Abstract
Three experiments were done to better assess the gastrointestinal (GI) site(s) of action of GLP-1 on food intake in rats. First, near-spontaneous nocturnal chow meal size (MS), intermeal intervals (IMI) length and satiety ratios (SR = MS/IMI) were measured after infusion of saline, 0.025 or 0.5 nmol/kg GLP-1 into the celiac artery (CA, supplying the stomach and upper duodenum), cranial mesenteric artery (CMA, supplying small and all of the large intestine except the rectum), femoral artery (FA, control) or portal vein (PV, control). Second, infusion of 0.5 nmol/kg GLP-1 was tested after pretreatment with the GLP-1 receptor (GLP-1R) antagonist exendin-4(3–39) via the same routes. Third, the regional distribution of GLP-1R in the rat GI tract was determined using rtPCR. CA, CMA and FA GLP-1 reduced first MS relative to saline, with the CMA route more effective than the others. Only CMA GLP-1 prolonged the IMI. None of the infusions affected second MS or later eating. CA and CMA GLP-1 increased the SR, with the CMA route more effective than the CA route. CMA exendin-4 (3–39) infusion reduced the effect of CMA GLP-1. Finally GLP-1R expression was found throughout the GI tract. The results suggest that exogenous GLP-1 acts in multiple GI sites to reduce feeding under our conditions and that GLP-1R in the area supplied by the CMA, i.e., the small and part of the large intestine, plays the leading role.
Keywords: GLP-1, Intermeal Interval, Satiety Ratio, Celiac Artery, Portal Vein
Introduction
Glucagon-like Peptide-1 (7–36)-amide (GLP-1) is a 30-amino acid peptide secreted by the L-cells of the gastrointestinal (GI) tract as well as some pancreatic cells and a small population of neurons in the nucleus tractus solitaries. Intestinal GLP-1 is secreted in response to neuroendocrine stimulation and macronutrient sensing. Through activation of a G-protein coupled receptor, GLP-1 receptor (GLP-1R), GLP-1 evokes responses including stimulation of insulin secretion, inhibition of gastric emptying and reduction of food intake (reviewed in (Holst, 2007)).
Evidence suggests that GLP-1 acts both peripherally and centrally to reduce food intake. In support of a peripheral action, systemic administration of albugon, a GLP-1-albumin fusion protein that does not cross the blood-brain barrier, reduced food intake (Baggio, Huang, Brown, & Drucker, 2004) and intraperitoneal injections of the GLP-1R antagonist exendin (9–39) increased food intake (Asarian, et al., 2012; Williams, Baskin, & Schwartz, 2009). In addition, total abdominal vagotomy or selective subdiaphragmatic vagal deafferentation attenuated the reduction of food intake by peripherally administered GLP-1 under some conditions (Abbott, et al., 2005; Ruttimann, Arnold, Hillebrand, Geary, & Langhans, 2009). On the other hand, in support of a central action, central injection of GLP-1 also decreased food intake, and central injection of exendin (9–39) increased it (Kinzig, D'Alessio, & Seeley, 2002; McMahon & Wellman, 1998; Turton, et al., 1996).
The peripheral sites where GLP-1 acts to reduce food intake are unknown. Rüttiman et al. found that GLP-1 infused via intraperitoneal catheters reduced the size of dark-onset meals in rats, whereas GLP-1 infused via hepatic portal-vein catheters did not, indicating that GLP-1R in the GI tract, pancreas or other extra-hepatic gut site mediated the effect. Therefore, to further specify the site(s) of action of peripheral GLP-1 on food intake, we compared the effects of GLP-1 infusions into (1) the celiac artery (CA), which supplies the stomach, upper duodenum, part of the pancreas and liver, (2) the cranial mesenteric artery (CMA), which supplies the duodenum, jejunum, ileum, cecum and colon (Robert, 1971; Roger, Cabanie, & Ferre, 1991; Sayegh, 2013a, 2013b; Snipes, 1981), (3) the femoral artery (FA), and (4) the hepatic portal vein (PV). Infusions were done just prior to the onset of the dark cycle, and near-spontaneous meal patterns were measured for 24 h. This represents the first test of the differential feeding effects of GLP-1 infused via different vascular routes. In addition to the better anatomical specificity, this technique permits the use of smaller doses of GLP-1 than necessary in intraperitoneal-injection tests, which may minimizes possible side effects. We previously used this intra-arterial catheterization technique to test CA and CMA infusions of cholecystokinin (CCK) and gastrin releasing peptide (GRP) (Sayegh, et al., 2015; Washington, Aglan, & Sayegh, 2014).
In a second experiment, we tested the effects of pretreated with the GLP-1 receptor antagonist exendin-4 (3–39) on the feeding effect of GLP-1 infusions into the CA, CMA, FA and PV. Finally, to support our hypothesis that GLP-1 acts in specific GI loci to control feeding, we determined GLP-1R expression in eleven different GI regions. Dunphy et al. (Dunphy, Taylor, & Fuller, 1998) previously identified GLP-1R in the rat GI tract, but did not provide information about differential expression in different GI loci.
Materials and Methods
The Tuskegee University Animal Care and Use Committee approved all animal protocols. Adult male Sprague Dawley rats weighing 400 – 450 g (n=28, divided into CA, CMA, FA and PV groups, n = 7 each) were individually housed in the BioDAQ E2 system (Research Diets, New Brunswick, NJ) in a controlled environment (12 h dark/12 h light cycle – lights off at 1800 h, 21.5°C), with water and pelleted rodent chow (Teklad, Madison, WI) available ad libitum.
Vascular Catheterization
One catheter was implanted in each rat, as described previously (Sayegh, et al., 2014; Washington, et al., 2014). Catheters (Micro-Renathane R-ITC-SP 9.5, Braintree Scientific, Braintree MA) were 24 cm long. The intravascular portion of the catheter was 0.25 mm OD × 0.12 mm ID, and the size of the remaining part was 0.84 mm OD × 0.36 mm ID. Catheterizations were performed using a surgical microscope (Carl Zeiss Opmi 160 12.5×/18B, 1×250, Monument, CO). General anesthesia, indicated by the absence of a pedal withdrawal reflex, was achieved with intramuscular injection of 1 ml/kg body weight of a mixture of 5.0 ml of Ketaset [100 mg/kg], 2.5 ml of Rompun® [xylazine 20 mg/kg], Bayer, Shawnee Mission, KS, 1.0 ml of acepromazine maleate® [10 mg/kg], Bayer, Shawnee Mission, KS and 1.5 ml of saline. The abdominal wall was clipped and cleaned with three alternating betadine solution and alcohol swabs. A ventral midline celiotomy was performed.
The CA was exposed and a temporary ligation was placed near the branch point from the aorta to prevent bleeding. The CA was punctured with a sterile 30 gauge needle 1–2 mm distal to this ligature, and the catheter was threaded into the artery and fixed in place using cyanoacrylate glue. The temporary ligation was removed, and the catheter was threaded out of the abdominal cavity subcutaneously, exteriorized between the scapulae and secured with sutures and cyanoacrylate glue. The CMA was similarly catheterized. The FA was exposed on the medial aspect of the right thigh, freed from the surrounding fat and connective tissue, clamped (MC6 double clamp 0.9 cm, Microsurgery Instruments, Inc. Bellaire, TX), and catheterized similarly. The PV was located and exposed on the ventral aspect of the liver and similarly catheterized.
The muscles of the abdominal wall were closed using a polydioxanone II (4-0) absorbable suture in a simple continuous pattern, and the skin was closed using surgical staples. Postoperative care included Metacam® (Meloxicam® [1.1 mg/kg]) subcutaneously for pain control, Boehringer Ingelheim, St. Joseph, MO and Baytril® (Enrofloxacin® [0.05 ml], Bayer, Shawnee Mission, KS) intramuscularly as an anti-bacterial medication, each given daily for 5 d. Rats were allowed two weeks of recovery time. The criteria for complete recovery following surgery included the absence of clinical signs (e.g., signs of pain, porphyria secretion, cold extremities, lethargy) and the return of food intake to pre-operative levels. Catheters were flushed twice daily (0900 h and 1700 h) with 0.3 ml heparinized saline.
The patency of CA and CMA catheters was confirmed during surgery, first, by injecting 0.5 ml sterile saline into the catheters and verifying pallor in the perfused tissue, and, second, by injecting 0.5 ml methylene blue and verifying dye in the perfused tissues. In addition, at the end of the experiment, all rats were sacrificed with an overdose of pentobarbital, and the catheters were infused with latex, whose distribution was verified. Verification of the PV and FA were done by injecting latex only.
Meal Patterns
The BioDAQ E2 Food and Water Intake system detects brief episodes of food intake while minimizing food spillage and hoarding and generates a computerized data stream including times of the initiation of intake activity, the period of the activity, and the weight consumed. The criterion for a meal was consumption of ≥ 0.2 g, and the criterion for intermeal interval (IMI) was no feeding activity for ≥ 15 min.
After two weeks of recovery from surgery, rats were habituated to the laboratory environment and the experimental design daily for two weeks. For the dose-response experiment, at 1700 h, 1 h before lights off, feeder gates were closed each rat was weighed, handled for a few minutes and received a 0.3 ml infusion of heparinized saline into its catheter. At 1800 h, lights were off, feeder gates were opened. For the antagonist experiment, the rats received two 0.3 ml infusions of heparinized saline, at 1750 h and at 1800 h. First MS, IMI and SR were determined and formed individual baselines for each of rats. These were compared later with the experimental data. If these did not match within two standard deviations, they were not included in the statistical analysis. Four of 28 rats were excluded from the dose-response experiment on this basis. All rats were included in the analysis of the antagonist study.
On Mondays, Wednesdays and Thursdays at 1800 h for the dose-response experiment and at 1750 h for the antagonist experiment, rats received a heparinized saline infusion. On Tuesdays, Thursdays and Saturdays, the dose-response rats received infusions of GLP-1(7–36) (GLP-1; 0, 0.025, 0.5 nmol/kg; Bachem, Torrance, CA, USA) at 1800 h and the antagonist rats received exendin-4 (3–39) (0 or 0.1 mg/kg; Bachem) at 1750 h followed by GLP-1 (0.5 nmol/kg) at 1800 h. Sundays were reserved for the maintenance, but the catheters were flushed with 0.3 ml of the heparinized saline solution twice a day including Saturdays and Mondays. Treatments were done in random order.
GLP-1R rtPCR
rtPCR was done as previously (Gulley, et al., 2005; Lateef, et al., 2012). Ten free-fed rats were sacrificed with an overdose of sodium pentobarbital (100 mg/kg, i.p.). Approximately 200 mg (1 cm2) samples were collected from the esophagus (mid-cervical region), gastric antrum and cardia, pylorus, proximal duodenum (0.5 cm aborad from the pylorus), distal duodenum (5 cm aborad from the pylorus), jejunum (20 cm aborad from the pylorus), ileum (5 cm orad from the cecum), cecum (body), colon (5 cm aborad from the cecum), and rectum. A hindbrain sample was taken immediately ventral to the cerebellum. Total RNA was isolated using the TRIZOL method (Invitrogen-Life Technologies, Inc., Carlsbad, CA) and according to the manufacturer's protocol, and the total RNA concentration was determined by ultraviolet absorbance at 260 nm (DU640, Beckman Coulter, Fullerton, CA). RNA from each sample was assessed for purity by determining the A260/280 ratios (ratios of 1.5 to 2 were used), and the integrity of each sample was assessed by samples produced on 2% agarose gels stained with ethidium bromide. RNA samples were treated with RNase-free DNase (Ambion, Foster City, CA) to remove residual DNA. Samples were selected based on the bright staining of the 18 s and 28 s ribosomal bands, with the latter showing twice the concentration of the former. First-strand cDNA was synthesized from 2 µg of total RNA using Reaction Ready™ First Strand cDNA Synthesis (Super Array Bioscience, Frederick, MD). mRNA levels were measured using RT-PCR in a 25 µL reaction mixture containing 12.5 µL of RT2Real-Time SYBR/Fluorescein Green PCR master mix, 1 µL of first-strand cDNA, 1 µL of RT2 validated PCR primer sets for CCK1 and CCK2 (Super Array Bioscience) and 10.5 µL PCR-grade water (Ambion). Samples were run in 96 well PCR plates (Bio-Rad, Hercules, CA) in duplicate, and the results were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPD). The amplification protocol was set at 95°C for 15 min, followed by 40 cycles each at 95°C for 30 s, 55°C f or 30 s, and 72°C for 30 s, followed by a melting curve determination between 55°C and 95°C to ensure the detection of a single PCR product.
Statistical Analysis
Meal size, IMI and SR were analyzed individually using two-way analyses of variance (route × treatment, with repeated measures on treatment), followed by Bonferroni-corrected t-tests for pairwise comparisons. Results were considered significant if p < 0.05. Data are displayed as the mean ± standard error of the mean (SEM). Comparisons for the GLP-1R expression was done by using a one-way analysis of variance followed by Bonferroni-corrected t-tests for pairwise comparisons.
Results
GLP-1
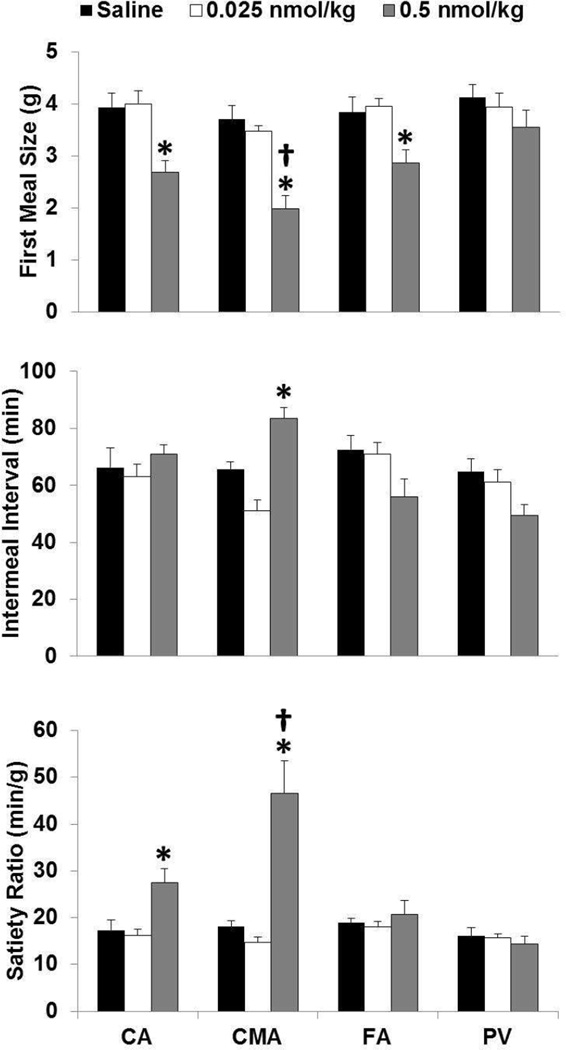
ANOVA revealed a main effect of treatments (F3, 20= 45.51, p=0.01) and routes (F3, 20=3.80, p=0.02) on the first nocturnal MS. Follow-up tests revealed that 0.025 nmol/kg GLP-1 did not affect MS significantly relative to saline control (Figure 1, upper panel). However, 0.5 nmol/kg GLP-1 infused via the CA, CMA and FA each reduced first MS relative to saline control, with the effect of CMA infusions larger than those of CA, FA and PV infusions and there was no difference between the CA and the PV effect (Figure 1, upper panel). None of the infusions affected second MS or later eating.
Figure 1.
Effects of GLP-1 infused into the CA, CMA, PV and FA on MS, IMI and SR. Upper panel: * 0.5 nmol/kg GLP-1 0.5 infused via the CA, CMA and FA reduced first nocturnal MS relative to saline control, p<0.05; † the CMA route was more effective than the remaining routes p<0.05. Middle panel: * 0.5 nmol/kg GLP-1 infused via the CMA route prolonged the IMI relative to saline control, p<0.05. Lower panel: * 0.5 nmol/kg GLP-1 infused via the CA and CMA increased the SR relative to saline control, p<0.05; † the CMA route was more effective than the CA routes, p<0.05.
IMI
ANOVA revealed a main effect of routes (F3, 20= 6.70, p=0.003) and treatments and routes (F3, 20=4.89, p=0.01) on the first IMI. Follow-up tests revealed that 0.025 nmol/kg GLP-1 did not affect the IMI length significantly relative to saline control (Figure 1, middle panel). However, 0.5 nmol/kg GLP-1 infused via the CMA prolonged the IMI length relative to saline control (Figure 1, middle panel).
SR
ANOVA revealed a main effect of treatments (F3, 20= 19.16, p=0.000), routes (F3, 20=10.44, p=0.000), and treatments and routes (F3, 20= 9.29, p=0.000). Follow-up tests revealed that 0.025 nmol/kg GLP-1 did not affect the SR significantly relative to saline control (Figure 1, lower panel). However, 0.5 nmol/kg GLP-1 infused via the CA and the CMA increased SR relative to saline control, with the effect of CMA infusions larger than the CA infusion (Figure 1, lower panel).
Exendin-4 (9–39)
First MS
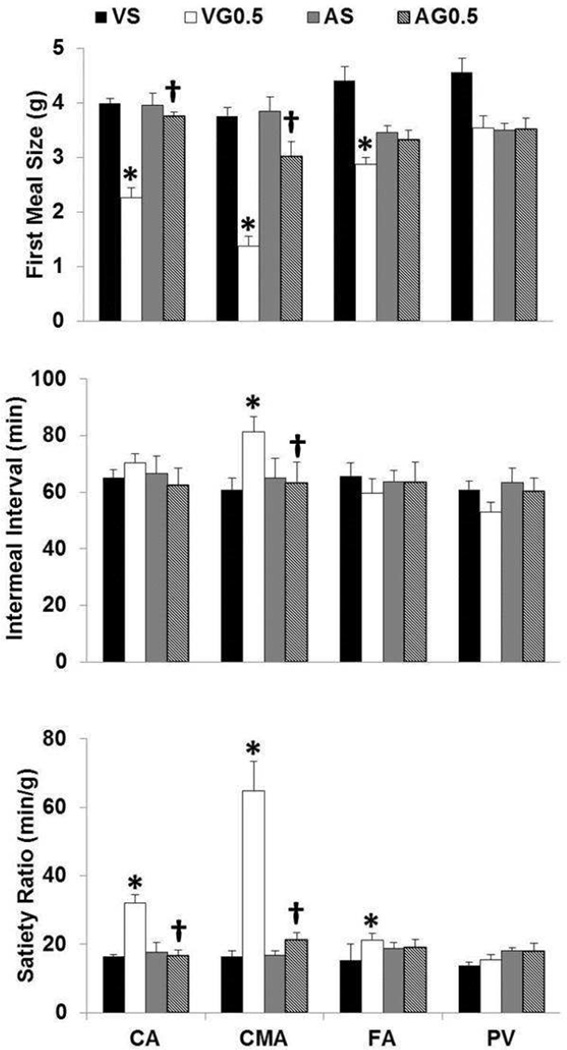
ANOVA revealed a main effect of treatments (F3, 24= 47.95, p<0.001), routes (F3, 24= 14.60, p<0.001), and treatments and routes (F3, 24= 5.86, p<0.001). Follow-up tests revealed that VG0.5 infused via the CA, CMA and FA reduced first MS relative to VS and AG 0.5 infused via the CA and CMA attenuated this effect – no difference between AG0.5 and AS and AG0.5 and VS (Figure 2, upper panel). None of the infusions affected second MS or later eating.
Figure 2.
Effects of Exendin-4 (9–39) infused into the CA, CMA, PV and FA on MS, IMI and SR. Upper panel: * VG0.5 infused in the CA, CMA and FA reduced MS relative to VS p<0.05; † AG0.5 infused in the CA and CMA attenuated this response relative to VG0.5, p<0.05. Middle panel: * VG0.5 infused in the CMA prolonged the IMI relative to VS p<0.05; † AG0.5 infused in the CMA attenuated this response relative to VG0.5, p<0.05. Lower panel: * VG0.5 infused in the CA, CMA and FA increased the SR relative to VS p<0.05; † AG0.5 infused in the CA and CMA attenuated this response relative to VG0.5, p<0.05. Abbreviations: VS, vehicle for antagonist / saline or vehicle for GLP-1; VG0.5, vehicle for the antagonist / GLP-1 (0.5 nmol/kg); AS, antagonist (0.1 mg/kg) / saline or vehicle for GLP-1; AG0.5, antagonist / GLP-1.
IMI
ANOVA revealed a main effect of routes (F3, 24= 3.93, p=0.02) and treatments and routes (F3, 24= 6.18, p=0.003). Follow-up tests revealed that VG0.5 infused via the CMA prolonged the IMI length relative to VS and AG0.5 infused via the CMA attenuated this effect – no difference between AG0.5 and AS and AG0.5 and VS (Figure 2, middle panel).
SR
ANOVA revealed a main effect of treatments (F3, 24= 31.78, p<0.001), routes (F3, 24= 23.02, p<0.001), and treatments and routes (F3, 24= 14.18, p<0.001). Follow-up tests revealed that VG0.5 infused via the CA, the CMA and the FA increased SR relative to VS, and AG0.5 infused via the CA and the CMA attenuated this effect – no difference between AG0.5 and AS and AG0.5 and VS (Figure 2, lower panel).
GLP-1R mRNA expression
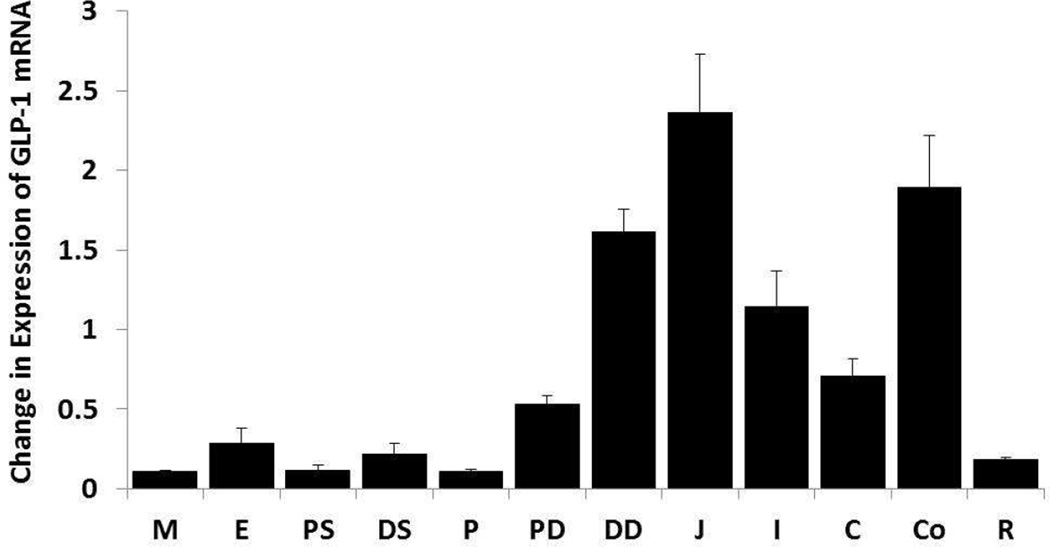
The expression of GLP-1R mRNA was determined by qRT-PCR on total RNA isolated from the GI and the hindbrain and quantified relative to the expression of GAPD. ANOVA revealed differences between intestinal levels (F11, 47= 21.76, p<0.001). GLP-1R mRNA expression was densest in the jejunum, colon and distal duodenum, was clear in the ileum, cecum and proximal duodenum, and sparse in the esophagus, distal stomach, rectum, proximal stomach, pylorus and medulla.
Discussion
The current study directly tested the hypothesis that the GI tract contains GLP-1R mediating the control of MS, IMI and SR by exogenous GLP-1 for the first time. Using a unique microvascular surgical technique, intra-arterial catheterization of the CA and the CMA, GLP-1 and its antagonist were delivered to specific GI locations. The principal findings were: first, that the vascular bed of the CMA supplies GLP-1R regulating MS by exogenous GLP-1; second, that exogenous GLP-1 (7–36) prolongs the IMI; third, that the vascular bed of the CMA supplies GLP-1R regulating IMI length by exogenous GLP-1, fourth, that GLP-1R expression is widespread in the GI tract, but densest in the jejunum (20 cm aborad from the pyloric sphincter) and the colon (5 cm aboard from the cecum), and, fifth, that the finding that exogenous GLP-1 (7–36) given in the FA reduced MS supports previous findings that there are also non-GI sites of action for GLP-1.
Others (Abbott, et al., 2005; Ruttimann, et al., 2009; Williams, et al., 2009) have reported that ip or iv GLP-1 reduces MS, but the current study is the first to compare GLP-1 infusions into the CA and CMA, which supply specific GI sites, with PV and FA infusions. In addition to localizing the sites of GLP-1R controlling the GLP-1-evoked decreases in MS, this technique also revealed that exogenous GLP-1 prolongs the IMI. Specifically, the vascular bed of the CMA contains sites of action regulating MS and IMI by exogenous GLP-1 because CMA infusions had more potent effects on MS and IMI than either CA or PV infusions. The localization of a site of action of exogenous GLP-1’s to the vascular bed of the CMA directly confirms a hypothesis based on several facts: (1) The main source of peripheral GLP-1 is GI L cells (Eissele, et al., 1992). (2) GLP-1 is secreted in response to the presence of food in the GI tract. (3) GLP-1R is heavily distributed in the GI tract (Figure 3). (4) Central injections of GLP-1 antagonist (exendin (3–39)) failed to attenuate the reduction of food intake by ip GLP-1 injection (Williams, et al., 2009), whereas ip injection of the same antagonist blocked reduction of food intake by ip GLP-1 (Williams, et al., 2009). (5) GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-IV) in the capillaries of the gut (Deacon & Holst, 2013), so that little GLP-1 reaches the general circulation after meals in rats (Arnold, Dai, Tso, & Langhans, 2012; Shin, Zheng, Townsend, Sigalet, & Berthoud, 2010). (6) In rats, vagal afferents supplying the GI tract express GLP-1R (Bucinskaite, et al., 2009; Nakagawa, et al., 2004; Vahl, et al., 2007). (7) Reduction of food intake by ip injection of GLP-1 (7–36) was attenuated by total subdiaphragmatic vagotomy (Abbott, et al., 2005). Finally, hepatic portal vein (HPV) and ip infusions of 10 nmol/kg GLP-1 reduced meal size similarly in sham-operated rats, but only HPV GLP-1 reduced meal size in subdiaphragmatic vagal deafferentation rats (Rüttiman et al., 2009) and selective vagal deafferentation (Rüttiman et al., 2009; Hayes et al., 2011), although not by selective or common hepatic branch vagotomy (Hayes, et al., 2011; Ruttimann, et al., 2009) or capsaicin treatment (Reidelberger, Haver, Anders, & Apenteng, 2014; Zhang & Ritter, 2012).
Figure 3.
Distribution of GLP-1R in the GI tract of the rat. GLP-1R expression was determined by rtPCR and quantified relative to expression of GADP. Abbreviations: jejunum, J; colon, Co; distal duodenum, DD; ileum, I; cecum, C; proximal duodenum, PD; esophagus, E; distal stomach, DS; rectum, R; proximal stomach, PS; pylorus, P; medulla, M.
The reduction of MS by exogenous GLP-1 given by the CA is consistent with either an upper GI or pancreatic site of action, because the CA also supplies the stomach, proximal duodenum and part of the pancreas. This possibility is also consistent with the finding that the stomach and proximal duodenum expressed GLP-1R (Figure 3). That CMA GLP-1 reduced food intake more than CA GLP-1, however, suggests that these sites are not the predominate sites of action. Nevertheless, further work to clarify this issue is warranted.
The GI distribution of GLP-1R has not been described in detail in rats previously. Dunphy et al. (Dunphy, et al., 1998) reported that GLP-1R were present in the GI tract, but did not localize them. Our findings extend this report. In addition, Korner et al. (2007) and Amato et al. (2014) described GLP-1R density / expression in human stomach, duodenum, ileum and colon, Rotondo et al. (2011) reported the expression of GLP-1R in the stomach, antrum and fundus of mice (Amato, Baldassano, Liotta, Serio, & Mule, 2014; Korner, Bessler, Inabnet, Taveras, & Holst, 2007; Rotondo, Amato, Lentini, Baldassano, & Mule, 2011) which are generally consistent with the present findings.
A role for the GI tract as a site of action for exogenous GLP-1 is further supported by the increase in the SR and the attenuation of MS and IMI effects of GLP-1 by the GLP-1 antagonist exendin-4(9–39) (Figures 1 and 2). Again, unlike the indirect evidence presented above, the present observations provide direct evidence in support of a GI site of action regulating MS and IMI length by exogenous GLP-1. In order to determine the specific site of action controlling MS and IMI length by GLP-1 the peptide should be infused in the branches of the CMA (i.e., caudal pancreatico-duodenal artery (supplies duodenum), jejunal arteries (supplies jejunum) and ileocolic artery (supplies ileum, cecum and colon)). Furthermore, the finding that exogenous GLP-1 was able to reduce MS by the other routes (e.g., CA and FA) reflects the wide distribution of GLP-1R and points to of both GI and non-GI sites that regulate food intake by GLP-1. In particular, many studies indicate that exogenous GLP-1 can act in the to reduce food intake (cite studies – best some reviews).
The finding that exogenous GLP-1 infused in the PV failed to reduce MS is consistent with a study by Rüttimann et al. (Ruttimann, et al., 2009), who showed that 0.33, but not 1 and 3, nmol/kg GLP-1 infused in the PV (or the vena cava) failed to reduce MS in rats. Here, GLP-1 0.5 nmol also failed to do so. The effect of the higher doses led the authors to speculate that GLP-1 acted at brain sites. The reduction in MS by GLP-1 given in the FA reduced MS.
Unlike the Ruttimann et al. (Ruttimann, et al., 2009), however, we found that GLP-1 given in the CMA prolonged the IMI. This is the first study in rats showing that intra-arterial infusion of GLP-1 (7–36) prolongs the IMI. Interestingly, unlike CMA infusions, which affected both MS and IMI, GLP-1 given in the CA or FA reduced MS but did not prolong the IMI. This suggests that the two feeding responses are controlled at different sites.
In two studies (Rüttimann et al., 2009; Hayes et al., 2011) subdiaphragmatic vagal deafferentation reduced or eliminated the reduction of MS by ip injections of GLP-1, a finding which has been challenged recently by Reidelberger et. al. (Reidelberger, et al., 2014), who found that reduction of food intake by 3 h jugular infusion of GLP-1 was not attenuated by capsaicin pretreatment. A possible resolution to this apparent discrepancy may be that capsaicin nonselectively lesions both vagal and non-vagal, small, unmyelinated sensory neurons, but does not lesion myelinated vagal fibers (Gamse, 1982).
The evidence that antagonism of endogenous GLP-1 signaling with exendin-4 (3–39) increase food intake in rats is mixed. Peripheral administration of exendin(9–39) increased food intake in only two studies to our knowledge (Asarian, et al., 2012; Williams, et al., 2009) and failed to do so in others (Dailey, Moghadam, & Moran, 2011; Punjabi, Arnold, Geary, Langhans, & Pacheco-Lopez; Ruttimann, Arnold, Geary, & Langhans, 2010). Although, the failure of the antagonist alone in the current study to increase food intake (Figure 2) does not support a GI site of action for endogenous GLP-1 on eating, higher doses of the antagonist infused at different times before, during and after a meal must be examined before this possibility is ruled out. Neverrtheless, that GLP-1R antagonist given in the CMA attenuated the reduction of MS and prolongation of the IMI by exogenous GLP-1 confirms a GI site of action regulating these feeding responses by exogenous GLP-1. Thus, both our agonist and antagonist studies suggest that the small and large intestinal areas supplied by the CMA contain sites of action controlling MS and IMI length by exogenous GLP-1.
Acknowledgments
Supported by grant 1SC1DK094972-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Amato A, Baldassano S, Liotta R, Serio R, Mule F. Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J Endocrinol. 2014;221:29–37. doi: 10.1530/JOE-13-0525. [DOI] [PubMed] [Google Scholar]
- Arnold M, Dai Y, Tso P, Langhans W. Meal-contingent intestinal lymph sampling from awake, unrestrained rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1365–R1371. doi: 10.1152/ajpregu.00497.2011. [DOI] [PubMed] [Google Scholar]
- Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. 2012;143:325–327. e322. doi: 10.1053/j.gastro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:e978–e978. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Dailey MJ, Moghadam AA, Moran TH. Jejunal linoleic acid infusions require GLP-1 receptor signaling to inhibit food intake: implications for the effectiveness of Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab. 2011;301:E1184–E1190. doi: 10.1152/ajpendo.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother. 2013;14:2047–2058. doi: 10.1517/14656566.2013.824966. [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol. 1998;141:179–186. doi: 10.1016/s0303-7207(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Gamse R. Capsaicin and nociception in the rat and mouse. Possible role of substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:205–216. doi: 10.1007/BF00510129. [DOI] [PubMed] [Google Scholar]
- Gulley S, Sharma SK, Mansour M, Sullivan CN, Moran TH, Sayegh AI. Strain differences in myenteric neuron number and CCK1 receptor mRNA expression may account for differences in CCK induced c-Fos activation. Brain Res. 2005;1058:109–119. doi: 10.1016/j.brainres.2005.07.074. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1479–R1485. doi: 10.1152/ajpregu.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lateef DM, Washington MC, Raboin SJ, Roberson AE, Mansour MM, Williams CS, Sayegh AI. Duodenal myotomy blocks reduction of meal size and prolongation of intermeal interval by cholecystokinin. Physiol Behav. 2012;105:829–834. doi: 10.1016/j.physbeh.2011.10.018. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–R29. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-Lopez G. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav. 2011;105:71–76. doi: 10.1016/j.physbeh.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Reidelberger R, Haver A, Anders K, Apenteng B. Role of capsaicin-sensitive peripheral sensory neurons in anorexic responses to intravenous infusions of cholecystokinin, peptide YY-(3–36), and glucagon-like peptide-1 in rats. Am J Physiol Endocrinol Metab. 2014;307:E619–E629. doi: 10.1152/ajpendo.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A. Proposed terminology for the anatomy of the rat stomach. Gastroenterology. 1971;60:344–345. [PubMed] [Google Scholar]
- Roger T, Cabanie P, Ferre JP. Microscopic and functional anatomy of the ileal papilla and caecocolonic valve in the rat. Acta Anat (Basel) 1991;142:299–305. doi: 10.1159/000147206. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Amato A, Lentini L, Baldassano S, Mule F. Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice. Peptides. 2011;32:60–64. doi: 10.1016/j.peptides.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav. 2010;100:291–296. doi: 10.1016/j.physbeh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake. Prog Mol Biol Transl Sci. 2013a;114:343–370. doi: 10.1016/B978-0-12-386933-3.00010-8. [DOI] [PubMed] [Google Scholar]
- Sayegh AI. The role of cholecystokinin receptors in the short-term control of food intake. Prog Mol Biol Transl Sci. 2013b;114:277–316. doi: 10.1016/B978-0-12-386933-3.00008-X. [DOI] [PubMed] [Google Scholar]
- Sayegh AI, Washington MC, Johnson RE, Johnson-Rouse T, Freeman C, Harrison A, Lucas J, Shelby M, Fisher B, Willis W, Reeve JJ., Jr Celiac and the cranial mesenteric arteries supply gastrointestinal sites that regulate meal size and intermeal interval length via cholecystokinin-58 in male rats. Horm Behav. 2014 doi: 10.1016/j.yhbeh.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Sayegh AI, Washington MC, Johnson RE, Johnson-Rouse T, Freeman C, Harrison A, Lucas J, Shelby M, Fisher B, Willis W, Reeve JJ., Jr Celiac and the cranial mesenteric arteries supply gastrointestinal sites that regulate meal size and intermeal interval length via cholecystokinin-58 in male rats. Horm Behav. 2015;67:48–53. doi: 10.1016/j.yhbeh.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes RL. Anatomy of the cecum of the laboratory mouse and rat. Anat Embryol (Berl) 1981;162:455–474. doi: 10.1007/BF00301871. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- Washington MC, Aglan AH, Sayegh AI. The stomach and/or upper duodenum contain sites of action that control meal size and intermeal interval length by exogenous rat gastrin releasing peptide. Peptides. 2014;55:41–46. doi: 10.1016/j.peptides.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302:R264–R273. doi: 10.1152/ajpregu.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]