Abstract
Purpose
Socioeconomic status (SES) is a known predictor of survival for several cancers and it has been suggested that SES differences affecting tumour stage at diagnosis may be the most important explanatory factor for this. However, only a limited number of studies have investigated SES differences in tumour stage at diagnosis of ovarian cancer. In a pooled analysis, we investigated whether SES as represented by level of education is predictive for advanced tumour stage at diagnosis of ovarian cancer, overall and by histotype. The effect of cigarette smoking and body mass index (BMI) on the association was also evaluated.
Methods
From 18 case-control studies, we obtained information on 10,601 women diagnosed with epithelial ovarian cancer. Study specific odds ratios (ORs) with corresponding 95% confidence intervals (CI) were obtained from logistic regression models and combined into a pooled odds ratio (pOR) using a random effects model.
Results
Overall, women who completed ≤high school had an increased risk of advanced tumour stage at diagnosis compared with women who completed >high school (pOR 1.15; 95% CI 1.03–1.28). The risk estimates for the different histotypes of ovarian cancer resembled that observed for ovarian cancers combined but did not reach statistical significance. Our results were unchanged when we included BMI and cigarette smoking.
Conclusion
Lower level of education was associated with an increased risk of advanced tumour stage at diagnosis of ovarian cancer. The observed socioeconomic difference in stage at diagnosis of ovarian cancer calls for further studies on how to reduce this diagnostic delay.
Keywords: Epidemiology, ovarian cancer, pooled analysis, socioeconomic status, tumour stage
1. Introduction
Ovarian cancer is the 5th most common malignancy among women in developed countries [1]. Furthermore, it is a highly fatal disease with the worst prognosis among the gynaecological cancers because it is often diagnosed at an advanced tumour stage [2]. As tumour stage at diagnosis is among the most important prognostic factors in ovarian cancer, detection at an early stage is essential. However, currently there are no efficient screening tools for ovarian cancer and as most women only experience vague symptoms, the disease is often detected at an advanced stage when survival is poor. Therefore, knowledge on predictors for advanced stage at diagnosis of ovarian cancer is crucial to reduce the mortality for this disease.
Socioeconomic status (SES) is a predictor of incidence and survival of a number of diseases and there is evolving evidence for socioeconomic differences in cancer survival for many cancer types [3;4]. However, in contrast to breast cancer, relatively few studies have addressed the association between SES and ovarian cancer survival and the results have been inconsistent. Five studies [5–9] found worse survival among women with low SES whereas two studies [10;11] found no association. The reasons for socioeconomic differences in cancer survival in general and ovarian cancer survival in particular are not well-understood [4]. Possible underlying causes can be separated into three groups: tumour characteristics (tumour stage at diagnosis and biological characteristics), health care factors (e.g., types of treatment received, medical expertise and utilization of screening), and patient characteristics (e.g., lifestyle factors and comorbidities) [4]. According to Woods et al. [4], SES differences in tumour stage at diagnosis is likely the most important explanation for differences in cancer survival for a number of cancer types; including breast- [12], endometrial- [13] and cervical cancer [14]. SES differences in tumour stage at diagnosis may be attributable to several reasons, including access to and acceptance of cancer screening technologies, awareness of cancer symptoms, health-seeking behaviour, access to health care, comorbidities, and lifestyle factors.
However, only a limited number of studies have investigated SES differences in tumour stage at diagnosis of ovarian cancer, and whereas the majority found no convincing evidence that tumour stage at diagnosis differed according to SES [15–18], one recent study showed that a lower level of education was associated with advanced tumour stage at diagnosis of ovarian cancer [8]. Many of the previous studies were limited by small sample sizes and lack of individual level SES data, and none of the studies investigated whether the association between SES and tumour stage at diagnosis differed by histotype.
Using data from 18 case-control studies included in the international Ovarian Cancer Association Consortium (OCAC), we performed a pooled analysis in order to evaluate the association between SES (represented by highest obtained level of education) and tumour stage at diagnosis, overall and by histotype. Furthermore, we aimed to investigate to what degree the association between SES and tumour stage at diagnosis was confounded by pre-diagnosis cigarette smoking or by body mass index (BMI).
2. Materials and Methods
The Ovarian Cancer Association Consortium (OCAC) described in details elsewhere [19] is an international collaboration of case-control studies founded in 2005 with the original aim to identify genetic polymorphisms associated with ovarian carcinogenesis. More recently, consortial activities have included the identification of risk factors and prognostic factors for ovarian cancer. In the present study, we obtained data from 18 studies that provided information about level of education and other required variables for the study [20–37] (Table 1). All data were checked for internal consistencies and clarifications were provided by the original investigators if needed. Among women diagnosed with ovarian cancer, we excluded from analyses those with missing data for level of education, those with non-epithelial ovarian tumours or epithelial tumours of low malignant potential (borderline ovarian tumours) and those who lacked information on age, race/ethnicity or tumour stage at diagnosis, leaving 10,601 women for analysis. All individual studies included in OCAC had institutional review board or ethics committee approvals and all study participants provided informed consent.
Table 1.
Characteristics of the 18 case-control studies included in the pooled analysis of level of education and stage at diagnosis of ovarian cancer.
| Region | Study | Study acronym |
Study period | Cases N |
Women diagnosed at advanced tumour stagea (%) |
Study type | Age range |
|---|---|---|---|---|---|---|---|
| Australia | Australian Ovarian Cancer Study and Australian Cancer Study (Ovarian Cancer) |
AUS | 2002–2006 | 1,073 | 950 (88.5) | Population-based | 20–80 |
| Europe | German Ovarian Cancer Study | GER | 1993–1996 | 219 | 178 (81.3) | Population-based | 21–75 |
| The Danish Malignant Ovarian Tumor Study | MAL | 1994–1999 | 551 | 478 (86.8) | Population-based | 32–80 | |
| Nijmegen Ovarian Cancer Study | NTH | 1989–2006 | 254 | 172 (67.7) | Hospital-based | 23–83 | |
| Polish Ovarian Cancer Case-Control Study | POL | 2000–2003 | 183 | 112 (61.2) | Population-based | 27–74 | |
| Study of Epidemiology and Risk Factors in Cancer Heredity |
SEA | 1998–2010 | 917 | 522 (56.9) | Population-based | 22–74 | |
| UK Ovarian Cancer Population Study | UKO | 2006–2010 | 524 | 443 (84.5) | Hospital-based | 19–89 | |
| United States (US) | Connecticut Ovarian Cancer Study | CON | 1998–2003 | 296 | 248 (83.8) | Population-based | 36–81 |
| Diseases of the Ovary and their evaluation | DOV | 2002–2005 | 592 | 504 (85.1) | Population-based | 35–74 | |
| Hawaii Ovarian Cancer Case-Control Study | HAW | 1993–2008 | 681 | 486 (71.4) | Population-based | 24–87 | |
| Novel Risk Factors and Potential Early Detection | HOP | 2003–2009 | 663 | 552 (83.3) | Population-based | 25–91 | |
| Markers for Ovarian Cancer | |||||||
| Mayo Clinic Ovarian Cancer Case-Control | MAY | 2000–2009 | 493 | 450 (91.3) | Clinic-based | 21–91 | |
| Control Study | |||||||
| North Carolina Ovarian Cancer study | NCO | 1999–2008 | 849 | 723 (85.2) | Population-based | 22–74 | |
| New England Case-Control Study | NEC | 1992–2003 | 827 | 570 (68.9) | Population-based | 21–77 | |
| New Jersey Ovarian Cancer Study | NJO | 2002–2008 | 230 | 184 (80.0) | Population-based | 30–81 | |
| Family Registry for Ovarian Cancer and Genetic Epidemiology of Ovarian Cancer |
STA | 1997–2001 | 488 | 376 (77.0) | Population-based | 21–64 | |
| University California Irvine Ovarian Study | UCI | 1993–2005 | 384 | 310 (80.7) | Population-based | 21–86 | |
| Los Angeles County Case-Control Studies of Ovarian Cancer |
USC | 1993–2005 | 1,377 | 1,073 (77.9) | Population-based | 20–84 | |
| TOTAL | 10,601 | 8,331 (78,6) | 19–91 |
Women diagnosed with regional or distant stage
2.1. Assessment of level of education
Information on highest attained level of education was obtained either from self-administered questionnaires (n = 8 studies) or from in-person interviews (n = 10 studies). For all included OCAC studies, information on highest level of education was harmonized and parameterized as a dichotomous variable (≤high school versus >high school).
2.2. Statistical analysis
Of the 18 studies included for analyses, 11 (AUS, GER, HOP, MAL, MAY, NCO, NEC, NTH, POL, SEA and UKO) used the FIGO staging system [38], while two studies (CON and NJO) used SEER staging manuals [39] to stage ovarian cancer. Five studies (DOV, HAW, STA, UCI and USC) had information on both FIGO and SEER tumour staging. In the common OCAC dataset, a harmonized summary tumour stage variable was created and reported in the following categories: localized tumour stage, regional tumour stage or distant tumour stage, using the following algorithm: localized = FIGO tumour stage IA, IB, I (not other specified (NOS)) or SEER tumour stage 1; regional = FIGO tumour stage IC, IIA, IIB, IIC, II (NOS) or SEER tumour stage 2, 3, 4, 5; distant = FIGO tumour stage IIIA, IIIB, IIIC, III (NOS), IV or SEER tumour stage 7. For studies with information on both FIGO and SEER tumour staging, the harmonized summary tumour stage variable represents the more advanced of FIGO and SEER tumour stage. In all analyses, the harmonized OCAC tumour stage variable was parameterized as a dichotomous comparison of localized tumour stage or advanced tumour stage (regional or distant).
To compare characteristics of the included women according to tumor stage at diagnosis (localized stage versus advanced stage), a Pearson's chi square statistical test was used when data were normally distributed (histology, level of education, smoking status and race/ethnicity) and a Wilcoxon rank sum statistical test was used when data were not normally distributed (age at diagnosis and BMI). We used a two-stage approach [40] to analyse the association between stage of ovarian cancer and level of education. First, study-specific odds ratios (ORs) were obtained by logistic regression models with adjustments for a priori selected potential confounding variables (described below). The study-specific estimates were then combined by random-effects inverse variance-weighted meta-analysis into a pooled odds ratio (pOR) with corresponding 95% confidence intervals (CIs) [41]. Statistical heterogeneity among studies was evaluated using the Cochran Q and I2 statistics. For all analyses, individual studies were included in the meta-analysis only if the following two requirements were met; i) five cases with complete data were available and ii) each level of the tumour stage variable had one or more subjects.
Two statistical models were fitted to evaluate the association of tumour stage at diagnosis of ovarian cancer according to level of education. Model 1 included adjustments for age at diagnosis (continuous variable) and race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or other, including unknown races). In Model 2, we additionally adjusted for pre-diagnosis cigarette smoking (never, former or current smoker) and BMI (determined either at one or five years prior to ovarian cancer diagnosis, depending on the study) as a continuous variable (per 5 kg/m2). Subgroup analyses were conducted for specific histotypes of ovarian cancers including serous, mucinous, endometrioid and clear cell tumours. Serous tumours were additionally categorized as low- (grade 1) or high- (grade 2+) grade tumours. Finally, we also performed additional sensitivity analyses to investigate whether the association between level of education and tumour stage at diagnosis of ovarian cancer overall differed according to study continent (US versus non-US studies), race/ethnicity (white (non-Hispanic or Hispanic White) versus all other races/ethnicities (Black, Asian and other)) or study type (population-based versus hospital-/clinic-based studies). All analyses were conducted using the environment R, version 3.1.0. A 5% significance level was used for all analysis.
3. Results
Characteristics of the 18 studies that contributed data from 10,601 women with epithelial ovarian cancer are shown in Table 1. Eleven studies were conducted in the United States (US), six in Europe and one in Australia. In the studies, the number of women with ovarian cancer ranged from 183 to 1377. Women were 19–91 years of age at diagnosis between 1989 and 2010. Fifteen studies were population-based and three were hospital/clinic-based. Almost four-fifths of the women (78.6%) had advanced tumour stage (regional or distant) at diagnosis of ovarian cancer.
Table 2 presents characteristics of the women included in the analysis according to tumour stage at diagnosis of ovarian cancer. Among women diagnosed at advanced tumour stage, median age at diagnosis was significantly higher (58.0 years) compared with women diagnosed at localized tumour stage (median age = 53.0 years). Furthermore, women diagnosed at advanced tumour stage of ovarian cancer were more often diagnosed with serous tumours, had completed ≤high school, had higher BMI, were less likely to be Asian and were more often a former smoker, compared with women diagnosed at a localized tumour stage (all p-values < 0.01).
Table 2.
Characteristics of women diagnosed with epithelial ovarian cancer, according to tumour stage at diagnosis.
| All (n = 10,601) |
Localized stage (n = 2,270) |
Advanced stageb (n = 8,331) |
P-value | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| Median | 57.0 | 53.0 | 58.0 | <0.001c |
| Interquartile range | 49.0–65.0 | 45.7–62.0 | 50.0–66.0 | |
| Histology | ||||
| Serous | 6,066 (57.2) | 501 (22.1) | 5,565 (66.8) | <0.001d |
| Serous low-grade | 479 (4.5) | 98 (4.3) | 381 (4.6) | |
| Serous high-grade | 5,030 (47.4) | 339 (14.9) | 4,691 (56.3) | |
| Endometrioid | 1,664 (15.7) | 665 (29.3) | 999 (12.0) | |
| Mucinous | 780 (7.4) | 480 (21.1) | 300 (3.6) | |
| Clear cell | 875 (8.3) | 412 (18.1) | 463 (5.6) | |
| Othera | 1,216 (11.5) | 212 (9.3) | 1,004 (12.1) | |
| Level of education | ||||
| ≤High school | 5,190 (49.0) | 1,054 (46.4) | 4,136 (49.6) | 0.008d |
| >High school | 5,411 (51.0) | 1,216 (53.6) | 4,195 (50.4) | |
| BMI | ||||
| Median | 24.0 | 23.6 | 24.1 | <0.001c |
| Interquartile range | 21.4–28.2 | 20.9–28.0 | 21.5–28.2 | |
| Smoking status | 0.02d | |||
| Never | 5,770 (54.4) | 1,227 (54.1) | 4,543 (54.5) | |
| Former | 3,391 (32.0) | 694 (30.6) | 2,697 (32.4) | |
| Current | 1,440 (13.6) | 349 (15.4) | 1,091 (13.1) | |
| Race/ethnicity | <0.001d | |||
| Non-Hispanic White | 9,129 (86.1) | 1,878 (82.7) | 7,251 (87.0) | |
| Hispanic White | 306 (2.9) | 62 (2.7) | 244 (2.9) | |
| Black | 265 (2.5) | 53 (2.3) | 212 (2.5) | |
| Asian | 565 (5.3) | 190 (8.4) | 375 (4.5) | |
| Other | 331 (3.1) | 87 (3.8) | 244 (2.9) |
Includes mixed cell, undifferentiated and tumours of unknown histology
Includes regional or distant stage
The P-value was calculated using the Wilcoxon rank sum statistical test as the data were not normally distributed
The P-value was calculated using the Pearson’s chi square statistical test at the data were normally distributed
Numbers may not sum up to total because of missing data
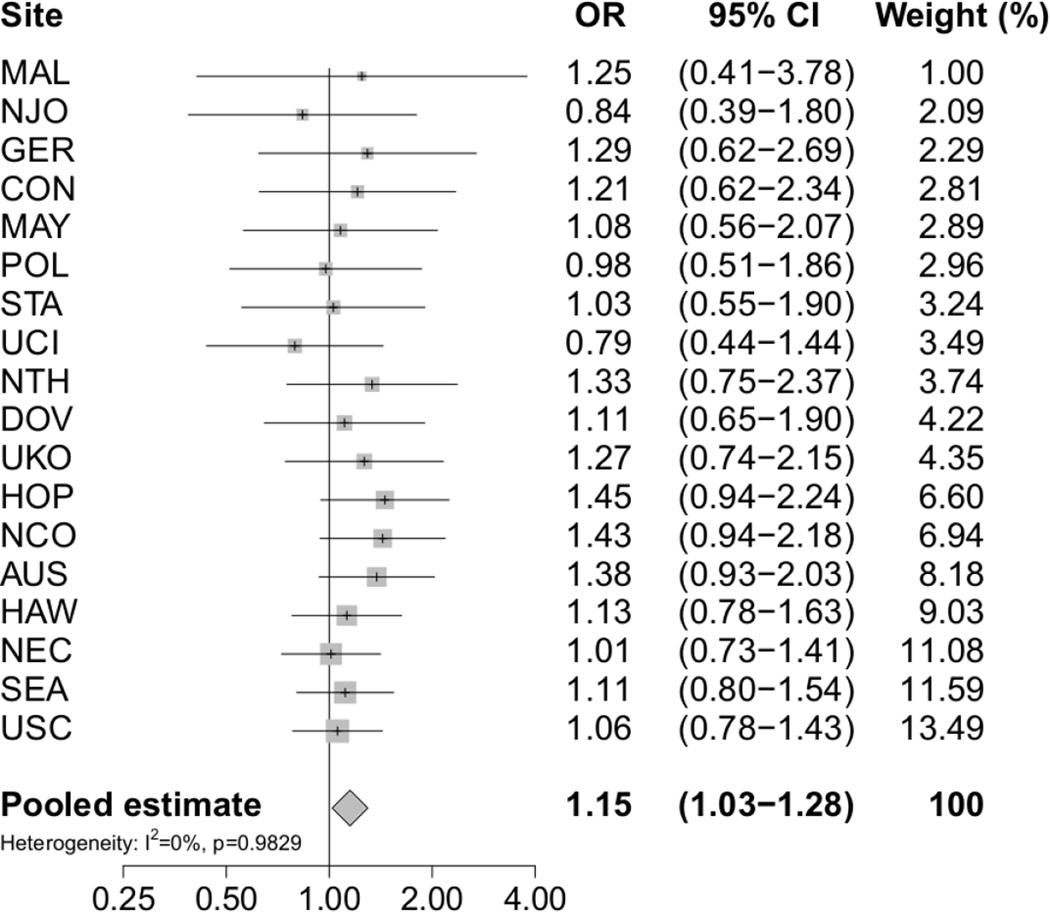
In Table 3 are presented the pooled odds ratios for advanced tumour stage at diagnosis of ovarian cancer overall and within histotypes according to level of education. The table shows pORs based on two analytic models: Model 1 includes adjustment for age and race/ethnicity and Model 2 includes additional adjustment for BMI and cigarette smoking status. Women who completed high school or less had an increased risk of advanced tumour stage at diagnosis of ovarian cancer (pOR 1.15; 95% CI 1.03–1.28) (Table 3, Model 1; Figure 1). The risk estimates for the various histotypes generally resembled that for ovarian cancer overall, though none of the risk estimates reached nominal statistical significance (Table 3, Model 1). Additional adjustments for BMI and cigarette smoking status made virtually no changes to the estimated associations between level of education and tumour stage at diagnosis of ovarian cancer (Table 3, Model 2). Heterogeneity was not evident for any of the analyses included in the present paper (All p-values > 0.4 and all I2 <5%).
Table 3.
Adjusted pooled odds ratios (pORs) and 95% confidence intervals (95% CI) for the association between level of education and advanced stage at diagnosis of ovarian cancer, overall and by histotype.
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| Cases (n = 10,601) | pOR (95% CI) | Cases (n = 10,457) | pOR (95% CI) | |
| Overall | ||||
| >high school | 5,411 | 1.00 | 5,362 | 1.00 |
| ≤high school | 5,190 | 1.15 (1.03–1.28) | 5,095 | 1.18 (1.05–1.32) |
| Serous | ||||
| >high school | 3,003 | 1.00 | 2,973 | 1.00 |
| ≤high school | 3,063 | 1.08 (0.87–1.34) | 3,009 | 1.13 (0.90–1.41) |
| Serous low-grade | ||||
| >high school | 143 | 1.00 | 142 | 1.00 |
| ≤high school | 228 | 1.10 (0.51–2.35) | 228 | 1.23 (0.49–3.12) |
| Serous high-grade | ||||
| >high school | 2,568 | 1.00 | 2,541 | 1.00 |
| ≤high school | 2,462 | 1.02 (0.78–1.32) | 2,413 | 1.09 (0.83–1.43) |
| Endometrioid | ||||
| >high school | 908 | 1.00 | 901 | 1.00 |
| ≤high school | 756 | 1.10 (0.86–1.42) | 749 | 1.17 (0.90–1.53) |
| Mucinous | ||||
| >high school | 349 | 1.00 | 349 | 1.00 |
| ≤high school | 271 | 0.97 (0.63–1.48) | 266 | 1.09 (0.68–1.76) |
| Clear cell | ||||
| >high school | 436 | 1.00 | 431 | 1.00 |
| ≤high school | 390 | 1.19 (0.84–1.71) | 376 | 1.21 (0.83–1.77) |
Adjusted for age (continuous variable) and race/ethnicity (Non-Hispanic White, Hispanic White, Black, Asian and other).
Adjusted for the two factors in Model 1 plus adjustment for BMI (continuous variable) and cigarette smoking status (never, former or current).
Fig. 1.
Risk of advanced tumour stage at diagnosis of ovarian cancer associated with level of education by study site and overall. Study-specific odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression models adjusted for age and race/ethnicity. The pooled odds ratio (pOR) with corresponding 95% CI was estimated using a random effects model. Level of education was parameterized as women who completed high school or less versus women who completed more than high school
Lastly, we performed sensitivity analyses to investigate whether the association between level of education and tumour stage at diagnosis of ovarian cancer overall differed according to study continent, race/ethnicity or study type. However, the direction and the magnitude of the associations were not markedly different from the associations obtained in the main analyses (Table 3). Further, the risk estimates did not differ statistically significantly between the US studies (pOR 1.11; 95% CI 0.97–1.28) and the non-US studies (pOR 1.22; 95% CI 1.01–1.47), between women of white race/ethnicity (pOR 1.15; 95% CI 1.02–1.29) and women of other races/ethnicities (pOR 1.13; 95% CI 0.84–1.51), as well as between population-based studies (pOR 1.14; 95% CI 1.01–1.28) and hospital-/clinic-based studies (pOR 1.28; 95% CI 0.88–1.73) (all p-values for pairwise comparisons >0.05).
4. Discussion
Tumour stage is the most important prognostic factor of survival in ovarian cancer. It is therefore important to identify factors that predict tumour stage at diagnosis. A potential candidate is socioeconomic status, which has been found to be associated with tumour stage at diagnosis for other gynaecological cancers [12–14]. The present large study evaluated the association between level of education and tumour stage at diagnosis of epithelial ovarian cancer. Our results showed that women who completed high school or less had a modest (15%) increased risk of advanced tumour stage at diagnosis of ovarian cancer compared with women who completed more than high school. Observed risk estimates for the histotypes resembled those for ovarian cancer overall.
Only a few studies have investigated SES differences in tumour stage at diagnosis of ovarian cancer. Our results are partly in line with results from a recent Danish cohort study. Ibfelt et al. [8], with data of 2873 women diagnosed with ovarian cancer, observed that women with medium level of education (10–12 years) had a 25% increased risk of advanced tumour stage at diagnosis of ovarian cancer compared with women with high level of education (>12 years). However, the authors found no association between risk of advanced tumour stage and short level of education (7–9 years). Other studies have found no convincing associations between various measures of SES and tumour stage at diagnosis of ovarian cancer [15–18]. For example, in the largest study to date using data from 16,228 American women with ovarian cancer, Morris et al. [18] found no association between a census-based measure of SES and tumour stage at diagnosis. An explanation for the divergent results may be that only our study and the study by Ibfelt et al. [8] used individual level measures of SES, whereas all other studies of this question have used various area-based/aggregate measures of SES as surrogates for individual SES. Area-based and aggregate measures of SES are known to be less precise (i.e., have higher risk of misclassification) than individual measures of SES and likely to bias relative risk toward the null in epidemiological studies [42]. No previous studies have examined whether or not the association between SES and tumour stage at diagnosis of ovarian cancer differs by histotype. We observed that the estimated risks for the histotypes of ovarian cancer resembled that for ovarian cancer overall. However, the numbers of cases for some of the histotypes were relatively small and additional confirmation would be warranted.
The observed association between educational level and tumour stage at diagnosis is likely to be explained by a complex interaction between several underlying factors, including patient access to regular health-care check-ups, patient awareness of cancer symptoms, adequate reaction to symptoms, access, barriers and quality of healthcare, time-period to referral to specialist care, lifestyle factors, and comorbidities. Cancer symptom awareness and interpretation of symptoms is poorer among those who are less educated and those with lower SES [43]. Though some ovarian cancers are asymptomatic, most women experience vague or non-specific symptoms, which are similar to those of other common illnesses [44]. Therefore, it is plausible that more highly educated women could be more aware of and able to recognize potential symptoms compared with less educated women and may therefore be more likely to seek medical care earlier, which would eventually lead to a diagnosis of ovarian cancer in an earlier tumour stage. However, compared with cancers that are generally screenable or present with clear clinical signs, the potential for socioeconomic status to have an influence on awareness of symptoms and health-care seeking in ovarian cancer are likely to be rather limited. Alternatively, women that are more educated might respond more promptly to their apparent signs or symptoms whereas less educated women may be more likely to ignore, discount or deny them until mounting discomfort becomes substantial in advanced tumour stage disease.
Regular visits to a primary care physician and the latency from date of visit at the general practitioner until referral to a gynaecologist are both factors that are potentially predictive for tumour stage at diagnosis of ovarian cancer. As low SES is associated with less frequent use of primary care [45] and likely increased time to referral to a specialist, these factors may combine to explain the observed association between educational level and tumour stage at diagnosis. In the present study, 11 of 18 individual studies were conducted in the USA, representing 65% of the women in our study population. In contrast to Europe and Australia, access to health care in the USA is not uniform and a larger proportion of well-educated American women are privately insured compared with less educated American women. It is plausible that women with private health insurance visit a primary care physician more regularly and are faster referred to a gynaecologist compared with women who are uninsured or covered by governmental insurance programs. Therefore, it would be reasonable to assume that the association between level of education and tumour stage at diagnosis of ovarian cancer would be more pronounced among the US studies than among the non-US studies. However, the results from our additional analysis stratified by study continent were not able to support this.
Finally, low SES is known to be associated with less unhealthy lifestyle, including factors such as poorer diet, less exercise, more cigarette smoking and higher BMI [46–48]. Both cigarette smoking and obesity accelerates carcinogenesis resulting in earlier progression and death, whereas obesity can blur ovarian cancer symptoms and delay diagnosis [49]. However, in the present study, BMI and cigarette smoking status had virtually no effect on the estimated associations between level of education and tumour stage at diagnosis. Therefore, BMI and cigarette smoking do not appear to have substantial influence on tumour stage difference by level of education.
A strength of the present study is the large number of ovarian cancer patients obtained by pooling data from 18 individual case-control studies. This collection strengthened the statistical power of the risk estimates and allowed us to examine associations both overall and separately for the various histotypes of ovarian cancer. In addition, the majority of the studies were population-based designs with information on education obtained from in-person interviews. The participating studies were not selected from among published studies. Therefore, our analyses included both positive and negative study-specific results, limiting the possibility of publication bias. The present analyses relied on individual data combined into a single dataset following careful central data harmonization. We considered differences in study design and data collection across studies and adjusted for relevant confounding factors across studies. However, we could not adjust for comorbidity, as this information was not available in our data. The degree of comorbidity is known to be inversely correlated with SES [50] and comorbidity may blur symptoms of cancer and may reduce individual resources when it comes to health care seeking. Hence, even though a recent cohort study showed that comorbidity only had a small impact on the differences in ovarian cancer stage and survival by SES [8], we cannot rule out that our results may have been slightly affected by unmeasured confounding from comorbidity. Furthermore, information on tumour stage was abstracted from hospital records or cancer registries and the majority of study sites performed pathology review in order to confirm histotype classifications. Nevertheless, not all ovarian tumours underwent systematic histopathologic review and therefore some degree of misclassification of subtype could have occurred. An additional potential limitation of the present study is that we only included one measure of SES - level of education - as only a limited number of OCAC studies obtained information on other measures such as income or marriage/cohabitation status. Even though a single measure of SES may show an association with the health outcome analysed, it may not encompass the entirety of the effect of SES on health, and inclusion of multiple measures of SES are always preferable [51]. Hence, by including only one measure of SES, we may only partly have explained the true association between SES and stage at diagnosis of ovarian cancer. However, level of education is considered to be a good and valid measure of SES with regard to health because it influences an individuals’ SES throughout life and it is highly associated with both income and occupation [6; 52]. Further, knowledge and skills obtained through education may affect cognitive functions and thereby strengthen the individuals’ comprehension of health messages and communication with health authorities [51]. Finally and perhaps most importantly, for most individuals, level of education does not change substantially throughout life compared with other measures of SES, including income and occupation, and can therefore be considered to be a robust measure of SES [51].
5. Conclusions
This large pooled analysis showed that lower educational level was associated with advanced tumour stage at diagnosis. BMI and cigarette smoking did not explain the association. Hence, in order to reduce diagnostic delays, it is important to identify which underlying factors (e.g., patient awareness of and response to cancer symptoms, access to healthcare and latency of referral to specialist care, lifestyle factors and comorbidities) that contribute to socioeconomic differences in tumour stage at diagnosis of ovarian cancer.
Acknowledgments
This work was supported by the European Commission’s Seventh Framework Programme grant agreement no. 223175 (HEALTH-F2-2009-223175). The work was also supported by the National Institutes of Health (R01 CA074850 and R01 CA080742 (CON), R01 CA112523 and R01 CA87538 (DOV), R01 CA58598, N01 CN55424 and N01 PC 67001 (HAW), MO1 – RR000056 (HOP), R01 CA61107 (MAL), R01 CA122443, P30 CA15083 and P50 CA136393 (MAY), R01 CA76016 (NCO), R01 CA54419 and P50 CA105009 (NEC), P30 CA072720, P30 CA008748, K07 CA095666, R01 CA83918 and K22 CA138563 (NJO), U01 CA71966, R01 CA16056, K07 CA143047 and U01 CA69417 (STA), R01 CA058860, R01 CA092044 and PSA 042205 (UCI), P30 CA14089, R01 CA61132 and N01 PC67010 (USC)); Danish Cancer Society (94 222 52 (MAL)); Mermaid 1 (MAL); U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729) (AUS), National Health & Medical Research Council of Australia (199600 and 400281) (AUS); Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania (AUS); Cancer Foundation of Western Australia (AUS); German Federal Ministry of Education and Research, Program of Clinical Biomedical Research (01GB9401 (GER)); German Cancer Research Center (GER); US Army Medical Research and Material Command (DAMD17-02-1-0669 (HOP), DAMD17-02-1-0666 (NCO) and W81XWH-10-1-02802 (NEC)); Mayo Foundation (MAY); Minnesota Ovarian Cancer Alliance (MAY); Fred C. and Katherine B. Andersen Foundation (MAY); The Cancer Institute of New Jersey (NJO); Radboud University Nijmegen Medical Centre (NTH); Intramural Research Program of the National Cancer Institute (POL); Cancer Research UK (C490/A10119 and C490/A10124 (SEA)); Lon V Smith Foundation (LVS-39420 (UCI)); Cancer Research UK (UKO, SEA); Eve Appeal (UKO); OAK Foundation (UKO); California Cancer Research Program (00-01389V-20170, N01 CN025403, R03 CA113148, R03 CA115195 (USC)); California Cancer Research Program (2II0200 (USC)); National Institute of Environmental Health Sciences T32ES013678 (USC) and US National Cancer Institute (P01 CA17054 (USC) and K07-CA80668 and P50-CA159981 (HOP)). The New Jersey State Cancer Registry is funded by the Center for Disease Control (5U58DP003931-02) and The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (HHSN 261201300021I NCI Control No. N01PC-2013-00021). A portion of this was done at UCLH/UCL within the ’Women’s Health Theme’ of the NIHR UCLH/UCL Comprehensive Biomedical Research Centre supported by the Department of Health (UKO). The German group thanks Ursula Eilber and Tanja Koehler for competent technical assistance (GER). The Australian group thanks all the clinical and scientific collaborators (http://www.aocstudy.org/) and the women for their contribution (AUS). The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged (CON). Certain data in the CON study were obtained from the Connecticut Tumor Registry, Connecticut Department of Public Health. The CON study assumes full responsibility for analyses and interpretation of these data. The MALOVA study is grateful to Nick Martinussen for data management assistance (MAL). The NJO group thanks the New Jersey State Cancer Registry staff, M. King and L. Rodriguez. The SEARCH group thanks the SEARCH team, Craig Luccarini, Caroline Baynes and Don Conroy. The UKOPS group thanks I. Jacobs, M. Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study (UKO).
Role of the funding sources:
The funding sources were not involved in the study design; collection, analysis or interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Abbreviations
- 95% CI
95% confidence interval
- BMI
Body mass index
- OCAC
Ovarian Cancer Association Consortium
- OR
odds ratio
- pOR
pooled odds ratio
- SES
socioeconomic status
Footnotes
Conflicts of interest: None
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. Bethesda, MD: National Cancer Institute; [Accessed on November 22]. SEER Cancer Statistics Review, 1975–2012. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 3.Kogevinas M, Porta M. Socioeconomic differences in cancer survival: a review of the evidence. IARC Sci Publ. 1997;138:177–206. [PubMed] [Google Scholar]
- 4.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 5.Jensen KE, Hannibal CG, Nielsen A, Jensen A, Nohr B, Munk C, Kjaer SK. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44:2003–2017. doi: 10.1016/j.ejca.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Braaten T, Weiderpass E, Lund E. Socioeconomic differences in cancer survival: the Norwegian Women and Cancer Study. BMC Public Health. 2009;9:178. doi: 10.1186/1471-2458-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anuradha S, Webb PM, Blomfield P, Brand AH, Friedlander M, Leung Y, Obermair A, Oehler MK, Quinn M, Steer C, Jordan SJ. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust. 2014;201:283–288. doi: 10.5694/mja14.00132. [DOI] [PubMed] [Google Scholar]
- 8.Ibfelt EH, Dalton SO, Hogdall C, Fago-Olsen CL, Steding-Jessen M, Osler M, Johansen C, Frederiksen K, Kjær SK. Do stage of disease, comorbidity or access to treatment explain socioeconomic differences in survival after ovarian cancer? - A cohort study among Danish women diagnosed 2005–2010. Cancer Epidemiol. 2015;39:353–359. doi: 10.1016/j.canep.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, Mutch DG, Cliby WA. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105:823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiderpass E, Oh JK, Algeri S, Bellocco R. Socioeconomic status and epithelial ovarian cancer survival in Sweden. Cancer Causes Control. 2014;25:1063–1073. doi: 10.1007/s10552-014-0407-1. [DOI] [PubMed] [Google Scholar]
- 11.O'Malley CD, Cress RD, Campleman SL, Leiserowitz GS. Survival of Californian women with epithelial ovarian cancer, 1994–1996: a population-based study. Gynecol Oncol. 2003;91:608–615. doi: 10.1016/j.ygyno.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, Goodman MT, Lynch CF, Schwartz SM, Chen VW, Bernstein L, Gomez SL, Graff JJ, Lin CC, Johnson NJ, Edwards BK. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–2111. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibfelt EH, Kjaer SK, Hogdall C, Steding-Jessen M, Kjaer TK, Osler M, Johansen C, Frederiksen K, Dalton SO. Socioeconomic position and survival after cervical cancer: influence of cancer stage, comorbidity and smoking among Danish women diagnosed between 2005 and 2010. Br J Cancer. 2013;109:2489–2495. doi: 10.1038/bjc.2013.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewster DH, Thomson CS, Hole DJ, Black RJ, Stroner PL, Gillis CR. Relation between socioeconomic status and tumour stage in patients with breast, colorectal, ovarian, and lung cancer: results from four national, population based studies. BMJ. 2001;322:830–831. doi: 10.1136/bmj.322.7290.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risser DR, Miller EA. Cancer in relation to socioeconomic status: stage at diagnosis in Texas, 2004–2008. South Med J. 2012;105:508–512. doi: 10.1097/SMJ.0b013e318268c752. [DOI] [PubMed] [Google Scholar]
- 17.Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, Greenberg DC. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. 2013;24:843–850. doi: 10.1093/annonc/mds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris CR, Sands MT, Smith LH. Ovarian cancer: predictors of early-stage diagnosis. Cancer Causes Control. 2010;21:1203–1211. doi: 10.1007/s10552-010-9547-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramus SJ, Vierkant RA, Johnatty SE, Pike MC, Van Den Berg DJ, Wu AH, Pearce CL, Menon U, Gentry-Maharaj A, Gayther SA, Dicioccio RA, McGuire V, Whittemore AS, Song H, Easton DF, Pharoah PD, Garcia-Glosas M, Chanock S, Lissowska J, Brinton L, Terry KL, Cramer DW, Tworoger SS, Hankinson SE, Berchuck A, Moorman PG, Schildkraut JM, Cunningham JM, Liebow M, Kjaer SK, Hogdall E, Hogdall C, Blaakaer J, Ness RB, Moysich KB, Edwards RP, Carney ME, Lurie G, Goodman MT, Wang-Gohrke S, Kropp S, Chang-Claude J, Australian Ovarian Cancer Study Group; Australian Cancer Study (Ovarian Cancer) Webb PM, Chen X, Beesley J, Chevenix-Trench G, Goode EL Ovarian Cancer Association Consortium. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123:380–388. doi: 10.1002/ijc.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 21.Risch HA, Bale AE, Beck PA, Zheng W. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1738–1741. doi: 10.1158/1055-9965.EPI-06-0272. [DOI] [PubMed] [Google Scholar]
- 22.Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23:1985–1994. doi: 10.1007/s10552-012-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95:370–374. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15:1055–1060. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, Brinton LA, Webb PM, Nagle CM, Jordan SJ, Australian Cancer Study Group, Australian Cancer Study (Ovarian Cancer) Risch HA, Rossing MA, Doherty JA, Goodman MT, Lurie G, Kjær SK, Hogdall E, Jensen A, Cramer DW, Terry KL, Vitonis A, Bandera EV, Olson S, King MG, Chandran U, Anton-Culver H, Ziogas A, Menon U, Gayther SA, Ramus SJ, Gentry-Maharaj A, Wu AH, Pearce CL, Pike MC, Berchuck A, Schildkraut JM, Wentzensen N Ovarian Cancer Association Consortium. Aspirin Nonaspirin Nonsteroidal Anti-inflammatory Drug, and Acetaminophen Use and Risk of Invasive Epithelial Ovarian Cancer: A Pooled Analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelemen LE, Sellers TA, Schildkraut JM, Cunningham JM, Vierkant RA, Pankratz VS, Fredericksen ZS, Gadre MK, Rider DN, Liebow M, Goode EL. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68:2498–2506. doi: 10.1158/0008-5472.CAN-07-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, Bock JE, Blaakaer J. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164:2253–2259. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 28.Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65:5974–5981. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schildkraut JM, Iversen ES, Wilson MA, Clyde MA, Moorman PG, Palmieri RT, Whitaker R, Bentley RC, Marks JR, Berchuck A. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, Widschwendter M, Vierkant RA, Larson MC, Kjaer SK, Birrer MJ, Berchuck A, Schildkraut J, Tomlinson I, Kiemeney LA, Cook LS, Gronwald J, Garcia-Closas M, Gore ME, Campbell I, Whittemore AS, Sutphen R, Phelan C, Anton-Culver H, Pearce CL, Lambrechts D, Rossing MA, Chang-Claude J, Moysich KB, Goodman MT, Dörk T, Nevanlinna H, Ness RB, Rafnar T, Hogdall C, Hogdall E, Fridley BL, Cunningham JM, Sieh W, McGuire V, Godwin AK, Cramer DW, Hernandez D, Levine D, Lu K, Iversen ES, Palmieri RT, Houlston R, van Altena AM, Aben KK, Massuger LF, Brooks-Wilson A, Kelemen LE, Le ND, Jakubowska A, Lubinski J, Medrek K, Stafford A, Easton DF, Tyrer J, Bolton KL, Harrington P, Eccles D, Chen A, Molina AN, Davila BN, Arango H, Tsai YY, Chen Z, Risch HA, McLaughlin J, Narod SA, Ziogas A, Brewster W, Gentry-Maharaj A, Menon U, Wu AH, Stram DO, Pike MC, Wellcome Trust Case-Control Consortium. Beesley J, Webb PM, Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer Study Group; Ovarian Cancer Association Consortium (OCAC) Chen X, Ekici AB, Thiel FC, Beckmann MW, Yang H, Wentzensen N, Lissowska J, Fasching PA, Despierre E, Amant F, Vergote I, Doherty J, Hein R, Wang-Gohrke S, Lurie G, Carney ME, Thompson PJ, Runnebaum I, Hillemanns P, Dürst M, Antonenkova N, Bogdanova N, Leminen A, Butzow R, Heikkinen T, Stefansson K, Sulem P, Besenbacher S, Sellers TA, Gayther SA, Pharoah PD Ovarian Cancer Association Consortium (OCAC) A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42:874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Closas M, Brinton LA, Lissowska J, Richesson D, Sherman ME, Szeszenia-Dabrowska N, Peplonska B, Welch R, Yeager M, Zatonski W, Chanock SJ. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC Cancer. 2007;7:60. doi: 10.1186/1471-2407-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song H, Ramus SJ, Quaye L, Dicioccio RA, Tyrer J, Lomas E, Shadforth D, Hogdall E, Hogdall C, McGuire V, Whittemore AS, Easton DF, Ponder BA, Kjaer SK, Pharoah PD, Gayther SA. Common variants in mismatch repair genes and risk of invasive ovarian cancer. Carcinogenesis. 2006;27:2235–2242. doi: 10.1093/carcin/bgl089. [DOI] [PubMed] [Google Scholar]
- 34.McGuire V, Felberg A, Mills M, Ostrow KL, Dicioccio R, John EM, West DW, Whittemore A. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160:613–618. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 35.Ziogas A, Gildea M, Cohen P, Bringman D, Taylor TH, Seminara D, Barker D, Casey G, Haile R, Liao SY, Thomas D, Noble B, Kurosaki T, Anton-Culver H. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:103–111. [PubMed] [Google Scholar]
- 36.Balogun N, Gentry-Maharaj A, Wozniak EL, Lim A, Ryan A, Ramus SJ, Ford J, Burnell M, Widschwendter M, Gessler SF, Gayther SA, Jacobs IJ, Menon U. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. J Clin Epidemiol. 2011;64:525–530. doi: 10.1016/j.jclinepi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124:1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 39.Adamo MB, Johnson CH, Ruhl JL, Dickie L, editors. 2013 SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute, NIH Publication number 13-5581; 2015. [Google Scholar]
- 40.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20:2115–2130. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer. 2010;116:4160–4167. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 43.Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101:S92–S101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb PM, Purdie DM, Grover S, Jordan S, Dick ML, Green AC. Symptoms and diagnosis of borderline, early and advanced epithelial ovarian cancer. Gynecol Oncol. 2004;92:232–239. doi: 10.1016/j.ygyno.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Dunlop S, Coyte PC, McIsaac W. Socio-economic status and the utilisation of physicians' services: results from the Canadian National Population Health Survey. Soc Sci Med. 2000;51:123–133. doi: 10.1016/s0277-9536(99)00424-4. [DOI] [PubMed] [Google Scholar]
- 46.Gustafsson PE, Persson M, Hammarstrom A. Socio-economic disadvantage and body mass over the life course in women and men: results from the Northern Swedish Cohort. Eur J Public Health. 2012;22:322–327. doi: 10.1093/eurpub/ckr061. [DOI] [PubMed] [Google Scholar]
- 47.Gray L, Leyland AH. Is the "Glasgow effect" of cigarette smoking explained by socio-economic status?: a multilevel analysis. BMC Public Health. 2009;9:245. doi: 10.1186/1471-2458-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am J Clin Nutr. 2006;83:139–145. doi: 10.1093/ajcn/83.1.139. [DOI] [PubMed] [Google Scholar]
- 49.Kjaerbye-Thygesen A, Frederiksen K, Hogdall EV, Glud E, Christensen L, Hogdall CK, Blaakaer J, Kjaer SK. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:798–803. doi: 10.1158/1055-9965.EPI-05-0897. [DOI] [PubMed] [Google Scholar]
- 50.Louwman WJ, Aarts MJ, Houterman S, van Lenthe FJ, Coebergh JW, Janssen-Heijnen ML. A 5090025; higher prevalence of life-shortening chronic conditions among cancer patients with low socioeconomic status. Br J Cancer. 2010;103:1742–1748. doi: 10.1038/sj.bjc.6605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2006;60:7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geyer S, Hemstrom O, Peter R, Vagero D. Education, income, and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against a common practice. J Epidemiol Community Health. 2006;60:804–810. doi: 10.1136/jech.2005.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]