. (2011)12 |
| 2 | MoCD | MOCS1 | Homozygous | Splice site | Intron 8 | c.1102 + 1G > A | Reiss et al. (1998)16 |
| 3 | MoCD | MOCS1 | Homozygous | Missense | 7 | c.971G > A (p.G324E) | Reiss. et al. (1998)16 |
| 4 | MoCD | MOCS1 | Homozygous | Nonsense | 2 | c.253C > T (p.Q85*) | Reiss et al. (2011)12 |
| 5 | MoCD | MOCS1 | Homozygous | Frame shift | 4 | c.722_722delT (p.L241Rfs*6) | Reiss et al. (2011)12 |
| 6 | MoCD | MOCS2 | Homozygous | Nonsense | 1 | c.3G > A p.M1I |
Novel |
| 7 | SOD | SUOX | Homozygous | Missense | 2 | c.713G > A (p.G238Q*) |
Novel |
| 8 | SOD | SUOX | Homozygous | Missense | 6 | c.884G > A (p.G295E) | Novel |
| 9 | SOD | SUOX | Homozygous | Missense | 6 | c.884G > A (p.G295E) | Novel |
Fig. 4.
Both Patient 7 and 8 were homozygous for a novel missense mutation in exon 6 of the SUOX gene. The mutation occurred in a fully conserved amino acid residue.
4. Discussion
MoCD and SOD are rare autosomal recessive inborn errors of metabolism with similar clinical presentations. Our study includes 6 MoCD and 3 SOD patients. All were products of consanguineous marriage and 6 patients had similarly affected siblings.
MoCD and SOD are characterized by intractable seizures that are usually seen immediately after birth.2,8,13 Eight patients in our series had the classic presentation with early onset intractable seizures (generalized tonic clonic and multifocal myoclonic), not responding to antiepileptic medications. Seizure onset ranged from day 2 to day 50 of life, and a single case had a late onset at the age of 1 year which is considered to be rare in comparison with other more common neonatal MoCD onset.8,17 The initial symptom of all our patients was seizures, which was reported to be the most common presenting symptom in both disorders.18,19
All the patients had accompanying severe developmental delay, and 7 had failure to thrive, which is in accordance with previous observations.20 Patient 6 in our cohort had a late onset at the age of 1 year with sudden deterioration and gradual progression of epilepsy which is reported in just one patient so far.21 Therefore, we think MoCD and SOD should also be in the differential diagnosis for patients presenting with progressive loss of milestones with or without seizures.
Our findings demonstrated feeding difficulties in 7 patients. Feeding difficulties and hypotonia are the most common symptoms after seizures as the presenting symptom in MoCD and SOD.18,19,21
Hyperekplexia (exaggerated startle) was present in 5 cases, and is described both in MoCD and SOD.22,23 Neonatal hyperekplexia can be seen in glycine neurotransmitter-conditions as well, and can alert the physician to consider genetic/metabolic conditions in the differential diagnosis.
All the patients in the present study were microcephalic with head circumferences ranging from −2.5 to −6 SD. Progressive microcephaly was reported in almost all patients with MoCD and SOD.8,24,25 Documenting the presence of progressive microcephaly especially in older patients with later onset can help with diagnosis in not so typical presentations.
Although, it is mentioned that dislocated lens is commonly observed in MoCD and SOD, none of our cases had lens subluxation.26,27 The fact that patients with MoCD develop lens dislocation late at the age of approximately 8 years may be the reason for the lack of this finding in our cohort as all our patients are well below this average age.28
Echocardiogram in one patient with MoCD revealed hypertrophic cardiomyopathy with severe left ventricular outlet (LVOT) obstruction. To the best of our knowledge this finding has not been previously reported in any MoCD case, and is not an obvious consequence of disease. Further reports will need to ascertain if this is a random finding or a rare presentation that may be linked with MoCD.
MoCD patients show parenchymal loss, cerebral atrophy and ventricular enlargement,2 which were also present in our MoCD patients as well as SOD patients. In our cohort, CT/MRI showed calcification of basal ganglia and/or thalami, abnormal signal and/or atrophy of basal ganglia, and bilateral hyperintensity of lentiform nucleus in the late onset MoCD patient. Subcortical cystic encephalomalacia was seen in 8 patients which is in accordance with previous reports, and should raise clinical suspicion of the disease.10,29
The distinctive biochemical analysis is an important tool in the diagnosis of MoCD and SOD, especially the evaluation of serum sulphocysteine.2,17 These entities are clinically similar, but normal xanthine, hypoxanthine, and uric acid levels are seen in SOD.30,31 Our findings demonstrated increased S-sulphocysteine in all patients. Xanthine level in urine was high in all patients with MoCD and serum uric acid was low in five, whereas serum uric acid and Xanthine were normal in all three SOD patients. It was suggested that rarely uric acid levels were normal in MoCD and the biochemical tests frequently do not correlate with the severity of the disease.12 This is quite notable in our case where uric acid was within normal limits in patient 6 and the level of the abnormal metabolites was not related to the clinical course.
The mutations leading to MoCD are not reported to correlate to severity of phenotype.3 They have been identified in the genes MOCS1 (type A deficiency), MOCS2 (type B deficiency), and in GPHN.12,14,20 It was documented that the most commonly mutated gene in MoCD patients was the MOCS1 gene, followed by MOCS2.19 Reiss et al., 2011 found 99% mutation detection with analysis of the MOCS1 and MOCS2 genes in MoCD diagnosed biochemically. Only in the case of negative results, sequencing of the genes GPHN and MOCS3 was suggested.12 Similarly, the majority (5/6) of mutations we observed in MoCD patients were in MOCS1, the remaining mutation was in MOCS2. SUOX is the only gene reported to be responsible for SOD so far.18 We have encountered SUOX mutations in all 3 meeting biochemical criteria for SOD. All were previously unreported mutations with the p.G295E allele detected in two patients, suggesting a founder allele.
Early diagnosis has become a key factor for MoCD type A, as a replacement therapy for the missing metabolite cPMP has yielded promising results. cPMP substitution therapy was undertaken in an infant diagnosed at 6 days of life. The treatment was started on day 36, and clinical improvements were observed, with decreased epileptic seizure frequency, and decreased urinary markers of sulfite, S-sulphocysteine, and thiosulfate. Xanthine and uric acid levels also returned to normal.20 A second successful treatment was reported in a patient who was prenatally diagnosed with MoCD type A, where substitution therapy was started 4 h following delivery. The patient showed a marked improvement in response to early start of therapy. At 21 months of age, there was only mild cognitive delay with normal fine and gross motor development.1 Further replacement therapies were undertaken in patients either diagnosed prenatally or very early in life, which reported favorable clinical and biochemical response.3 The limited data suggests that early initiation might be associated with better outcomes, although further studies are needed.
5. Conclusion
Early diagnosis and confirmatory gene analysis of these devastating disorders is crucial to providing precise genetic counseling and prenatal testing, and for potential therapeutic intervention considering the favorable outcomes for early replacement therapy in MoCD type A. We have discussed the clinical and radiological features in our series, and compared it with existing data to aid physicians in diagnosing these ultra-rare disorders early in infancy.
Acknowledgments
We would like to thank Pharmagene lab in Cairo, directed by Dr. Mohamed S. Rashed for performing the biochemical tests.
Footnotes
Conflict of interest
No conflict of interest.
REFERENCES
- 1.Hitzert MM, Bos AF, Bergman KA, et al. Favorable outcome in a newborn with molybdenum cofactor type A deficiency treated with cPMP. Pediatrics. 2012;130:e1005–e1010. doi: 10.1542/peds.2011-3330. [DOI] [PubMed] [Google Scholar]
- 2.Alkan M, Kip G, Sahin S, Atabek D. Choice of anesthesia in molybdenum cofactor deficiency: a case report. J Res Med Sci. 2014;19:1103–1105. [PMC free article] [PubMed] [Google Scholar]
- 3.Schwahn BC, Van Spronsen FJ, Belaidi AA, et al. Efficacy and safety of cyclic pyranopterin monophosphate substitution in severe molybdenum cofactor deficiency type A: a prospective cohort study. Lancet. 2015;386:1955–1963. doi: 10.1016/S0140-6736(15)00124-5. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz G, Mendel RR. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu Rev Plant Biol. 2006;57:623–647. doi: 10.1146/annurev.arplant.57.032905.105437. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Yuichiro Y, Sass JO, et al. Clin Chim Acta. 2012;414:158–160. doi: 10.1016/j.cca.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Bayram E, Topcu Y, Karakaya P, et al. Molybdenum cofactor deficiency: review of 12 cases (MoCD and review) Eur J Paediatr Neurol. 2013;17:1–6. doi: 10.1016/j.ejpn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Serrano M, Lizarraga I, Reiss J, et al. Cranial ultrasound and chronological changes in molybdenum cofactor deficiency. Pediatr Radiol. 2007;37:1043–1046. doi: 10.1007/s00247-007-0558-2. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi K, Hamano S, Mochizuki H, Ichida K, Ida H. Molybdenum cofactor deficiency mimics cerebral palsy: differentiating factors for diagnosis. Pediatr Neurol. 2012;47:147–149. doi: 10.1016/j.pediatrneurol.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Brucknerova I, Behulova D, Bzduch V, et al. Newborn with neonatal form of molybdenum cofactor deficiency – the first patient in the Slovak Republic. Neuro Endocrinol Lett. 2010;31(Suppl. 2):5–7. [PubMed] [Google Scholar]
- 10.Vijayakumar K, Gunny R, Grunewald S, et al. Clinical neuroimaging features and outcome in molybdenum cofactor deficiency. Pediatr Neurol. 2011;45:246–252. doi: 10.1016/j.pediatrneurol.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Kugler S, Hahnewald R, Garrido M, Reiss J. Long-term rescue of a lethal inherited disease by adeno-associated virus-mediated gene transfer in a mouse model of molybdenum-cofactor deficiency. Am J Hum Genet. 2007;80:291–297. doi: 10.1086/511281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiss J, Hahnewald R. Molybdenum cofactor deficiency: mutations in GPHN, MOCS1, and MOCS2. Hum Mutat. 2011;32:10–18. doi: 10.1002/humu.21390. [DOI] [PubMed] [Google Scholar]
- 13.Westerlinck H, Meylaerts L, Van Hoestenberghe MR, Rossi A. Sulfite oxidase deficiency in a newborn. JBR-BTR. 2014;97:113–114. doi: 10.5334/jbr-btr.40. [DOI] [PubMed] [Google Scholar]
- 14.Seidahmed MZ, Alyamani EA, Rashed MS, et al. Total truncation of the molybdopterin/dimerization domains of SUOX protein in an Arab family with isolated sulfite oxidase deficiency. Am J Med Genet A. 2005;136:205–209. doi: 10.1002/ajmg.a.30796. [DOI] [PubMed] [Google Scholar]
- 15.Rashed MS, Saadallah AA, Rahbeeni Z, Eyaid W, Seidahmed MZ, et al. Determination of urinary S-sulphocysteine, xanthine and hypothanxine by liquid chromatography-electrospray tandem mass spectrometry. Biomed Chromatogr. 2005;19:223–230. doi: 10.1002/bmc.439. [DOI] [PubMed] [Google Scholar]
- 16.Reiss J, Christensen E, Kurlemann G, Zabot MT, Dorche C. Genomic structure and mutational spectrum of the bicistronic MOCS1 gene defective in molybdenum cofactor deficiency type A. Hum Genet. 2005;103:639–644. doi: 10.1007/s004390050884. [DOI] [PubMed] [Google Scholar]
- 17.Edwards M, Roeper J, Allgood C, et al. Meta Gene. 2015;3:43–49. doi: 10.1016/j.mgene.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan WH, Eichler FS, Hoda S, et al. Isolated sulfite oxidase deficiency: a case report with a novel mutation and review of the literature. Pediatrics. 2005;116:757–766. doi: 10.1542/peds.2004-1897. [DOI] [PubMed] [Google Scholar]
- 19.Mechler K, Mountford WK, Hoffmann GF, Ries M. Ultra-orphan diseases: a quantitative analysis of the natural history of molybdenum cofactor deficiency. Genet Med. 2015;17:965–970. doi: 10.1038/gim.2015.12. [DOI] [PubMed] [Google Scholar]
- 20.Veldman A, Santamaria-Araujo JA, Sollazzo S, et al. Successful treatment of molybdenum cofactor deficiency type A with cPMP. Pediatrics. 2010;125:e1249–e1254. doi: 10.1542/peds.2009-2192. [DOI] [PubMed] [Google Scholar]
- 21.Arenas M, Fairbanks LD, Vijayakumar K, et al. An unusual genetic variant in the MOCS1 gene leads to complete missplicing of an alternatively spliced exon in a patient with molybdenum cofactor deficiency. J Inherit Metab Dis. 2009;32:560–569. doi: 10.1007/s10545-009-1151-7. [DOI] [PubMed] [Google Scholar]
- 22.Holder JL, Jr, Agadi S, Reese W, Rehder C, Quach MM. Infantile spasms and hyperekplexia associated with isolated sulfite oxidase deficiency. JAMA Neurol. 2014;71:782–784. doi: 10.1001/jamaneurol.2013.5083. [DOI] [PubMed] [Google Scholar]
- 23.Macaya A, Brunso L, Fernandez-Castillo N, Arranz JA, Ginjaar HB, et al. Molybdenum cofactor deficiency presenting as neonatal hyperekplexia: a clinical, biochemical and genetic study. Neuropediatrics. 2005;36:389–394. doi: 10.1055/s-2005-872877. [DOI] [PubMed] [Google Scholar]
- 24.Gumus H, Ghesquiere S, Per H, et al. Maternal uniparental isodisomy is responsible for serious molybdenum cofactor deficiency. Dev Med Child Neurol. 2010;52:868–872. doi: 10.1111/j.1469-8749.2010.03724.x. [DOI] [PubMed] [Google Scholar]
- 25.Grings M, Moura AP, Parmeggiani B, et al. Disturbance of brain energy and redox homeostasis provoked by sulfite and thiosulfate: potential pathomechanisms involved in the neuropathology of sulfite oxidase deficiency. Gene. 2013;531:191–198. doi: 10.1016/j.gene.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Struys EA, Nota B, Bakkali A, et al. Pyridoxine-dependent epilepsy with elevated urinary alpha-amino adipic semialdehyde in molybdenum cofactor deficiency. Pediatrics. 2012;130:e1716–e1719. doi: 10.1542/peds.2012-1094. [DOI] [PubMed] [Google Scholar]
- 27.Hughes EF, Fairbanks L, Simmonds HA, Robinson RO. Molybdenum cofactor deficiency-phenotypic variability in a family with a late-onset variant. Dev Med Child Neurol. 1998;40:57–61. doi: 10.1111/j.1469-8749.1998.tb15357.x. [DOI] [PubMed] [Google Scholar]
- 28.Parini R, Briscioli V, Caruso U, et al. Spherophakia associated with molybdenum cofactor deficiency. Am J Med Genet. 1997;73:272–275. doi: 10.1002/(sici)1096-8628(19971219)73:3<272::aid-ajmg8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Chen LW, Tsai YS, Huang CC. Prenatal multicystic encephalopathy in isolated sulfite oxidase deficiency with a novel mutaion. Pediatr Neurol. 2014;51:181–182. doi: 10.1016/j.pediatrneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Bindu PS, Christopher R, Mahadevan A, Bharath RD. Clinical and imaging observations in isolated sulfite oxidase deficiency. J Child Neurol. 2011;26:1036–1040. doi: 10.1177/0883073811401399. [DOI] [PubMed] [Google Scholar]
- 31.Belaidi AA, Arjune S, Santamaria-Araujo JA, Sass JO, Schwarz G. Molybdenum cofactor deficiency: a new HPLC method for fast quantification of s-sulphocysteine in urine and serum. JIMD Rep. 2012;5:35–43. doi: 10.1007/8904_2011_89. [DOI] [PMC free article] [PubMed] [Google Scholar]