Abstract
Acinetobacter johnsonii rarely causes human infections. While most A. johnsonii isolates are susceptible to virtually all antibiotics, strains harboring a variety of β-lactamases have recently been described. An A. johnsonii Aj2199 clinical strain recovered from a hospital in Buenos Aires produces PER-2 and OXA-58. We decided to delve into its genome by obtaining the whole genome sequence of the Aj2199 strain. Genome comparison studies on Aj2199 revealed 240 unique genes and a close relation to strain WJ10621, isolated from the urine of a patient in China. Genomic analysis showed evidence of horizontal genetic transfer (HGT) events. Forty-five insertion sequences and two intact prophages were found in addition to several resistance determinants such as blaPER-2, blaOXA-58, blaTEM-1, strA, strB, ereA, sul1, aacC2 and a new variant of blaOXA-211, called blaOXA-498. In particular, blaPER-2 and blaTEM-1 are present within the typical contexts previously described in the Enterobacteriaceae family. These results suggest that A. johnsonii actively acquires exogenous DNA from other bacterial species and concomitantly becomes a reservoir of resistance genes.
Introduction
Human infections caused by non- A. baumannii members of the Acinetobacter genus have been recently recognized due to the implementation of new technologies in clinical diagnostic laboratories [1–6]. Forty-seven distinctive Acinetobacter species with valid names have been described and more species are identified each year [7, 8]. Acinetobacter johnsonii is usually found in the environment and animals [9], it can occasionally colonize human skin [10] and cause clinical infections such as catheter-related bloodstream infections [11] or peritonitis associated with peritoneal dialysis [12].
Although multidrug resistance has not been widespread among non-baumannii Acinetobacter species [13], A. johnsonii strains harboring a variety of β-lactamase genes are being isolated at different geographic locations [12–19]. One such isolate, recently described by Rodriguez et al., is strain (Aj2199) which produces PER-2 and OXA-58 [12]. These findings strongly suggest that A. johnsonii can acquire exogenous DNA as these genes were previously described in other bacterial genus and species. The detection of β-lactamase genes and other resistance determinants among Acinetobacter species demonstrates their potential to acquire and stably maintain resistance determinants.
In this study, we determined the complete genome sequence of the Aj2199 strain. The presence of a variety of traits like antibiotic resistance genes, insertion sequences, phage sequences, and numerous unique genes are indicative of active horizontal genetic transfer (HGT).
Materials and Methods
Whole-genome sequence of Aj2199 clinical strain
Genomic DNA was extracted using a MasterPure DNA Purification kit from Epicentre Biotechnologies. Whole-genome shotgun sequencing was performed using Illumina MiSeq- I, with Nextera XT libraries for sample preparation. De novo assembly was performed with SPADES assembler version 3.1.0 [14], using a pre-assembly approach with Velvet [15]. RAST server was used to predict open reading frames [16] and BLAST (version 2.0) software was utilized to confirm the predictions. tRNAscan-SE was used to predict tRNA genes [17]. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LVIB00000000. The version described in this paper is version LVIB01000000.
Genome Sequences for comparative genomics
All available assemblies of A. johnsonii strains were downloaded from NCBI via ftp at ftp.ncbi.nlm.nih.gov/genomes/ASSEMBLY_BACTERIA. Other complete genomes, draft genomes and assemblies were also downloaded from NCBI via ftp and used for comparative studies. Up to three randomly selected genomes for each species were included in the analysis in order to avoid over-representation of some species. Reference genomes of the genus: A. baumannii AYE and A. baumannii ACICU were also analyzed (See S1 Table for the complete list of the 97 genomes sequences used in this work). Assemblies were annotated by means of RAST server [16] and the SEED source for genome annotations [18] with default parameters. GO ontology annotation was performed by means of HMM profile searches against bacterial EggNog 4.5 database [19] using HMMER 3.1 software, available at http://hmmer.org/.
Clustering of homologous genes and phylogenetic analysis
Identification of homologous genes among the analyzed genomes was carried out using the OrthoMCL method [20] by means of the get homologous software [21]. Blastp searches were done with a minimum e-value of 1.10−5, a minimum identity value of 30% and minimum query coverage of 75%. 301 groups of putative orthologous sequences were identified among genomes of the family Moraxellaceae (Class: Gammaproteobacteria, Order: Pseudomonadales). Protein sequences alignments were done using Clustal Omega v1.2.0 [22]. Phylogenies were inferred using the maximum-likelihood method with an amino acid LG+G (8 categories) model by means of Phyml version 3.1 [23] with 5 random starting trees. The default SH-like test was used to evaluate branch supports in each analysis [24]. Finally, a consensus tree was inferred from the 301 trees using the sumtrees.py script [25]. In the consensus tree, node support represents the number of phylogenies in which certain node appeared.
Average nucleotide identity
Average nucleotide identity (ANI) between A. johnsonii Aj2199 and other closely related genomes (all A. johnsonii strains plus A. lwoffii WJ10621) was estimated. The ANI was used to delineate species using genome sequence data [26], two genomes displaying an ANI value equal or higher than 95% belong to the same species. Two-way ANI (reciprocal best hits based comparison) was estimated by means of the ani.rb script developed by Luis M. Rodriguez-R and available at enveomics.blogspot.com.
Genomic comparison, gene content and sequences analysis
Sequence analysis was carried out using BLAST (version 2.0) software (http://www.ncbi.nlm.nih.gov/BLAST/).
ARG-ANNOT and ISfinder softwares were used to identify antibiotic resistance genes and insertion sequences within the genome of Aj2199, respectively [27, 28]. Furthermore, phages and prophages prediction was done using PHAST (PHAge Search Tool) bioinformatic tool [29]. PlasmidFinder was used to detect the presence of Enterobacteriacea plasmids[30]. plasmidSPAdes software, which distinguishes plasmid sequences via the read coverage of contigs, was also used [31].
Prediction of small non-coding RNAs was done using RNA families from the Rfam database and the software Infernal [32].
Other bacterial strains
Four A. johnsonii strains (Aj205, Aj286, Aj289, Aj306) from the bacterial collection of the Hospital de Clínicas José de San Martín were included in the present study (Table 1). The four strains were identified as A. johnsonii by MALDI-TOF MS (Bruker Daltonik), rpoB amplification, and sequencing.
Table 1. Other Acinetobacter johnsonii strains included in this study.
| MIC (mg/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Year | Sample | AMP | SAM | FOX | CTX | CAZ | IMP | MEM | AMK | GEN | CIP | COL | CEF |
| Aj306 | 2013 | Urine | ≤2 | ≤2 | ≤4 | ≤1 | ≤1 | ≤1 | ≤0.25 | ≤2 | ≤1 | ≤0.25 | 2 | ≤1 |
| Aj289 | 2013 | BAL | ≤2 | ≤2 | ≥ 64 | 4 | 4 | ≤1 | ≤0.25 | ≤2 | ≤1 | 0.5 | ≤0.5 | 4 |
| Aj286 | 2013 | Bone | ≥ 32 | ≥ 32 | ≥ 64 | 4 | 2 | ≤1 | ≤0.25 | ≤2 | ≤1 | 0.5 | ≤0.5 | 4 |
| Aj205 | 2013 | Urine | ≤2 | ≤2 | ND | ≤1 | ≤1 | ≤1 | ≤0.25 | ≤2 | ≤1 | ND | ≤0.5 | ≤1 |
BAL, bronchoalveolar lavage; AMP, ampicilin; SAM, ampicilin/sulbactam; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; IPM, imipenem; MEM, meropenem; AMK, amikacin; GEN, gentamicin; CIP, ciprofloxacin; COL, colistin; CEF, cefepime; ND, no determinated.
General molecular techniques
Total DNA extraction was carried out using Wizard® Genomic DNA Purification Kit according to manufacturer instructions (Promega, Madison, WI). PCR reactions using the extracted DNA as template were carried out to determine the presence of resistance determinants (blaPER-2, blaOXA-58, blaTEM-1, strA, strB, ereA, sul1, aacC2) previously identified in Aj2199. The reactions were performed using the GoTaq enzyme according to manufacturer’s instructions (Promega, Madison, USA). Plasmid extraction and repAcil gene amplification was carried out to search for the presence of the replicase gene of plasmids harboring blaOXA-58 [33]. Plasmid DNA extraction was performed using the alkaline lysis method as described [34] with or without further purification using the QIAfilter Midi prep Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s recommendations.
Conjugation assays were carried out to determine if blaTEM-1 and blaPER-2 are plasmid located. Briefly, Aj2199 and E. coli J53-2 cells grown with agitation in Luria Bertani (LB) broth were mixed (1:10 donor:recipient) and incubated for 18 h at 30°C. Transconjugants were selected on LB agar supplemented with sodium azide (100 μg/ml) and ampicillin (100 μg/ml). Transformation assays were carried out using as hosts competent E. coli TOP10 or the naturally competent A. baumannii A118 [35]. After transformation E. coli cells were plated on LB-agar containing ampicillin (100 μg/ml) or ceftazidime (4μg/ml). Transformation of A. baumannii A118 was carried out as previously described [35]. Briefly, fresh LB was inoculated with cells at late stationary phase followed by addition of 200 ng plasmid DNA and incubated for 1 hour at 37°C. Cells were then plated on LB-agar containing ceftazidime (4μg/ml). Electroporation was performed using the A. baumannii strains A118 and ATCC 17978, and A. baylyi ADP1 as described before [36]. Cells were plated on LB-agar containing ceftazidime (4μg/ml).
Results
Aj2199 genome sequence, features and comparative genomic analysis
The whole genome sequence of Aj2199 has a total of 1,167,708 high quality paired-end reads with an average insertion size of 514 bp [15]. 99.9% of the generated reads were mapped, resulting in mean nucleotide coverage of 65X (and a k-mer coverage of 29.9). Corrected reads show an average length of 251 bp.155 contigs larger than 500 bp were assembled, comprising 3,803,969 bp. The draft genome has a N50 contig size of 70,198 bp with a maximum contig length of 286,583 bp and a G+C content of 41.4%. A total of 3,737 open reading frames were predicted using the RAST server [16]. Using tRNAscan-SE, a total of 114 tRNA genes were identified [17] (Table 2).
Table 2. General features of the A. johnsonnii Aj2199 genome.
| Features | Chromosome |
|---|---|
| Total number of base pairs | 3,803,969 |
| G+C content (%) | 41.4% |
| Number of possible ORFs | 3,737 |
| Number of tRNA genes | 114 |
| Number of Insertion Sequences (ISs) | 45 |
| Number of prophages | 4 |
Prophages include partial and complete sequences.
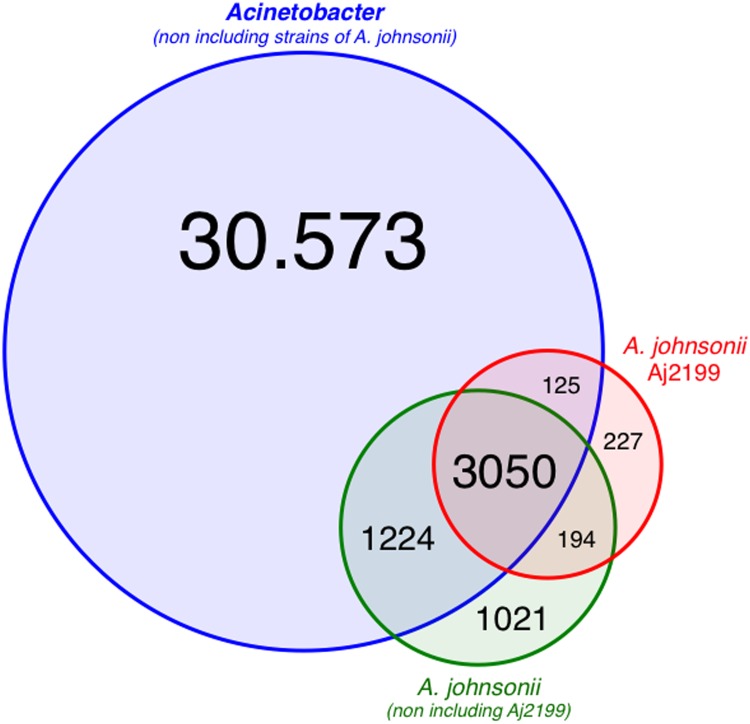
We performed a genome comparison between Aj2199 and 79 Acinetobacter genomes available in the GenBank and identified 36,414 homologous gene families. The data could be interpreted as a rough estimation of the pangenome of Acinetobacter. Minimum values of 30% amino acid identity and 75% coverage was set for grouping genes in homologous families. Among the analyzed genomes, a total of 1,442 gene families were predicted as unique to A. johnsonii strains (S2 Table), including Aj2199. Homologous identification suggests that more than 63% of the gene families exclusively found in A. johnsonii are strain-specific, which means that they are only found in one genome. On the other hand, only nine gene families are conserved among the strains and not found in any other analyzed Acinetobacter genome. These nine gene families may represent a molecular signature of the species (S2 Table). There are 227 gene families comprised of 240 genes distributed within 84 contigs, that are exclusively found in strain Aj2199 (Fig 1). 161 genes were annotated as hypothetical proteins and 24 as mobile elements. Among the other 55 unique genes in Aj2199 we found genes coding for antibiotic resistance determinants, DNA binding and integration proteins, integral component of membrane, transferases, and phage proteins (S2 Table). Some genes coding for proteins related to ion transport and regulation were found in this genome. Interestingly, genes coding for resistance to toxic metal ions were also found. Finally an aquaporin protein coding gene (homologous to aqpZ), two pilus biogenesis proteins (PilO-M) and two glutathione S-transferases (EC: 2.5.1.18), among others were also present (S2 Table).
Fig 1. Comparative analysis of the gene families found within the 79 Acinetobacter genomes.
Venn diagram representing: i) shown in blue is the number of gene families found in the pangenome of Acinetobacter, except A johnsonii strains, ii) green corresponds to the pangenome of A. johnsonii, except Aj2199 strain, and iii) in red all gene families found in Aj2199 genome are shown.
Assembled contigs, comprising 246.615bp, were predicted as putative plasmid sequences by plasmidSPAdes software [31]. These sequences were re-annotated resulting in 225 coding sequences. All of them were already annotated as part of the Aj2199 genome. Among them we found seven genes that are part of toxin-antitoxin systems, six genes part of restriction modification systems, ParA and ParB partitioning proteins, at least 20 metal resistance genes and four cation transporters (S3 Table). Note that any of the antibiotic resistance genes described in this work were predicted to be located in plasmids according to the plasmidSPAdes prediction. The role and functional implications of these genes will be further investigated in the future.
Further analysis of the genomes revealed the presence of 301orthologs among 98 complete selected genomes of the Moraxellaceae family, 97 from the NCBI database plus Aj2199 (S1 Table) that were used for phylogenetic analysis. The 301 orthologous sets were aligned and then a Maximum Likelihood phylogenetic tree was built for each alignment. The 301 phylograms, considering nodes with significant statistical support (SH-like test > 50%), were merged into a consensus tree shown in Fig 2. The resulting phylogeny clustered Aj2199 with A. johnsonii strains with 82% node support, which indicates that the node appears in 82% of the total phylograms. We found that the WJ10621 strain, reclassified as A. johnsonii considering the whole genome comparison, is the closest strain to Aj2199. The two-way average nucleotide identity (ANI) among closely related strains strongly supported the phylogenetic results and the 16S rDNA sequence analysis. The ANI between Aj2199, WJ10621 and the other A. johnsonii strains was above 96% (S4 Table). In summary, the estimated two-way ANI values suggest that all the A. johnsonii genomes grouped as a monophyletic cluster belong to the same species, which is comprised of SH046, ANC_3681, TG19625, CIP64.6, TG19605, DSM_6963, MB44, XBB1, Aj2199 and also WJ10621.
Fig 2. Consensus phylogenetic tree obtained for 98 complete genomes of the Moraxellaceae family plus Aj2199 strain.
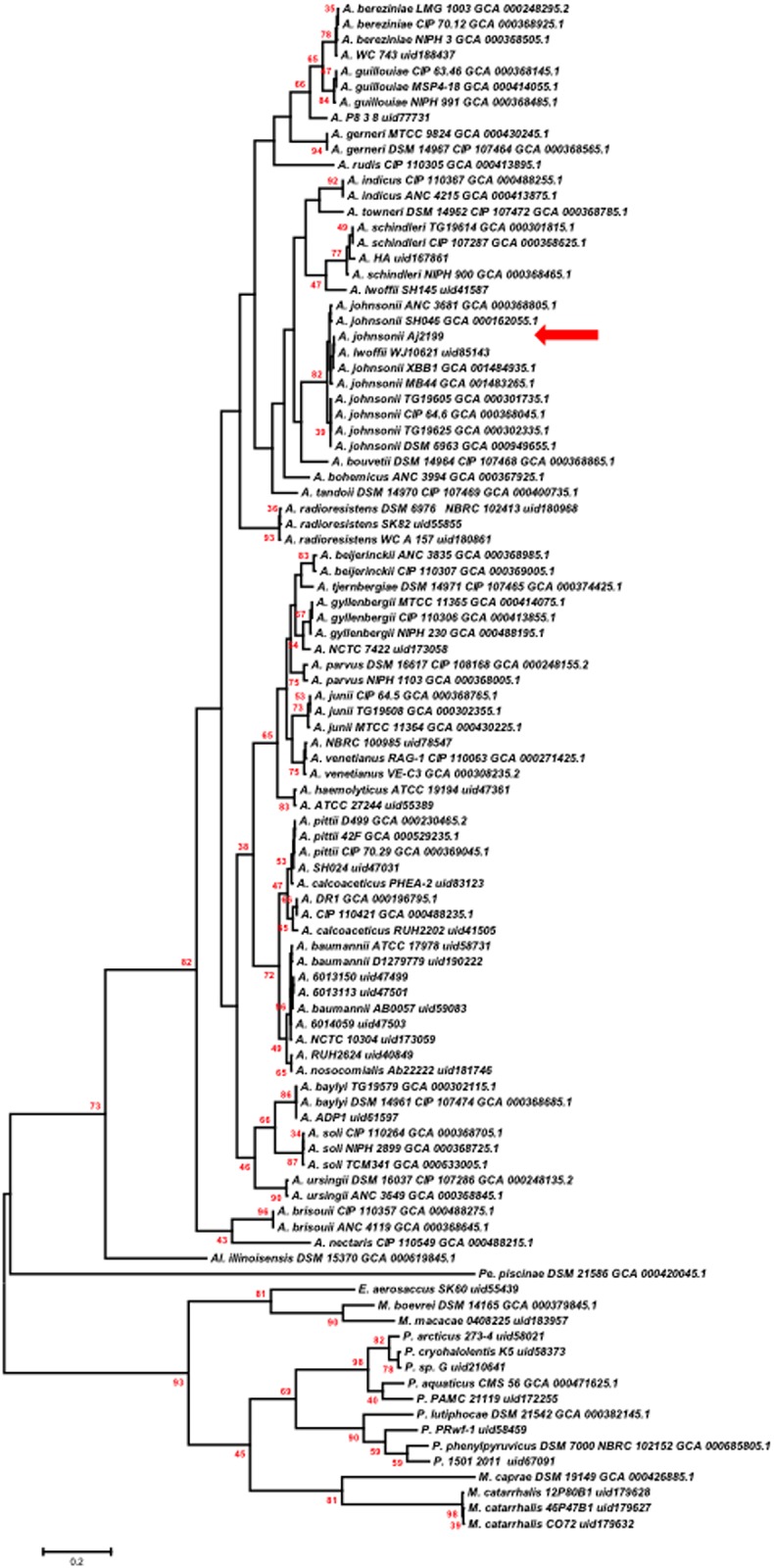
Phylogenetic trees were inferred for 301orthologous protein sequences using the maximum-likelihood method with an amino acid LG+G model. The default SH-like test was used to evaluate branch supports in each case. The consensus tree was inferred from the 301 phylograms, considering only nodes equal or above 50%. Note that node values represent the number of orthologous protein analysis that support a certain group and that node support values bellow 33% are not shown in the Fig The tree was arbitrarily rooted using the mid-point rooting method. A red arrow indicates the position of Aj2199 strain in the tree. In the phylogeny A. indicates Acientobacter, M. indicates Moraxella, E. indicates Enhydrobacter, P. indicates Psychrobacter, Al. indicates Alkanindiges and Pe. Indicates Perlucidibaca genus.
Genetic context and description of a new variant of blaOXA-211 in A. johnsonii
As was previously reported by Perichon et al 2014, OXA-like genes were found frequently in Acinetobacter species. In A. johnsonnii three variant of blaoxa-211, so called blaoxa-211, blaoxa-280 and blaoxa-281, were described [37].
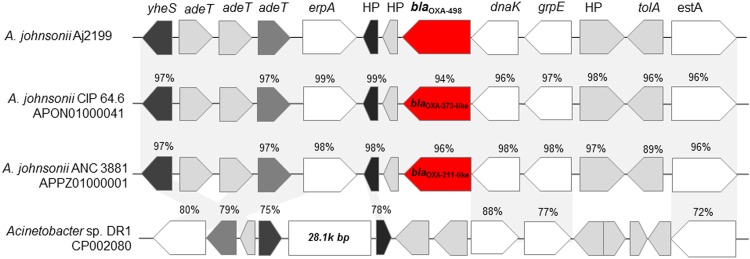
The genome of Aj2199 contains a new variant of the chromosomally encoded oxacillinase OXA-211 possessing 10 amino acid substitutions compared to the formerly defined OXA-211 (GenBank accession number AEV91550). This blaOXA, now called blaOXA-498, has a 95% nucleotide identity to previously described OXA genes, such as blaOXA-211 (GenBank accession number KF453234), blaOXA-212 (GenBank accession number JN861780), and blaOXA-309 (GenBank accession number HF947514), all of which are found in A. johnsonii strains. Furthermore, blaOXA-498 has up to a 96% identity with other blaOXA-211 variants such as those in A. johnsonii strains CIP 64.6 (GenBank accession number APON01000041) and ANC 3681 (GenBank accession number APPZ01000001). A comparison using BLAST between OXA sequences of other Acinetobacter species shows that blaOXA-498 has up to a 72% identity, which indicate that these OXA variants are unique to A. johnsonii. Located upstream of blaOXA-498 in Aj2199, we found dnaK, grpE, hypothetical protein (HP) gene, tolA and estA genes. Among the identified genes, dnaK, grpE, and estA are responsible for the coding of a chaperone, co-chaperone, and esterase A protein, respectively. A similar genetic context lacking the presence of an upstream OXA gene was described in other Acinetobacter sp. (GenBank accession numbers CP003847, CP002080, CP002177).
The genetic context of OXA variants was conserved among all A. johnsonii strains (Fig 3). The region between grpE and estA, containing HP and tolA, is only conserved in A. johnsonii. Moreover, from our analysis, we observed dnaK with 75% identity in Moraxella catarrhalis BBH18 strain. However, grpE present in M. catarrhalis BBH18 did not show any significant similarities to those found in A. johnsonii strains.
Fig 3. Genetic context of a new variant of blaOXA-211 in Aj2199.
Analysis of the genetic context of blaOXA-211 and its variants.
A hypothetical protein (HP, shown in black in Fig 3), located downstream of blaOXA-498 is conserved in multiple Acinetobacter species. Farther downstream of blaOXA-498, we found erpA and three different adeT genes, followed by yheS. The genetic context between yheS and the conserved HP is larger in other Acinetobacter sp. (ranging from 28.1 kbp to 29.7 kbp) compared to Aj2199 (3.9 kbp). The yheS gene is reversely oriented in Acinetobacter sp. DR1 compared to Aj2199 (Fig 3).
This analysis showed that the genes located upstream and downstream of blaOXA-211 and variants are conserved among A. johnsonii strains indicating the ubiquity of the gene.
Resistance determinants present in Aj2199 and their genetic contexts supports the occurrence of HGT
To determine the repertoire of resistance genes present in Aj2199 genome we have used ARG-ANNOT database to predict them. A total of ten resistance gene sequences, excluding blaOXA-498, were identified. Among them we found genes encoding for β-lactamases (blaPER-2, blaOXA-58 and blaTEM -1), aminoglycoside modification enzymes (strA, strB and aacC2), sulfonamide-resistant dihydropteroate synthase (sul1), erythromycin esterase (ereA) and macrolide efflux protein and phosphotranferase (msrE and mphE).
In order to analyze the organization and context of the β-lactamase genes, in-depth analysis of the genome sequences of Aj2199 was performed.
Analysis of blaOXA-58 genetic context
The blaOXA-58 genomic context analysis showed the presence of a truncated ISAba3 upstream of the gene and a complete ISAba3 downstream of it (Fig 4A). The ΔISAba3 located upstream (5’ end) has lost the right inverted repeat (IR-R). The presence of truncated versions of ISAba3 in the 5 ‘end of the blaOXA-58 gene were previously described by others showing that in some cases ISAba3 is interrupted by another IS, such as ISAba2 [38, 39] (Fig 4A). The genetic context downstream (3’ end) blaOXA-58 resembles the genetic structure previously described in A. baumannii strains and in the A. nosocomialis AG13TU119 strain [40] [39], where ISAba3 is followed by araC1 and lysE. In contrast, the region flanking the 5’ end of the blaOXA-58 was not previously described. Directly upstream ΔISAba3-blaOXA-58 we found a hypothetical protein (HP) with 86% of identity to another HP described only in Dendroctonus ponderosae (GenBank accession number APGK01000113) and two streptomycin resistance determinants, strB and strA, are preceding it (Fig 4A). This region containing strB and strA is derived from Tn5393 [41] and was previously described in some species such as Erwinia amylovora, A. baumannii, Escherichia coli, Pseudomonas aeruginosa (GenBank accession numbers M96392, CP010779, CP010373 and KP975076, respectively).
Fig 4. Resistance determinants present in Aj2199 and corresponding genetic contexts supports the occurrence of HGT.
(A) Analysis of blaOXA-58 genetic context compared to other blaOXA-58 genetic contexts described. (B) Schematic representation of blaPER-2 and blaTEM -1 flanking regions.
A blast analysis was performed and it showed that 62 blaOXA-58 sequences are available in the GenBank. However, the genetic context of blaOXA-58 was only described in 36 out of the 62 deposited sequences. Within the 36 descriptions, at least one copy of ISAba3 was associated to blaOXA-58 in the in the 5’ or 3’ end of the gene. The association of blaOXA-58 with ISAba3 seems to be a stable and successful platform for this carbapenemase.
Other genetic contexts, some of them described in plasmid, have been reported in the literature, e.g. the association of blaOXA-58 with a ISAba825, ISAba2 and IS18 upstream of blaOXA-58 [38, 39, 42–44].
A new genetic environment flanking the upstream (5’ end) region of the ΔISAba3 was found in Aj2199 genome suggesting the acquisition of this gene from at least two different species.
Genetic context analysis of blaPER-2 and blaTEM-1 exhibits the presence of foreign DNA acquisition
We analyzed the genetic context of blaTEM-1 in Aj2199 and found that blaTEM-1 is embedded in a truncated version of Tn3, in which the tnpA gene is absent and the tnpR gene is complete and shared 94%, 100% and 95% of identity to the previously tnpR described in the Tn1, Tn2 and Tn3 transposons, respectively [45]. Located downstream blaTEM-1 is aacC2, which has been shown to confer resistance to gentamicin (Fig 4B). The analysis of the contig (named contig_8) harboring blaTEM-1 demonstrated it was 24,769 bp away (from the 3’ end of the blaTEM-1 until the 3’ end of blaPER-2) from another important β-lactamases blaPER-2. Some of the genes located in the region between blaTEM-1 and blaPER-2 where previously described in the context of genomic islands (umuC and umuD genes) [46, 47], or in plasmids as the genes present in the Tn21 mer operon [48].
Our analysis of the blaTEM-1 genetic context showed 99% identity to a previously described genetic context in Klebsiella pneumoniae (GenBank accession number LM994717). A complete IS26 was located upstream of tnpR and preceded by HP that shared 97% identity with a HP described in an A. pittii (GenBank accession number WP_044100499) (Fig 4B).
Futhermore, the analysis of the genetic context of blaPER-2 showed that this gene was flanked by a complete ISPa12 upstream and a glutathione S-transferase downstream (Fig 4B). The association of this later gene with blaPER-2 was previously described in Citrobacter freundii (GenBank accession number AM409516) [49]. Preceding ISPa12 was a HP with 34% identity to a HP described in Paraglaciecola polaris (GenBank accession number WP_007103537). Moreover, a top-like gene with 97% identity to a transposes reported in Methylophaga frappieri–a bacteria typically isolated from marine environments or brackish waters–(GenBank accession number CP003380), was found 1,422 bp downstream of blaPER-2. This particular transposase was also identified in Klebsiella oxytoca (GenBank accession number CP003684) and Vibrio salmonicida (GenBank accession number AJ289135). Downstream this transposase in Aj2199 was a region containing a complete gene cassette (ereA), qacEΔ1, and sul1. The analysis of blaTEM-1 and blaPER-2 in Aj2199 indicates the occurrence of different recombination events. There was a great degree of mosaicism, suggesting an ability to acquire exogenous DNA from different species as well as exposing a high degree of genomic plasticity.
Since blaTEM-1 and blaPER-2 have been found to be located within plasmids or chromosomes [49–53], we performed various plasmid extraction techniques and used the products in transformation assays of competent E. coli, A. baumannii and A. baylyi cells. In addition, we carried out conjugation assays using different bacteria as host cells. Resistance could not be detected in any of the assays suggesting that blaTEM-1 and blaPER-2 are likely chromosomally located. Moreover, using a bioinformatic approach we searched for Enterobacteriaceae plasmid replicon sequences (n = 116) [30] and obtained negative results. In addition, as noted above, neither of these genes are included within the contigs predicted to be part of plasmid structures by the plasmidSpades software. Also, the repAcil PCR amplification gave negative results. In summary, although not definitive, the available evidence suggest that blaTEM-1 and blaPER-2 are not plasmid-mediated.
Identification of potential prophages and phage sequences in Aj2199 genome
Using PHAST tool to predict phage sequences, we identified two intact prophages, a questionable prophage and an incomplete prophage in the Aj2199 genome.
One of the intact prophages contained 50,055 bp and a G+C content of 41.46% with 61 predicted open reading frames. This prophage has the corresponding attL (TATACAATGATTTTAG) and attR (TATACAATGATTTTAG) recognition sites. The phage integrase had a 99% identity with an integrase described in the genome of A. johnsonii (GenBank accession number WP_035326457) and an 83% identity with an integrase found in A. bereziniae genome (GenBank accession number WP_005029089). The genes required for the head, capsid, tail tape, tail fiber as well as the tail assembly protein encoding genes were present.
The second intact prophage contained 22,820 bp a G+C content of 43% with 29 predicted open reading frames. The genes encoding for putative head, capsid, tail tape and tail fiber were present. However, the attL and attR recognition sites as well as the integrase-encoding gene were not found.
Furthermore, a questionable prophage (24,313 bp), for which only phage proteins and transposases were recognized by PHAST, was identified.
The incomplete predicted prophage, composed of 21,414 bp, possessed 39 predicted open reading frames including the phage integrase, which had a 100% identity with an integrase in the A. johnsonii SH046 (GenBank accession number EEY97814). The attL (ATATCTAAGCAT) and attR (ATATCTAAGCAT) recognition sites were also present. The analyses of non-phage genes into the predicted prophage showed the presence of putative transposases and genes with unknown functions.
In agreement with previous published results, our findings revealed the presence of phage related sequences in the Aj2199 genome [54].
Identification of insertion sequence elements in Aj2199 genome
The Aj2199 genome contains a large variety of insertion sequence (IS) elements, which reinforces the theory of extensive acquisition events of mobile genetic elements (MGEs) in this strain. A total of 19 complete ISs and 26 partial ISs were identified (Table 3). As was observed for other Acinetobacter sp., the most abundant IS elements within Aj2199 are ISAba-like. Multiple copies of complete ISAba12 (n = 6), ISAba19 (n = 2), and ISAba8 (n = 2) were present in the genome. Furthermore, multiple copies of truncated ISs were found, including five copies of ΔISAba19 and five copies of ΔISAba11. The ISAba12 and ISAba19, in their complete and truncated versions, were the most abundant IS within Aj2199 genome. ISAba12 was found downstream or upstream of different genes in all instances. Genes found upstream of ISAba12 were involved in the synthesis of lipid A, which is known to be an activator of monocytes triggering a proinflammatory response and is recognized as a Toll-like receptor 4, TLR4 [55, 56]. A copy of ISAba12 was found downstream of Ton-B dependent receptor gene, which codes for bacterial outer membrane proteins that transports siderophores, vitamin B12, nickel complexes, and carbohydrates [57]. We also observed other copies of ISAba12 in different genetic context, e.g. near to hypothetical proteins or putative genes related to zinc metabolism or UV radiation resistance.
Table 3. Insertion sequences identified in A. johnsonii Aj2199 genome.
| Insertion sequences | Origin | Complete/partial sequences | Amount |
|---|---|---|---|
| IS26 | Proteus vulgaris | 1/0 | 1 |
| ISAba2 | A. baumannii | 0/1 | 1 |
| ISAba3 | A. baumannii | 1/1 | 2 |
| ISAba5 | A. baumannii | 0/1 | 1 |
| ISAba7 | A. baumannii | 0/1 | 1 |
| ISAba8 | A. baumannii | 2/0 | 2 |
| ISAba11 | A. baumannii | 0/5 | 5 |
| ISAba12 | A. baumannii | 6/4 | 10 |
| ISAba14 | A. baumannii | 1/0 | 1 |
| ISAba19 | A. baumannii | 2/5 | 7 |
| ISAba21 | A. baumannii | 0/1 | 1 |
| ISAba25 | A. baumannii | 0/1 | 1 |
| ISAba31 | A. baumannii | 0/1 | 1 |
| ISAha2 | A. haemolyticus | 1/0 | 1 |
| ISAjo1 | A. johnsonnii | 1/0 | 1 |
| ISAjo2 | A. johnsonnii | 0/1 | 1 |
| ISKpn11 | Klebsiella pneumoniae | 1/0 | 1 |
| ISKpn12 | Klebsiella pneumoniae | 1/0 | 1 |
| ISKpn18 | Klebsiella pneumoniae | 0/1 | 1 |
| ISOur1 | Oligella urethralis | 1/0 | 1 |
| ISPa12 | Pseudomonas aeruginosa | 1/0 | 1 |
| ISPa14 | Pseudomonas aeruginosa | 0/1 | 1 |
| ISPst3 | Pseudomonas stutzeri | 0/1 | 1 |
| ISSwi1 | Salmonella enterica | 0/1 | 1 |
Additionally, IS elements previously described in K. pneumoniae such as ISKpn11, ISKpn12 and ISKpn18 were identified. ISKpn11 and ISKpn12 were found flanking the RNA polymerase σ70 factor (rpoD), which may allow for the movement of rpoD within the genome as well to be transferred to another bacterial cell. This σ70 factor was also described in plasmids and in the chromosome of many other species, such as K. pneumoniae, A. baumannii, E. coli, Serratia marcescens and Shigella sonnei [58].
ISOur1 was also found in Aj2199 genome. This IS, which has been previously reported as a strong promoter when localized upstream of ampC in Oligella urethralis [59], is found upstream of a putative gene belonging to an UvrABC system.
The present data exposed the importance of ISs in modeling Aj2199 genome and its ability to acquire and later recombine exogenous DNA within its genome.
Presence of putative RNA regulators in Aj2199 genome
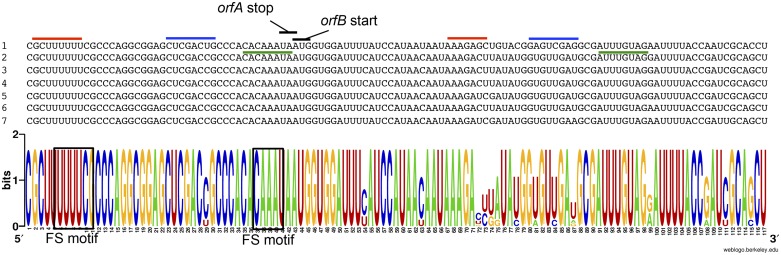
In-silico prediction of putative regulatory RNAs, including small RNA, antisense RNA, thermoregulators, riboswitches and cis-regulators, as well as other small non-coding RNA involved in cellular processes, such as CRISPRs and antitoxins was assessed in the Aj2199 genome. To perform the search we used 720 covariance models resulting in 66 potential candidates. Among them, twenty-eight showed significant e-values corresponding to four riboswitches (families FMN, TPP, cobalamin, glycine), one thermoregulator (CspA), 13 sRNA (CsrC, MicC, t44, RsmZ, RsmY, IsrG, IsrK, SraL, rli40, STnc40, STnc370, Aar, sau-50, Atu_C9), one asARNA (C4) and four cisRNA (yybP-ykoY, Alpha_RBS, ykkC-yxkD, ALIL pseudoknot). While most of these candidates have previously been studied [60, 61], the role of many such as the apical loop-internal loop RNA (ALIL) pseudoknot is still unclear and poorly understood, and thus was further analyzed in this study. In particular, the apical loop-internal loop RNA (ALIL) pseudoknot, was further analyzed. This RNA is directly involved in the stimulation of transposition in IS3-like elements [62]. RNA prediction of ALIL pseudoknot retrieved seven positive sequences that were located within an IS (Fig 5). Five of them were part of an ISAba19 and the remaining pseudoknots were found in ISAba2. Sequence analysis of the pseudoknot sequences showed a highly conserved sequence with two main palindromic regions that are marked with red and blue lines in Fig 5. In Fig 5 the base pairing interaction involved in the pseudoknot was marked with green lines. Noteworthy, ISAba19 was the second most predominant after ISAba12 within Aj2199 genome. The role of the ALIL regulatory RNA in A. johnsonii in the activity of ISAba19 will be studied in future studies.
Fig 5. Conserved sequence of ALIL-pseudoknot associated to ISAba insertion sequences.
Sequences involved in the predicted RNA secondary structure are indicated with red and blue lines. Putative pseudoknot pairing is indicted with green lines. Frame-shift motives are marked with black squares.
Our analysis exposed a wide variety of regulatory elements in Aj2199 genome. The presence of several MGEs and RNA regulators within this genome suggests a potential contribution of MGEs in the mobilization of RNA elements that have an impact on genome evolution and bacteria adaptation.
Dispersion of resistance determinants in other A. johnsonii clinical strains
In order to determine if other A. johnsonii isolates recovered from the same hospital harbored some of the resistance determinants present in Aj2199, we tested four A. johnsonii strains (Aj205, Aj286, Aj289, Aj306) (Table 1). Among the tested isolates Aj286 and Aj289 were resistant to FOX. Moreover, Aj286 was resistant to AMP and SAM. The other two isolates, Aj205 and Aj306, were susceptible to all antibiotics tested (Table 1). PCR amplification for blaPER-2, blaOXA-58, blaTEM-1, strA, strB, ereA, sul1, and aacC2 were performed. Positive amplification results were only obtained for strA and strB in Aj289, Aj286, and Aj306.
Discussion
In the last few years, the increase of antibiotic resistant bacteria has posed a serious threat to human health. The recoveries of species not previously recognized as a menace in the hospital environment have been widely reported [63–65].
In the present study we have delved into the genome of a multidrug-resistant A. johnsonii strain (Aj2199) that was recovered from a hospital in Buenos Aires and it exhibited the occurrence of resistance genes previously reported in other species. By the implementation of different bioinformatics tools, we analyzed in depth its genome to identify particular features and the presence of HGT events within it.
A wide variety of MGEs and traces of HGT were identified in Aj2199. These results are in accordance with previous studies that reported great number of MGEs in Acinetobacter genomes [42, 54, 66–69]. Ten resistance genes (blaPER-2, blaOXA-58, blaTEM-1, strA, strB, ereA, sul1, aacC2, msrE and mphE) were identified demonstrating the potential of other non-baumannii Acinetobacter species to evolve towards the development of high levels of antibiotic resistance. Moreover, a new variant of blaOXA-211, called blaOXA-498, was also identified reinforcing the idea that some Acinetobacter sp. harbor a chromosomally encoded oxacillinase. blaOXA-498 has up to 72% amino acid identity with other oxacillinases described in Acinetobacter species. This supports the uniqueness of the OXA variant to A. johnsonii.
Regarding other relevant β-lactamases present in Aj2199 genome, analysis of blaPER-2 and blaTEM-1 genetic contexts revealed a great degree of mosaicism evidencing its ability to acquire exogenous DNA from different species as well as exposing a high degree of genomic plasticity. The presence of genetic contexts previously described in the Enterobacteriaceae family supported the occurrence of DNA exchange within them. The analysis of blaOXA-58 genetic environment exposed that the flanking region upstream (5’ end) the ΔISAba3 was novel. The 3’ end resembled the genetic environment previously described in other Acinetobacter strains [40] [39].
The results obtained here showed that blaOXA-58, blaPER-2, and blaTEM-1 are likely chromosomally located. This differs from a recent study where the presence of different β-lactamase genes, such as blaNDM-1, blaOXA-58 and blaPER-1, were plasmid located [50].
The role of IS in the development of antibiotic resistance and genome plasticity in A. baumannii strains has been extensively mentioned in the literature [69, 70]. However, the role, quantity, and identity of ISs in other species have been poorly studied. In the Aj2199 genome a large number of complete or partial IS were found. A total of 45 IS-related sequences belonging to different families were identified.
IS elements previously described in K. pneumoniae such as ISKpn11, ISKpn12 and ISKpn18 were identified, which reinforces the idea of extensive acquisition of mobile genetic elements and the importance of ISs in modeling Aj2199 genome and its ability to acquire and later recombine exogenous DNA within its genome.
Different phages, prophages and phage related proteins have been found in A. baumannii, as well as in other species of this genus [54, 71, 72]. Touchon et al. found that temperate phages account for a great proportion of Acinetobacter’s genome identifying over 260 prophages among the genomes in their study [54]. It is well known that phages play a crucial role in HGT as well as in the evolution and dynamics of bacterial genomes [54, 73]. There are multiple descriptions of phage related genes, cryptic prophages and prophage structures in Acinetobacter sp. [54, 71, 72, 74]. However, the role of phages in Acinetobacter is poorly understood. We have identified two intact phages and other phages related sequences within Aj2199 genome, supporting the general idea that phages impact in the evolution of bacterial genomes.
To the best of our knowledge, RNA regulatory sequences have not yet been identified in A. johnsonii. Only one article has addressed the role of regulatory RNAs in Acinetobacter specifically A. baylyi [75]. In that manuscript the Aar RNA regulator was described, which is an RNA element involved in amino acid metabolism [75]. Noteworthy, A. johnsonii Aj2199 also has a putative Aar regulator along with several other regulatory elements. In addition, it encodes the ALIL regulatory RNA, which we found within the second most abundant IS distributed in A. johnsonii Aj2199 genome. Future studies to address its function(s) will be conducted.
The great number of MGEs in the Aj2199 genome suggests a direct impact in the evolution and adaptability of this strain to extreme environment due to the fact that it showed an increasing number of antibiotic resistance determinants within it genome.
The data presented here strongly suggested that A. johnsonii actively acquires exogenous DNA from other bacterial species and concomitantly becomes a reservoir of resistance genes.
Supporting Information
(XLSX)
GO term is added next to each gene family as functional annotation. Contig and position of each Aj2199 specific gene is also indicated.
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We want to acknowledge the Argentinian Consortium of Genomic Technology (ACGT), which is funded by the PPL project of the Science, Technology, and Productive Innovation office (Mincyt) Argentina AECID A1/041041/11, D/024562/09, and INTA, for technical support.
Data Availability
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LVIB00000000. The version described in this paper is version LVIB01000000.
Funding Statement
This work was supported by grants from the “Secretaría de Ciencia y Técnica de la Universidad de Buenos Aires” (UBACyT) and PICT 0120 to MSR, Buenos Aires, Argentina. MSR and CQ is a member of the CONICET research career and SM, GMT, and GPDN have a Doctoral Fellowship from CONICET. KC was supported by grant MHIRT 2T37MD001368 from the National Institute on Minority Health and Health Disparities, National Institute of Health. AI is a researcher from the Sistema Nacional de Investigadores (ANII), Uruguay.
References
- 1.Karah N, Haldorsen B, Hegstad K, Simonsen GS, Sundsfjord A, Samuelsen O, et al. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. The Journal of antimicrobial chemotherapy. 2011;66(4):738–44. 10.1093/jac/dkq521 . [DOI] [PubMed] [Google Scholar]
- 2.Sousa C, Botelho J, Silva L, Grosso F, Nemec A, Lopes J, et al. MALDI-TOF MS and chemometric based identification of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex species. International journal of medical microbiology: IJMM. 2014;304(5–6):669–77. 10.1016/j.ijmm.2014.04.014 . [DOI] [PubMed] [Google Scholar]
- 3.Sousa C, Silva L, Grosso F, Nemec A, Lopes J, Peixe L. Discrimination of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex species by Fourier transform infrared spectroscopy. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2014;33(8):1345–53. 10.1007/s10096-014-2078-y . [DOI] [PubMed] [Google Scholar]
- 4.Turton JF, Shah J, Ozongwu C, Pike R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. Journal of clinical microbiology. 2010;48(4):1445–9. 10.1128/JCM.02467-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Broek PJ, van der Reijden TJ, van Strijen E, Helmig-Schurter AV, Bernards AT, Dijkshoorn L. Endemic and epidemic acinetobacter species in a university hospital: an 8-year survey. Journal of clinical microbiology. 2009;47(11):3593–9. 10.1128/JCM.00967-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traglia GM, Almuzara M, Vilacoba E, Tuduri A, Neumann G, Pallone E, et al. Bacteremia caused by an Acinetobacter junii strain harboring class 1 integron and diverse DNA mobile elements. Journal of infection in developing countries. 2014;8(5):666–9. 10.3855/jidc.3747 . [DOI] [PubMed] [Google Scholar]
- 7.Krizova L, Maixnerova M, Sedo O, Nemec A. Acinetobacter albensis sp. nov., isolated from natural soil and water ecosystems. Int J Syst Evol Microbiol. 2015. 10.1099/ijsem.0.000511 . [DOI] [PubMed] [Google Scholar]
- 8.Krizova L, McGinnis J, Maixnerova M, Nemec M, Poirel L, Mingle L, et al. Acinetobacter variabilis sp. nov. (formerly DNA group 15 sensu Tjernberg & Ursing), isolated from humans and animals. Int J Syst Evol Microbiol. 2015;65(Pt 3):857–63. 10.1099/ijs.0.000028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guardabassi L, Dalsgaard A, Olsen JE. Phenotypic characterization and antibiotic resistance of Acinetobacter spp. isolated from aquatic sources. J Appl Microbiol. 1999;87(5):659–67. . [DOI] [PubMed] [Google Scholar]
- 10.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. Journal of clinical microbiology. 1997;35(11):2819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifert H, Strate A, Schulze A, Pulverer G. Vascular catheter-related bloodstream infection due to Acinetobacter johnsonii (formerly Acinetobacter calcoaceticus var. lwoffi): report of 13 cases. Clin Infect Dis. 1993;17(4):632–6. . [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez CH, Nastro M, Dabos L, Barberis C, Vay C, Famiglietti A. First isolation of Acinetobacter johnsonii co-producing PER-2 and OXA-58 beta-lactamases. Diagn Microbiol Infect Dis. 2014;80(4):341–2. 10.1016/j.diagmicrobio.2014.09.013 . [DOI] [PubMed] [Google Scholar]
- 13.Espinal P, Roca I, Vila J. Clinical impact and molecular basis of antimicrobial resistance in non-baumannii Acinetobacter. Future Microbiol. 2011;6(5):495–511. 10.2217/fmb.11.30 . [DOI] [PubMed] [Google Scholar]
- 14.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–9. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014;42(Database issue):D206–14. 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huerta-Cepas J, Szklarczyk D, Forslund K, Cook H, Heller D, Walter MC, et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016;44(D1):D286–93. 10.1093/nar/gkv1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Stoeckert CJ Jr., Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–89. 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras-Moreira B, Vinuesa P. GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl Environ Microbiol. 2013;79(24):7696–701. 10.1128/AEM.02411-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5):696–704. . [DOI] [PubMed] [Google Scholar]
- 24.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–21. 10.1093/sysbio/syq010 . [DOI] [PubMed] [Google Scholar]
- 25.Sukumaran J, Holder MT. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26(12):1569–71. 10.1093/bioinformatics/btq228 . [DOI] [PubMed] [Google Scholar]
- 26.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57(Pt 1):81–91. 10.1099/ijs.0.64483-0 . [DOI] [PubMed] [Google Scholar]
- 27.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrobial agents and chemotherapy. 2014;58(1):212–20. 10.1128/AAC.01310-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(Database issue):D32–6. 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39(Web Server issue):W347–52. 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrobial agents and chemotherapy. 2014;58(7):3895–903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antipov Dmitry H N, Shen Max, Raiko Mikhail, La Alla, Pevzner PA. plasmidSPAdes: Assembling Plasmids from Whole Genome Sequencing Data. bioRxiv. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29(22):2933–5. 10.1093/bioinformatics/btt509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel L, Figueiredo S, Cattoir V, Carattoli A, Nordmann P. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrobial agents and chemotherapy. 2008;52(4):1252–6. 10.1128/AAC.01304-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolmasky ME, Crosa JH. Molecular cloning and expression of genetic determinants for the iron uptake system mediated by the Vibrio anguillarum plasmid pJM1. J Bacteriol. 1984;160(3):860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez MS, Don M, Merkier AK, Bistue AJ, Zorreguieta A, Centron D, et al. Naturally competent Acinetobacter baumannii clinical isolate as a convenient model for genetic studies. Journal of clinical microbiology. 2010;48(4):1488–90. 10.1128/JCM.01264-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yildirim S, Thompson MG, Jacobs AC, Zurawski DV, Kirkup BC. Evaluation of Parameters for High Efficiency Transformation of Acinetobacter baumannii. Sci Rep. 2016;6:22110 10.1038/srep22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perichon B, Goussard S, Walewski V, Krizova L, Cerqueira G, Murphy C, et al. Identification of 50 class D beta-lactamases and 65 Acinetobacter-derived cephalosporinases in Acinetobacter spp. Antimicrobial agents and chemotherapy. 2014;58(2):936–49. 10.1128/AAC.01261-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iacono M, Villa L, Fortini D, Bordoni R, Imperi F, Bonnal RJ, et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrobial agents and chemotherapy. 2008;52(7):2616–25. 10.1128/AAC.01643-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poirel L, Nordmann P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2006;50(4):1442–8. 10.1128/AAC.50.4.1442-1448.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Jiang J, Zhou H, Jiang Y, Fu Y, Yu Y, et al. Characterization of a novel plasmid type and various genetic contexts of bla OXA-58 in Acinetobacter spp. from multiple cities in China. PloS one. 2014;9(1):e84680 10.1371/journal.pone.0084680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiou CS, Jones AL. Nucleotide sequence analysis of a transposon (Tn5393) carrying streptomycin resistance genes in Erwinia amylovora and other gram-negative bacteria. J Bacteriol. 1993;175(3):732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravasi P, Limansky AS, Rodriguez RE, Viale AM, Mussi MA. ISAba825, a functional insertion sequence modulating genomic plasticity and bla(OXA-58) expression in Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2011;55(2):917–20. 10.1128/AAC.00491-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, Khan AU, et al. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrobial agents and chemotherapy. 2008;52(11):4115–20. 10.1128/AAC.00366-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Nocera PP, Rocco F, Giannouli M, Triassi M, Zarrilli R. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 2011;11:224 10.1186/1471-2180-11-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey JK, Pinyon JL, Anantham S, Hall RM. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. The Journal of antimicrobial chemotherapy. 2011;66(4):745–51. 10.1093/jac/dkq529 . [DOI] [PubMed] [Google Scholar]
- 46.Hare JM, Bradley JA, Lin CL, Elam TJ. Diverse responses to UV light exposure in Acinetobacter include the capacity for DNA damage-induced mutagenesis in the opportunistic pathogens Acinetobacter baumannii and Acinetobacter ursingii. Microbiology. 2012;158(Pt 3):601–11. 10.1099/mic.0.054668-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rapa RA, Islam A, Monahan LG, Mutreja A, Thomson N, Charles IG, et al. A genomic island integrated into recA of Vibrio cholerae contains a divergent recA and provides multi-pathway protection from DNA damage. Environ Microbiol. 2015;17(4):1090–102. 10.1111/1462-2920.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebert CA, Hall RM, Summers AO. Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol Rev. 1999;63(3):507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power P, Di Conza J, Rodriguez MM, Ghiglione B, Ayala JA, Casellas JM, et al. Biochemical characterization of PER-2 and genetic environment of blaPER-2. Antimicrobial agents and chemotherapy. 2007;51(7):2359–65. 10.1128/AAC.01395-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Y, Yang P, Wang X, Zong Z. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. The Journal of antimicrobial chemotherapy. 2016;71(1):71–5. 10.1093/jac/dkv324 . [DOI] [PubMed] [Google Scholar]
- 51.Zong Z. The complex genetic context of blaPER-1 flanked by miniature inverted-repeat transposable elements in Acinetobacter johnsonii. PloS one. 2014;9(2):e90046 10.1371/journal.pone.0090046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basmaci R, Bidet P, Bercot B, Jost C, Kwon T, Gaumetou E, et al. First identification of a chromosomally located penicillinase gene in Kingella kingae species isolated in continental Europe. Antimicrobial agents and chemotherapy. 2014;58(10):6258–9. 10.1128/AAC.03562-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehlberg LC, da Silva Nogueira K, Cayo da Silva R, Nicoletti AG, Palmeiro JK, Gales AC, et al. Detection of PER-2-producing Enterobacter cloacae in a Brazilian liver transplantation unit. Antimicrobial agents and chemotherapy. 2014;58(3):1831–2. 10.1128/AAC.01260-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Touchon M, Cury J, Yoon EJ, Krizova L, Cerqueira GC, Murphy C, et al. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol Evol. 2014;6(10):2866–82. 10.1093/gbe/evu225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–5. 10.1038/nature07830 . [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Akira S. Lipid A receptor TLR4-mediated signaling pathways. Adv Exp Med Biol. 2010;667:59–68. 10.1007/978-1-4419-1603-7_6 . [DOI] [PubMed] [Google Scholar]
- 57.Zimbler DL, Arivett BA, Beckett AC, Menke SM, Actis LA. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect Immun. 2013;81(9):3382–94. 10.1128/IAI.00540-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho BK, Kim D, Knight EM, Zengler K, Palsson BO. Genome-scale reconstruction of the sigma factor network in Escherichia coli: topology and functional states. BMC Biol. 2014;12:4 10.1186/1741-7007-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mammeri H, Poirel L, Mangeney N, Nordmann P. Chromosomal integration of a cephalosporinase gene from Acinetobacter baumannii into Oligella urethralis as a source of acquired resistance to beta-lactams. Antimicrobial agents and chemotherapy. 2003;47(5):1536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43(6):880–91. 10.1016/j.molcel.2011.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9(8):578–89. 10.1038/nrmicro2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazauric MH, Licznar P, Prere MF, Canal I, Fayet O. Apical loop-internal loop RNA pseudoknots: a new type of stimulator of -1 translational frameshifting in bacteria. J Biol Chem. 2008;283(29):20421–32. 10.1074/jbc.M802829200 . [DOI] [PubMed] [Google Scholar]
- 63.Chusri S, Chongsuvivatwong V, Rivera JI, Silpapojakul K, Singkhamanan K, McNeil E, et al. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrobial agents and chemotherapy. 2014;58(7):4172–9. 10.1128/AAC.02992-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Endo S, Yano H, Kanamori H, Inomata S, Aoyagi T, Hatta M, et al. High frequency of Acinetobacter soli among Acinetobacter isolates causing bacteremia at a tertiary hospital in Japan. Journal of clinical microbiology. 2014;52(3):911–5. 10.1128/JCM.03009-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai HY, Cheng A, Liu CY, Huang YT, Lee YC, Liao CH, et al. Bacteremia caused by Acinetobacter junii at a medical center in Taiwan, 2000–2010. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2012;31(10):2737–43. 10.1007/s10096-012-1622-x . [DOI] [PubMed] [Google Scholar]
- 66.Harmer CJ, Hall RM. IS26-Mediated Precise Excision of the IS26-aphA1 a Translocatable Unit. MBio. 2015;6(6):e01866–15. 10.1128/mBio.01866-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopes BS, Amyes SG. Role of ISAba1 and ISAba125 in governing the expression of blaADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J Med Microbiol. 2012;61(Pt 8):1103–8. 10.1099/jmm.0.044156-0 . [DOI] [PubMed] [Google Scholar]
- 68.Mugnier PD, Poirel L, Nordmann P. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J Bacteriol. 2009;191(7):2414–8. 10.1128/JB.01258-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roca I, Espinal P, Vila-Farres X, Vila J. The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Frontiers in microbiology. 2012;3:148 10.3389/fmicb.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imperi F, Antunes LC, Blom J, Villa L, Iacono M, Visca P, et al. The genomics of Acinetobacter baumannii: insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life. 2011;63(12):1068–74. 10.1002/iub.531 . [DOI] [PubMed] [Google Scholar]
- 71.Hare JM, Ferrell JC, Witkowski TA, Grice AN. Prophage induction and differential RecA and UmuDAb transcriptome regulation in the DNA damage responses of Acinetobacter baumannii and Acinetobacter baylyi. PloS one. 2014;9(4):e93861 10.1371/journal.pone.0093861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z, Ling B, Zhou L. Prevalence of 16S rRNA methylase, modifying enzyme, and extended-spectrum beta-lactamase genes among Acinetobacter baumannii isolates. J Chemother. 2015;27(4):207–12. 10.1179/1973947814Y.0000000190 . [DOI] [PubMed] [Google Scholar]
- 73.Menouni R, Hutinet G, Petit MA, Ansaldi M. Bacterial genome remodeling through bacteriophage recombination. FEMS Microbiol Lett. 2015;362(1):1–10. 10.1093/femsle/fnu022 . [DOI] [PubMed] [Google Scholar]
- 74.Renda BA, Dasgupta A, Leon D, Barrick JE. Genome instability mediates the loss of key traits by Acinetobacter baylyi ADP1 during laboratory evolution. J Bacteriol. 2015;197(5):872–81. 10.1128/JB.02263-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schilling D, Findeiss S, Richter AS, Taylor JA, Gerischer U. The small RNA Aar in Acinetobacter baylyi: a putative regulator of amino acid metabolism. Arch Microbiol. 2010;192(9):691–702. 10.1007/s00203-010-0592-6 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
GO term is added next to each gene family as functional annotation. Contig and position of each Aj2199 specific gene is also indicated.
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LVIB00000000. The version described in this paper is version LVIB01000000.