ABSTRACT
Tight junctions form a continuous intercellular barrier between epithelial cells that is required to separate tissue spaces and regulate selective movement of solutes across the epithelium. They are composed of strands containing integral membrane proteins (e.g., claudins, occludin and tricellulin, junctional adhesion molecules and the coxsackie adenovirus receptor). These proteins are anchored to the cytoskeleton via scaffolding proteins such as ZO-1 and ZO-2. In salivary glands, tight junctions are involved in polarized saliva secretion and barrier maintenance between the extracellular environment and the glandular lumen. This review seeks to provide an overview of what is currently known, as well as the major questions and future research directions, regarding tight junction expression, organization and function within salivary glands.
KEYWORDS: cell adhesion, epithelium, salivary glands, saliva secretion, tight junction
Introduction
In epithelial cells, tight junctions (TJs) are considered to be the principal structures that contribute to cell polarity by acting as a barrier preventing lateral movement of proteins between the apical and basolateral membranes.1 TJs also form the primary barrier against paracellular diffusion of solutes,2 thus maintaining the selective transepithelial ion gradients needed for saliva secretion.3 Moreover, TJs serve as targets and effectors of signaling pathways that control gene expression, cell differentiation and proliferation.4 TJs appear as continuous belt-like structures in electron microscopy pictures5 and as a branching anastomosing network of strands in freeze fracture replicas.6 These strands are composed of transmembrane proteins embedded within plasma membranes of neighboring cells in which extracellular domains interact to seal the intercellular junctions.7 The main TJ proteins are claudins, occludin, tricellulin, junctional adhesion molecules (JAMs) and the coxsackie adenovirus receptor (CAR).8 These transmembrane proteins are linked to the actin cytoskeleton via membrane-anchored scaffolding proteins such as ZO-1 and ZO-2,9 indicating that the TJ complex is regulated by the actin cytoskeleton.10 In salivary glands, TJs allow unidirectional saliva secretion and maintain a cellular barrier between blood and tissue fluids.11 Several studies have shown that TJ expression and organization change during physiological and pathological processes. For instance, salivary gland acini allow paracellular flux of water while ducts remain impermeable to water during saliva secretion.12 In Sjögren’s syndrome (SS, an autoimmune disease that causes salivary gland hypofunction), TJ expression and organization are altered,13,14 indicating that they have a significant role in maintaining intact salivary gland functioning. This review seeks to provide an overview of what is currently known, as well as the major questions and future research directions, regarding tight junction expression, organization and function within salivary glands.
Structure of salivary glands
Salivary glands comprise multiple secretory units (defined as acini) that are connected to the oral cavity by way of salivary ducts. Humans and rodents possess 3 pairs of major salivary glands (parotid, submandibular and sublingual) as well as hundreds of minor salivary glands located throughout the oral cavity.15 Salivary glands are also classified according to their secretory product. For instance, serous glands produce a watery secretion comprised almost exclusively of proteins,16 while mucous glands produce a viscous secretion rich in glycoproteins (i.e., mucins).16 Likewise, there are also mixed serous/mucous glands that produce both types of secretion. The cells of the serous acini have a conical shaped appearance and the nucleus is round and centrally located in the cytoplasm; by contrast, the cells of the mucous acini have a flattened nucleus, as the mucous secretion tends to push it to the periphery of the acinus.15 Parotid glands are predominantly serous and both sublingual and minor salivary glands are almost exclusively mucous. Approximately 90% of saliva is produced by the major salivary glands, with the remainder deriving from the minor salivary glands.17
Myoepithelial cells are thin and spindle-shaped and are sandwiched between the acinar and small ductal epithelial cells, at one level, and the basement membrane at another. They display features of both smooth muscle and epithelium, such as numerous microfilaments with focal densities in the cytoplasmic processes and desmosomes, which attach them to the epithelial cells. The cells of the intercalated duct are cuboidal and mainly serve to connect acini with ducts. The cells from the striated duct posses basal infoldings at the basolateral side of the plasma membrane (typical of ion-pumping activity by the numerous mitochondria) and connect the intercalated ducts to the interlobular duct.16 The structure of the salivary glands and the presence of TJs within them are summarized in Figure 1.
Figure 1.
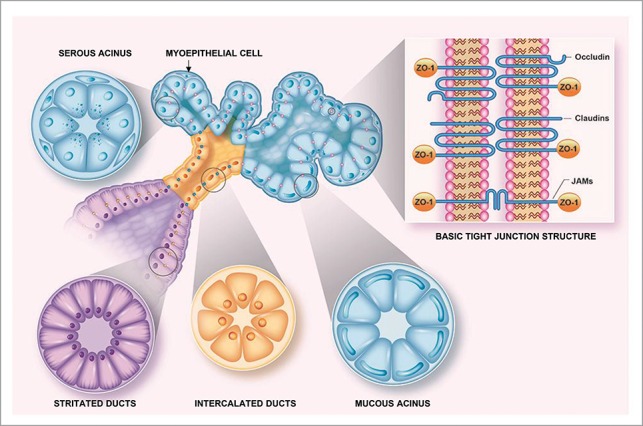
Tight junction localization in salivary glands. This diagram depicts the basic structure of a salivary gland. In it, TJs are represented in contrasting colors on the basis of their localization within the gland (i.e., pink within the acinus, blue in intercalated ducts and yellow in striated ducts) to illustrate changes in TJ composition due to differing functions within various salivary gland compartments. Finally, the inset shows tight junction transmembrane proteins (i.e., occludin, claudins and JAM family members) that are linked to the cytoskeleton via cytoplasmic ZO-1.
Saliva secretion
Saliva allows for the performance of basic functions within the oral cavity (e.g., swallowing, digestion and prevention of oral infections) and is a complex mixture composed primarily of ions, electrolytes, proteins, glycoproteins and some lipids.18 Saliva from the major salivary glands is first produced and secreted within the acini (stage 1) and then modified as it passes through the salivary ducts (stage 2).19 Fluid and electrolyte secretion in acinar cells is initiated through stimulation of parasympathetic nerves by the cholinergic agonist acetylcholine,20 whereas protein secretion is initiated through stimulation of sympathetic nerves by the β-adrenergic agonist adrenaline.21 During fluid and electrolyte secretion, acetylcholine stimulates G-protein coupled muscarinic receptors, resulting in increased intracellular-free calcium concentration [Ca2+]i as well as activation of Ca2+-dependent apical Cl- and basolateral K+ channels.19 The stimulated efflux of Cl- and K+ produces a transepithelial potential difference that triggers transcellular water secretion (via the water channel aquaporin-5) as well as paracellular Na+ and water diffusion (via TJs).12 Primary saliva becomes hypotonic as it passes through the ducts, due both to actions of reabsorption (of Na+ and Cl-) and secretion (of K+ and HCO3-).22 During protein secretion, adrenaline or norepinephrine stimulate G-protein-coupled β-adrenergic receptors, thereby increasing cyclic adenosine monophosphate (cAMP) and leading to exocytosis.21
Previous studies suggest that TJ composition and organization change not only during water secretion but also during secretory granule exocytosis. Regarding water secretion, studies using MDCK cells demonstrated that TJs are water permeable and that paracellular permeability is determined by the molecular composition of the TJs.23 Furthermore, a lack of aquaporin-5 causes reduction of water transport through both the plasma membrane and the salivary gland TJs.12 Concerning exocytosis, previous studies showed that the driving force required to complete the collapse of secretory granules is provided by the recruitment of F-actin and nonmuscle myosin II on the granule membranes in salivary glands.21 Likewise, studies involving intestinal epithelium demonstrated that F-actin and myosin contraction regulate TJs.24,25 Together, these studies indicate that water secretion and secretory granule exocytosis in salivary glands are regulated by changes in TJ composition and organization.
Transmembrane tight junction proteins
Several TJ transmembrane proteins have been detected in human and rodent salivary glands (e.g., claudins, occludin and JAMs) will be discussed in this section; their structure is shown in Figure 1 and their classification is summarized in Table 1.
Table 1.
Tight junction expression in salivary glands. This table summarizes TJs detected to date (both in salivary glands and related cell lines). Abbreviations within it are as follows: SMG: submandibular gland, PG: parotid gland, MSG: Major salivary glands, mSG: minor salivary glands, Par-C10 and Pa4: rat parotid cell lines, HSG: human submandibular cell line and MG: Matrigel.
| Claudin-1 | Human SMG | Human MSG | Human MSG Human Msg Rat PG | Par-C10 HSG | |
| Claudin-2 | Human SMG | Human MSG | Human MSG | SMG-C6 HSG on MG | |
| Claudin-3 | Human SMG Rabbit SMG | Human MSG Mouse MSG | Human MSG Mouse MSG | Par-C10 SMG-C6 HSG on MG | |
| Claudin-4 | Human SMG | Human MSG | Human MSG Rat PG Mouse SMG | Par-C10 SMG-C6 HSG on MG | |
| Claudin-5 | Human SMG | Human MSG Rat MSG | |||
| Claudin-6 | E mouse SMG | ||||
| Claudin-7 | Human SMG Mouse SMG | Human mSG | |||
| Claudin-8 | E mouse SMG | E mouse SMG | |||
| Claudin-10 | E mouse SMG E rat SMG | Par-C10 | |||
| Claudin-11 | Human SMG | Human mSG | |||
| Claudin-12 | Mouse SMG | ||||
| Claudin-16 | Human MSG | Human MGS | |||
| Occludin | Human MSG Human mSG Mouse SMG | Human MSG Human mSG Mouse SMG | Human MSG | Pa-4 Par-C10 HSG on MG | |
| JAM-A | Human MSG | Human MSG | Par-C10 HSG on MG | ||
| ZO-1 | Human SMG | Human MSG Human mSG Mouse SMG | Human MSG Human mSG Mouse SMG | Human MSG | Par-C10 SMG-C6 |
Claudins
Claudins, of which there are <27 members in humans and rodents, are the backbone of TJ stands in that they are capable of forming TJ without the aid of any other transmembrane TJ proteins.7 Claudins span the cellular membrane 4 times, with both N and C-terminal ends located within the cytoplasm.26 Their C-terminal ends vary among subtypes, as claudins posses multiple phosphorylation sites and binding domains.27 Additionally, they have 2 well-conserved extracellular loops that participate in a variety of homophilic and heterophilic interactions (on the basis of their location within a given tissue).26
Claudins can be functionally divided into 3 groups: a) those that seal the TJs (claudins 1, 3, 4, 5, 8, 11, 14 and 19), b) those that provide TJ paracellular permeability (claudins 2 and 10), and c) those that perform both functions (claudins 7, 12, 15 and 16).28 Structurally, claudins can be subdivided into 2 groups, one of which is highly homologous (claudin 1–10, 14, 15, 17 and 19) and the other which is non-homologous (claudin 11–13, 16, 18 and 20–27).29 Tissue-specific expression of various claudin combinations have been shown to determine barrier characteristics.30 Specifically, expression of claudin-5 tightly seals the paracellular TJ cleft,31 while claudin-2 facilitates paracellular ion permeability.32 The pore-forming function of specific claudins is mainly determined by the first extracellular loop,32 while the tightening of the paracellular TJ cleft is primarily regulated by the second of these loops.33 Claudin-claudin interactions in salivary glands have not yet been characterized; however, multiple claudin combinations may give rise to functional specificity in acinar and ductal cells and are likely to regulate salivary gland functions.11 To date, claudins 1-8, 10, 11, 12 and 16 have been detected in salivary glands and are detailed below.
Claudin-1
Claudin-1 was the first in its family to be identified34 and studies ablating claudin-1 expression in mice have demonstrated that this protein is essential for epidermal barrier functioning. Specifically, claudin-1-deficient mice lose high amounts of water due to compromised epidermal barrier (despite the presence of intact layering of keratinocytes). As a consequence, mice had wrinkled skin and died within 1 d of birth.35 Furthermore, the first extracellular loop of claudin-1 possesses a sequence motif that contributes to the regulation of TJ structure and function and has been shown to be critical for epithelial barrier integrity.36
Claudin-1 has been detected both in the intercalated and striated ducts from human major and minor salivary glands as well as in rat parotid glands.37,38,39 Functional studies using Par-C10 cells showed that claudin-1 downregulation was associated with disruption of TJ structure and function,40 and based on these studies, claudin-1 is thought to determine barrier function in serous salivary glands. However, future studies will be necessary to understand how claudin-1 interacts with the full range of claudins and TJs for salivary gland regulation.
Claudin-2
Similar to claudin-1, claudin-2 is capable of inducing the formation of networks of strands and grooves at cell–cell contact sites when introduced into fibroblasts lacking TJs.34 Claudin-2 is mostly expressed in cation-leaky epithelia (e.g., kidney proximal tubules); however, it is absent in the remaining distal nephrons, which are considered to be tight epithelia.23,41,42 Furthermore, mice that are deficient in this protein have reduced rates of reabsorption for Na+ in the proximal tubule, further supporting its role in paracellular transport.43
In human major salivary glands, claudin-2 is known to be expressed in mucous and serous acini as well as in striated and intercalated ducts;39 by contrast, it has not been detected in minor salivary glands.37,38 Previous studies have shown that claudin-2 contributes to permeability of water and Na+ in the kidney proximal tubules.42,43 Therefore, it is likely that this permeability function may also occur in salivary gland acini; however, future studies will be needed for confirmation.
Claudin-3
Claudin-3 expression has been demonstrated in respiratory, urinary, gastrointestinal and mammary epithelia.7 Additionally, this protein has been shown to be part of the blood-brain and blood-testis barriers.44,45 Studies using kidney epithelium as a model have determined that claudin-3 acts as a sealing component of the TJ for ions of both charged and uncharged solutes.46 In human and mouse salivary glands, claudin-3 is expressed in mucous and serous acini as well as in intercalated and striated ducts.37-39 Claudin-3 is expressed in the SMG-C6 rat submandibular cell line.40,47 Particularly, treatment of these cells with the pro-inflammatory cytokine TNF-α reduced transepithelial resistance (TER) and increased FITC-dextran flux. In these studies, claudin-3 was down-regulated and redistributed, whereas other claudin family members were unaffected, thereby demonstrating a selective effect for this protein in submandibular epithelial barrier function.47 Furthermore, overexpression of claudin-3 in these cells prevented effects on barrier function typically caused by TNF-α. Likewise, mechanistic studies demonstrated that TNF-α-mediated alteration of claudin-3 was dependent on Erk1/2 and slug signaling.47 These results suggest claudin-3 plays a key role in the barrier function of the salivary epithelium.
Claudin-4
Claudin-4 has been detected in diverse epithelia, including renal, lung, intestinal and epidermal cells.7 Previous studies have found that claudin-4 is specifically induced during epithelial repair, while higher claudin-4 expression levels are associated with intact barrier functioning in injured human lungs.48 Moreover, recent studies involving claudin-4 knockout mice suggest it may help to protect against acute lung injury.49
In human salivary glands, claudin-4 has been detected in both acinar and ductal cells,38,39 whereas in rat parotid and mouse submandibular glands claudin-4 was detected only in ductal cells.37,50 Studies involving SMG-C6 cells showed that claudin-4 is required for paracellular permeability during agonist-mediated fluid secretion51 and plays a crucial role in AMPK-modulated paracellular permeability in these cells.52 Taken together, these findings suggest that claudin-4 is involved in regulation of barrier functioning during agonist-stimulated secretion in salivary epithelium.
Claudin-5
Claudin-5 has been shown to be expressed in endothelial cells from brain and lung vasculatures. This protein is also expressed in liver and dermal vascular endothelia as well as in the blood-brain barrier.53 Claudin-5 is a key component of TJ strands, the role of which is to selectively decrease their permeability to ions.33 Furthermore, claudin-5 has been shown to improve paracellular solute and water movement across endothelial monolayers.53 The function of TJs relies largely on homo- and hetero-philic interactions of claudin-5 with other claudins.44 The expression of claudin-5 in the major salivary glands of humans and rats has been shown to be restricted to endothelial cells surrounding acinar and ductal cells.37,39 The function of claudin-5 in salivary glands is unknown; however, based on its location, it is likely involved in controlling nutrient supply from blood to salivary glands, a hypothesis that must be confirmed by further studies.
Claudin-6
Claudin-6 is mostly expressed during prenatal developmental stages.54 In adult mammalian tissues, claudin-6 was reported in the kidney,55 taste buds56 and mammary gland.57 In embryos and neonates, claudin-6 has been found in the kidney,55 liver 58 and periderm of the skin.59 Other studies have shown that claudin-6 is present in undifferentiated stem cells and its expression decreases together with a downregulation of the pluripotent factors Oct4, Nanog and Sox2.60 In mouse salivary glands, claudin-6 is expressed apically in the ducts at embryonic day 16 and completely absent after birth.50 These studies suggest that claudin-6 may play a role in epithelial differentiation from stem cells; however, future studies will be needed to determine whether claudin-6 is also involved in salivary gland morphogenesis.
Claudin-7
Claudin-7 is expressed in various epithelial tissues, with the lung and the aldosterone-sensitive distal nephron of the kidney having the highest levels.61,62 Mice lacking claudin-7 were shown to have urinary salt wasting, dehydration, and growth retardation as well as to die within 12 d of birth.63 It appears to act as a paracellular Cl- pore, given that the distal nephron mediates electrogenic Na+ reabsorption and is accompanied by passive paracellular Cl- transport. Another unique feature of claudin-7 is that it is strongly expressed in the basolateral membrane of many tissues and interacts with various adhesion molecules, all of which suggests a role in regulating cell-cell adhesion and cell motility.64
In human minor salivary glands, claudin-7 is expressed in ductal cells across the lifespan (i.e., from the early developmental stages through adulthood).38 Finally, previous studies in parotid glands have shown that mice lacking the water channel aquaporin-5 displayed claudin-7 downregulation.12 These results further indicate that claudin-7 plays an important role in the regulation of water transport in salivary glands.
Claudin-8
Claudin-8 is expressed in intestinal, kidney, inner ear, mammary and bladder epithelium.61,65,66 The function of this protein can be highlighted in mice lacking claudin-8, which have been shown to develop hypotension, hypochloremia, and metabolic alkalosis.67 Claudin-8 functions as anion-selective channel and as a Na+ barrier or a Cl- pore.68. In mouse submandibular glands, claudin-8 has been detected in the ducts and terminal tubules at both the pre- and post-natal stages;50 however, further research is needed to determine its function and expression patterns in human salivary glands.
Claudin-10
Claudin-10 is expressed in a variety of tissues, including the kidney, intestine, lung and heart.69 The 2 isoforms of claudin-10 are 10a and 10b; while 10a serves as an anion pore, claudin-10b acts as a strong cation-permeating channel.69 In mouse submandibular glands, claudin-10 is expressed in the terminal tubules, where it is co-localized with ZO-1;50 however, studies involving rat major salivary glands indicate that claudin-10 is also present at the basolateral region of acinar cells, demonstrating an ectopic subcellular localization where TJ strands do not exist.70
Claudin-11
Claudin-11 appears to have a wide variety of functions in mammals. First, it determines the permeability between layers of myelin sheaths.71 Second, claudin-11 is present in Sertoli cells and is apparently involved in spermatogenesis,72 based on the observation that male claudin-11 knockout mice lose spermatocyte differentiation and are consequently sterile.72 Third, these mice display inner ear deafness due to a disappearance of TJs from the basal cells and the resulting loss of endocochlear potential, thereby indicating a further role of claudin-11 in hearing.73 Taken together, these studies indicate the claudin-11 possesses a wide variety of functions depending upon the cell system in which it is involved. As for the specific role in this protein in salivary glands, relatively little is known. To date, we know that claudin-11 is expressed in cytoplasm from ductal cells but is absent in acinar cells38 and that it is also expressed in the terminal tubules (precursors of acini) and ducts, where it is co-localized with ZO-1.50 Consequently, further investigation of the role of this protein is warranted (particularly in light of its varied expression patterns, as detailed above).
Claudin-12
Claudin-12 is atypical in that its extracellular loops display a low level of homology with other claudins. Furthermore, this protein is unlikely to interact with ZO-1, as it lacks the C-terminal PDZ binding domain found in other claudins.29 Claudin-12 has been localized in the blood-brain barrier, inner ear and intestinal epithelium74 and it appears necessary for vitamin D-dependent Ca2+ absorption in intestinal epithelium.75 Regarding its role in salivary glands, recent studies found that claudin-12 is expressed in mouse submandibular glands and that it was up-regulated in submandibular glands from NOD/ShiLtJ SS mouse model (as compared to healthy mice).14 These studies indicate a role for claudin-12 in salivary gland inflammation; however, future studies will be necessary to better understand the role of claudin-12 in salivary glands as well as in other tissues.
Claudin-16
Claudin-16 is expressed in several tissues (e.g., in the kidney,76 mammary glands77 and enamel)78 and mutations of this protein cause familial hypomagnesemia, hypercalciuria, and nephrocalcinosis.79 When claudin-16 is missing, magnesium does not return from the renal tubules to the blood and is lost in the urine,80 that in turn leads to hypomagnesemia. These studies indicate that claudin-16 provides a cation-selective channel in the renal tubule, which is significant because similar functions could occur in salivary glands.
In human major salivary glands, claudin-16 has been detected at the basolateral pole of the acini and the apical region of the ducts. While its significance for acini functioning is unknown, the ductal placement of this protein strongly suggests a role in calcium and magnesium transport.81 As such, further studies are needed to confirm the significance of cluadin-16 for ductal functioning and exploratory studies are warranted to determine its potential significance in the acini.
Occludin
Occludin is a transmembrane TJ protein formed by a multidomain tetraspan structure, with individual domains exhibiting distinct functions and regulatory features.82 The extended C-terminus is essential for occludin interactions with ZO-1, subsequently mediating its intracellular trafficking to the plasma membrane TJ site.83 The C-terminus also has essential signaling functions and mediates occludin dimerization,84 with previous studies suggesting a possible copolymerization with claudins for proper stabilization of TJ strands.85 Occludin is localized in a variety of tissues (e.g., in renal, intestinal and gastric epithelia as well as in endothelial and brain cells).86 There is an ongoing controversy regarding the roles of occludin in TJ regulation in various epithelia.87,88 Specifically, occludin knockout mice have not demonstrated permeability defects;88 likewise, siRNA mediated knockdown of occludin have not been shown to affect TJ structure and permeability.89 Furthermore, although overexpression of dominant-negative occludin constructs 90 or cell exposure to occludin-derived peptides appear to disrupt TJ,82 these effects may suggest high levels of hydrophobic polypeptides and do not reliably indicate the patterning of epithelial TJs.
In human major salivary glands, occludin was detected in ductal and acinar cells as well as in endothelial cells surrounding the epithelium.39 Furthermore, occludin has been shown to be expressed in acini and ducts from human minor salivary and mouse submandibular glands;14 likewise, this protein has been found in cell lines of salivary gland origin (e.g., in polarized Par-C10 and SMIE cells).40,91 Finally, mice lacking occludin showed loss of cytoplasmic granules in striated ducts from salivary glands,92 suggesting that occludin may control salivary gland phenotype in both acinar and ductal structures.
The functional role of occludin in salivary gland TJs has been demonstrated though studies involving polarized cell lines. In rat parotid Pa-4 cells, transfection of an oncogenic Raf-1 resulted in a complete loss of TJ function (and the acquisition of a stratified phenotype that lacked cell–cell contact growth control).93 Specifically, occludin and claudin-1 expression was downregulated and ZO-1 and E-cadherin organization patterns were altered. Interestingly, introduction of the human occludin gene into Raf-1–activated Pa-4 cells restored the monolayer phenotype and function.93 Another study involving murine submandibular gland carcinoma cells expressed an N-terminally truncated occludin construct that decreased TER and paracellular permeability to 4-42 kDa tracers.94 Together, the above results indicate that occludin plays a critical role in TJ barrier functioning within salivary epithelium.
Junctional adhesion molecules (JAM)
Junction adhesion molecules (JAM) are a family of proteins that includes JAM-A, JAM-B, JAM-C, JAM-4, JAM-L and CAR.95 Unlike occludin and claudins, JAM proteins have a single transmembrane domain.96 JAM-A has been detected in polarized epithelial and endothelial cells and is also believed to contribute to the adhesion and transmigration of monocytes through endothelial cells.97 In fact, studies involving JAM-A-deficient epithelial cells and JAM-A knockout mice indicate that this protein is an important regulator of epithelial paracellular permeability.98 More recently, it was shown to be required for establishment of viremia and viral spread to sites of secondary replication.99 In human major salivary glands, JAM-A is the only member of its family that has been detected in acini and ducts.39 Given the role of JAM-A in regulating paracellular permeability, it appears likely that a similar function may occur in salivary glands; however, further studies are needed to explore this possibility.
Tight junction plaque proteins
The TJ plaque is a region of cytoplasm underlying transmembrane TJs that contains multiple protein complexes with varied functions (e.g., scaffolding of membrane proteins, regulation of cytoskeletal organization and establishment of polarity and cell signaling).100 The TJ plaque is mainly formed by proteins containing PDZ domains that contribute to TJ assembly and epithelial barrier formation;100 however, TJ plaque also contains proteins lacking PDZ domains that are involved in the regulation of signaling.101 TJ plaque proteins that contain PDZ domains are: 1) ZO proteins: ZO-1, ZO-2 and ZO-3; 2) membrane-associated guanylate kinase inverted proteins (MAGIs): MAGI-1 and MAGI-3; 3) the multi-PDZ protein MUPP1; 4) the Ras target protein AF-6/afadin and 5) PAR proteins: PAR-3, PAR-6, PALS-1, and PATJ.102 TJ plaque proteins that do not contain PDZ domains are: 1) the cingulin and JACOP/paracingulin proteins, which seem to be specifically expressed in epithelial tissues and mainly interact with ZO-1; 2) angiomotin family members: Angiomotin (Amot), JEAP (Angiomotin-like-protein1) and MASCOT (Angiomotin-like-protein-2), which appear to be important for tissue morphogenesis; 3) small GTPases, which regulate assembly of TJs and 4) Symplekin (a protein with dual location in TJs and cell nucleus), which contribute to TJ integrity of the epithelial monolayer and cellular polarity.101 The TJ plaque proteins listed above are numerous; however, among them only the ZO-1 protein has been found in mammalian salivary gland epithelium to date (and for that reason the discussion will focus on this protein). As such, the unexplored TJ plaque proteins within salivary plaque present a much needed and likely fruitful area for future research.
ZO-1 is a scaffolding protein of the membrane-associated guanylate kinases (MAGUK) family. It possesses 3 PDZ domains, one SH3 domain, and one guanylate kinase (GuK) domain.103 ZO-1 also interacts with other cytoplasmic proteins (e.g., ZO-2 and ZO-3, which are homologues of ZO-1) and with the actin cytoskeleton,104 as well as with F-actin and transmembrane TJ proteins.105 In addition to their structural function at cell-cell contacts, ZO-1 appears to participate in the regulation of cell growth and proliferation.106 Furthermore, the amino acid sequences of ZO-1, ZO-2 and ZO-3 exhibit conserved functional nuclear localization and export motifs.107 ZO proteins also interact with dual residency proteins found within the plasma membrane and the nucleus,108 and its primary function is highlighted in studies involving use of ZO-1 knockout mice that die during gestation (highlighted by the fact that no viable embryos lacking this protein were found beyond embryonic day 11.5). Additionally, these mice showed disturbed yolk sac angiogenesis and delayed embryonic growth. Interestingly, deficiency of ZO-1 did not exert any effects on the localization of ZO-2/ZO-3 at junctional sites but did induce mislocalization of endothelial JAMs in the yolk sac,109 which might explain the disturbance of vascular development. ZO-1 is expressed in acini, ducts, and inter-glandular endothelial cells from human major salivary glands and mouse submandibular glands 14,39,81 and is also co-localized with claudin-16 at the excretory duct of human major salivary glands.81 ZO-1 has been used as a marker for salivary gland polarity and differentiation;110 however, further studies are needed to determine the molecular mechanisms by which ZO-1 modulates TJs in salivary glands.
Models available to study tight junctions in salivary glands
Several salivary gland epithelial cells lines are available to the research community for the study of TJs. Specifically, rat parotid Par-C10 and Par-C5 cell lines, rat submandibular SMG-C6, SMG-C10 and SMIE cell lines, the human parotid HSY cell line as well as the human submandibular HSG cell line have each been useful for understanding the barrier functioning of salivary epithelium.111 Par-C10 cells form polarized monolayers (expressing occludin, claudin-1, -3, -4, -10, JAM-A and ZO-1) when cultured on permeable supports (i.e., in a 2-dimensional culture) and acinar spheres when single cells are grown on Matrigel (i.e., in a 3-dimensional culture).40,112 Studies using SMG-C6 have demonstrated that claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptors in epithelial cells,51 while additional studies with the same cell line have indicated that claudin-3 plays a role in TNF-α-modulated paracellular permeability.47 Moreover, SMIE cells have been shown to display selective barrier functioning and fluid transport,91,113 while it has been demonstrated that HSY cells form polarized monolayers that respond to growth factors related to salivary gland morphogenesis.114 Finally, HSG cells are known to express TJ proteins (i.e., occludin, claudin-1, -2, -3, -4, JAM-A and ZO-1) as well and aquaporin-5 when grown on permeable supports and coated with Matrigel but do not display these features when grown on plastic.115 In summary, the above models have proven useful for examining TJ expression, morphology, organization, ion transport and barrier functioning and present many options for future research on TJs (i.e., individual properties and interactions) in salivary glands.
Several recent studies have demonstrated that TJs can be formed from primary salivary in vitro,110,116-119 an advance which can be used to study barrier function of the salivary epithelium in a system that more closely resembles native tissue. Furthermore, the use of knockout mice for the various TJ proteins will also prove useful in determining the role of these proteins for in vivo salivary gland functioning. Finally, further study is needed to determine the role of specific TJs in modulating fluid secretion and secretory granule exocytosis.
Tight junctions in salivary gland dysfunction
SS is an autoimmune disease characterized by chronic inflammation of salivary and lacrimal glands that progressively decreases secretion of saliva and tears, thereby leading to dryness in the mouth and eyes. 120 The diminished function of exocrine glands in SS is commonly associated with extensive lymphocytic infiltration, acinar destruction, and local production of proinflammatory cytokines (e.g., tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, IL-6, and IL-10).121 Despite extensive molecular, histological, and clinical studies, the cause and cure for SS remain largely unknown. Alternation of TJ expression and organization has been demonstrated in salivary glands with SS, suggesting a central role for these proteins in the study of salivary gland dysfunction. Specifically, ZO-1 and occludin were shown to be down-regulated, claudin-1 and claudin-4 were overexpressed in minor salivary glands from SS patients, and claudin-1 and -4 were redistributed from the apical to the basolateral side of acinar cells.13 Moreover, SS-related pro-inflammatory cytokines have been shown to compromise TJ integrity, thereby resulting in salivary epithelial dysfunction in vitro.40,112 Particularly, the pro-inflammatory cytokines TNF-α and/or IFN-γ have been shown to disrupt barrier functioning in Par-C10 cell monolayers. Such disruption is associated with changes in cell and TJ morphology as well as decreased expression of the TJ protein claudin-1, alterations that correlate with decreases in TER and agonist-induced anion secretion along with increases in paracellular permeability of normally impermeant proteins.40 Thus, TNF-α and IFN-γ contribute to secretory dysfunction in SS by disrupting TJ integrity. Taken together, these studies suggest that TJ structure and function are altered in connection with SS; however, further investigation is needed to confirm and extend these findings.
Finally, TJs have not been studied in relation to several important salivary gland conditions (e.g., salivary gland cancer, hyposalivation due to head and neck radiation therapy related to this and other cancers, medications with xerostomia as a side effect, and all developmental disorders with a salivary gland component). Such areas of study are wide open for future investigation and offer a high degree of reward for efforts to pursue them.
Conclusion
TJ expression patterns in human and rodent salivary glands have been detailed and the current models available for the study of barrier functioning have been described in this review. The benefits of studying TJs in salivary glands is well-established; moreover, rapidly improving tissue culture techniques and bioengineering approaches, together with the availability of knockout mouse models for many of the TJs, offer the promise of continued advances for this endeavor.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the NIH-NIDCR grants R01DE022971, R01DE021697, R01DE021697S1 and R01DE021697S2.
References
- [1].Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol 2006; 16:181-8; PMID:16537104; http://dx.doi.org/ 10.1016/j.tcb.2006.02.006 [DOI] [PubMed] [Google Scholar]
- [2].Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci 2001; 16:126-30; PMID:11443232 [DOI] [PubMed] [Google Scholar]
- [3].Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA, Melvin JE. Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 2015; 94:715-21; PMID:25680367; http://dx.doi.org/ 10.1177/0022034515570943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farkas AE, Capaldo CT, Nusrat A. Regulation of epithelial proliferation by tight junction proteins. Ann N Y Acad Sci 2012; 1258:115-24; PMID:22731724; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06556.x [DOI] [PubMed] [Google Scholar]
- [5].Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17:375-412; PMID:13944428; http://dx.doi.org/ 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Claude P, Goodenough DA. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol 1973; 58:390-400; PMID:4199658; http://dx.doi.org/ 10.1083/jcb.58.2.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Capaldo CT, Nusrat A. Claudin switching: Physiological plasticity of the Tight Junction. Semin Cell Dev Biol 2015; 42:22-9; PMID:25957515; http://dx.doi.org/ 10.1016/j.semcdb.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [8].Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 2008; 27:6930-8; PMID:19029935; http://dx.doi.org/ 10.1038/onc.2008.344 [DOI] [PubMed] [Google Scholar]
- [9].Gonzalez-Mariscal L, Dominguez-Calderon A, Raya-Sandino A, Ortega-Olvera JM, Vargas-Sierra O, Martinez-Revollar G. Tight junctions and the regulation of gene expression. Semin Cell Dev Biol 2014; 36:213-23; PMID:25152334; http://dx.doi.org/ 10.1016/j.semcdb.2014.08.009 [DOI] [PubMed] [Google Scholar]
- [10].Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, Rao R. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2014; 306:G947-58; PMID:24722904; http://dx.doi.org/ 10.1152/ajpgi.00396.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baker OJ. Tight junctions in salivary epithelium. J Biomed Biotechnol 2010; 2010:278948; PMID:20182541; http://dx.doi.org/ 10.1155/2010/278948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kawedia JD, Nieman ML, Boivin GP, Melvin JE, Kikuchi K, Hand AR, Lorenz JN, Menon AG. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci U S A 2007; 104:3621-6; PMID:17360692; http://dx.doi.org/ 10.1073/pnas.0608384104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ewert P, Aguilera S, Alliende C, Kwon YJ, Albornoz A, Molina C, Urzúa U, Quest AF, Olea N, Pérez P, et al. Disruption of tight junction structure in salivary glands from Sjogren's syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum 2010; 62:1280-9; PMID:20131287; http://dx.doi.org/ 10.1002/art.27362 [DOI] [PubMed] [Google Scholar]
- [14].Mellas RE, Leigh NJ, Nelson JW, McCall AD, Baker OJ. Zonula occludens-1, occludin and E-cadherin expression and organization in salivary glands with Sjogren's syndrome. J Histochem Cytochem 2015; 63:45-56; PMID:25248927; http://dx.doi.org/ 10.1369/0022155414555145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci 2014; 24:1-13; PMID:24862590; http://dx.doi.org/ 10.1159/000358776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tandler B. Introduction to mammalian salivary glands. Microsc Res Tech 1993; 26:1-4; PMID:8219370; http://dx.doi.org/ 10.1002/jemt.1070260102 [DOI] [PubMed] [Google Scholar]
- [17].Tandler B, Gresik EW, Nagato T, Phillips CJ. Secretion by striated ducts of mammalian major salivary glands: review from an ultrastructural, functional, and evolutionary perspective. The Anatomical Record 2001; 264:121-45; PMID:11590591; http://dx.doi.org/ 10.1002/ar.1108 [DOI] [PubMed] [Google Scholar]
- [18].Carpenter GH. The secretion, components, and properties of saliva. Annu Rev Food Sci Technol 2013; 4:267-76; PMID:23464573; http://dx.doi.org/ 10.1146/annurev-food-030212-182700 [DOI] [PubMed] [Google Scholar]
- [19].Catalan MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest 2009; 56 Suppl:192-6; PMID:20224180; http://dx.doi.org/ 10.2152/jmi.56.192 [DOI] [PubMed] [Google Scholar]
- [20].Proctor GB. Muscarinic receptors and salivary secretion. J Appl Physiol 2006; 100:1103-4; PMID:16540706; http://dx.doi.org/ 10.1152/japplphysiol.01546.2005 [DOI] [PubMed] [Google Scholar]
- [21].Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci U S A 2011; 108:13552-7; PMID:21808006; http://dx.doi.org/ 10.1073/pnas.1016778108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martinez JR, Holzgreve H, Frick A. Micropuncture study of submaxillary glands of adult rats. Pflugers Arch Gesamte Physiol Menschen Tiere 1966; 290:124-33; PMID:5233669; http://dx.doi.org/ 10.1007/BF00363690 [DOI] [PubMed] [Google Scholar]
- [23].Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 2010; 123:1913-21; PMID:20460438; http://dx.doi.org/ 10.1242/jcs.060665 [DOI] [PubMed] [Google Scholar]
- [24].Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Frontiers in bioscience : a journal and virtual library 2008; 13:6662-81; PMID:18508686; http://dx.doi.org/ 10.2741/3180 [DOI] [PubMed] [Google Scholar]
- [25].Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010; 177:512-24; PMID:20581053; http://dx.doi.org/ 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci 2000; 915:129-35; PMID:11193568; http://dx.doi.org/ 10.1111/j.1749-6632.2000.tb05235.x [DOI] [PubMed] [Google Scholar]
- [27].Li J, Li YX, Chen MH, Li J, Du J, Shen B, Xia XM. Changes in the phosphorylation of claudins during the course of experimental colitis. Int J Clin Exp Pathol 2015; 8:12225-33; PMID:26722407 [PMC free article] [PubMed] [Google Scholar]
- [28].Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol 2012; 2:1819-52; PMID:23723025 [DOI] [PubMed] [Google Scholar]
- [29].Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525-69; PMID:23589827; http://dx.doi.org/ 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta 2008; 1778:631-45; PMID:18036336; http://dx.doi.org/ 10.1016/j.bbamem.2007.10.018 [DOI] [PubMed] [Google Scholar]
- [31].Staat C, Coisne C, Dabrowski S, Stamatovic SM, Andjelkovic AV, Wolburg H, Engelhardt B, Blasig IE. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015; 54:9-20; PMID:25907035; http://dx.doi.org/ 10.1016/j.biomaterials.2015.03.007 [DOI] [PubMed] [Google Scholar]
- [32].Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. Claudin-2-dependent paracellular channels are dynamically gated. Elife 2015; 4:e09906; http://dx.doi.org/ 10.7554/eLife.09906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci 2010; 67:2131-40; PMID:20333434; http://dx.doi.org/ 10.1007/s00018-010-0332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539-50; PMID:9647647; http://dx.doi.org/ 10.1083/jcb.141.7.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 2002; 156:1099-111; PMID:11889141; http://dx.doi.org/ 10.1083/jcb.200110122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mrsny RJ, Brown GT, Gerner-Smidt K, Buret AG, Meddings JB, Quan C, Koval M, Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol 2008; 172:905-15; PMID:18349130; http://dx.doi.org/ 10.2353/ajpath.2008.070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peppi M, Ghabriel MN. Tissue-specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat 2004; 205:257-66; PMID:15447685; http://dx.doi.org/ 10.1111/j.0021-8782.2004.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lourenco SV, Coutinho-Camillo CM, Buim ME, Uyekita SH, Soares FA. Human salivary gland branching morphogenesis: morphological localization of claudins and its parallel relation with developmental stages revealed by expression of cytoskeleton and secretion markers. Histochem Cell Biol 2007; 128:361-9; PMID:17687562; http://dx.doi.org/ 10.1007/s00418-007-0322-6 [DOI] [PubMed] [Google Scholar]
- [39].Maria OM, Kim JW, Gerstenhaber JA, Baum BJ, Tran SD. Distribution of tight junction proteins in adult human salivary glands. J Histochem Cytochem 2008; 56:1093-8; PMID:18765838; http://dx.doi.org/ 10.1369/jhc.2008.951780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol 2008; 295:C1191-201; PMID:18768927; http://dx.doi.org/ 10.1152/ajpcell.00144.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002; 115:4969-76; PMID:12432083; http://dx.doi.org/ 10.1242/jcs.00165 [DOI] [PubMed] [Google Scholar]
- [42].Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 2001; 281:F966-74; PMID:11592954; http://dx.doi.org/ 10.1152/ajprenal.0021.2001 [DOI] [PubMed] [Google Scholar]
- [43].Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 2010; 107:8011-6; PMID:20385797; http://dx.doi.org/ 10.1073/pnas.0912901107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, Wolburg H, Krause G, Piontek J. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem 2014; 289:7641-53; PMID:24478310; http://dx.doi.org/ 10.1074/jbc.M113.531012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chihara M, Ikebuchi R, Otsuka S, Ichii O, Hashimoto Y, Suzuki A, Saga Y, Kon Y. Mice stage-specific claudin 3 expression regulates progression of meiosis in early stage spermatocytes. Biol Reprod 2013; 89:3; PMID:23677978; http://dx.doi.org/ 10.1095/biolreprod.113.107847 [DOI] [PubMed] [Google Scholar]
- [46].Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta 2010; 1798:2048-57; PMID:20655293; http://dx.doi.org/ 10.1016/j.bbamem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- [47].Mei M, Xiang RL, Cong X, Zhang Y, Li J, Yi X, Park K, Han JY, Wu LL, Yu GY. Claudin-3 is required for modulation of paracellular permeability by TNF-alpha through ERK1/2/slug signaling axis in submandibular gland. Cell Signal 2015; 27:1915-27; PMID:26148935; http://dx.doi.org/ 10.1016/j.cellsig.2015.07.002 [DOI] [PubMed] [Google Scholar]
- [48].Rokkam D, Lafemina MJ, Lee JW, Matthay MA, Frank JA. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol 2011; 179:1081-7; PMID:21741940; http://dx.doi.org/ 10.1016/j.ajpath.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, Kim KJ, Crandall ED, Borok Z. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307:L524-36; PMID:25106430; http://dx.doi.org/ 10.1152/ajplung.00077.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hashizume A, Ueno T, Furuse M, Tsukita S, Nakanishi Y, Hieda Y. Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Dev Dyn 2004; 231:425-31; PMID:15366020; http://dx.doi.org/ 10.1002/dvdy.20142 [DOI] [PubMed] [Google Scholar]
- [51].Cong X, Zhang Y, Li J, Mei M, Ding C, Xiang RL, Zhang LW, Wang Y, Wu LL, Yu GY. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J Cell Sci 2015; 128:2271-86; PMID:25948584; http://dx.doi.org/ 10.1242/jcs.165878 [DOI] [PubMed] [Google Scholar]
- [52].Xiang RL, Mei M, Cong X, Li J, Zhang Y, Ding C, Wu LL, Yu GY. Claudin-4 is required for AMPK-modulated paracellular permeability in submandibular gland cells. J Mol Cell Biol 2014; 6:486-97; PMID:25503106; http://dx.doi.org/ 10.1093/jmcb/mju048 [DOI] [PubMed] [Google Scholar]
- [53].Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 1999; 147:185-94; PMID:10508865; http://dx.doi.org/ 10.1083/jcb.147.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Turksen K, Troy TC. Claudin-6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dyn 2001; 222:292-300; PMID:11668606; http://dx.doi.org/ 10.1002/dvdy.1174 [DOI] [PubMed] [Google Scholar]
- [55].Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T. Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1856-62; PMID:18367650; http://dx.doi.org/ 10.1152/ajpregu.00862.2007 [DOI] [PubMed] [Google Scholar]
- [56].Michlig S, Damak S, Le Coutre J. Claudin-based permeability barriers in taste buds. J Comp Neurol 2007; 502:1003-11; PMID:17447253; http://dx.doi.org/ 10.1002/cne.21354 [DOI] [PubMed] [Google Scholar]
- [57].Quan C, Lu SJ. Identification of genes preferentially expressed in mammary epithelial cells of Copenhagen rat using subtractive hybridization and microarrays. Carcinogenesis 2003; 24:1593-9; PMID:12896909; http://dx.doi.org/ 10.1093/carcin/bgg129 [DOI] [PubMed] [Google Scholar]
- [58].Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, Song X, Ding M, Deng H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol 2007; 81:12465-71; PMID:17804490; http://dx.doi.org/ 10.1128/JVI.01457-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Morita K, Furuse M, Yoshida Y, Itoh M, Sasaki H, Tsukita S, Miyachi Y. Molecular architecture of tight junctions of periderm differs from that of the maculae occludentes of epidermis. J Invest Dermatol 2002; 118:1073-9; PMID:12060405; http://dx.doi.org/ 10.1046/j.1523-1747.2002.01774.x [DOI] [PubMed] [Google Scholar]
- [60].Wang L, Xue Y, Shen Y, Li W, Cheng Y, Yan X, Shi W, Wang J, Gong Z, Yang G, et al. Claudin 6: a novel surface marker for characterizing mouse pluripotent stem cells. Cell Res 2012; 22:1082-5; PMID:22565286; http://dx.doi.org/ 10.1038/cr.2012.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 2004; 286:F1063-71; PMID:14722018; http://dx.doi.org/ 10.1152/ajprenal.00384.2003 [DOI] [PubMed] [Google Scholar]
- [62].Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol 2012; 302:L193-205; PMID:22003091; http://dx.doi.org/ 10.1152/ajplung.00349.2010 [DOI] [PubMed] [Google Scholar]
- [63].Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol 2010; 298:F24-34; PMID:19759267; http://dx.doi.org/ 10.1152/ajprenal.00450.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Heiler S, Mu W, Zoller M, Thuma F. The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities. Cell Commun Signal 2015; 13:29; PMID:26054340; http://dx.doi.org/ 10.1186/s12964-015-0105-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 2007; 215:147-59; PMID:17516019; http://dx.doi.org/ 10.1007/s00232-007-9014-3 [DOI] [PubMed] [Google Scholar]
- [66].Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem 2006; 54:933-44; PMID:16651389; http://dx.doi.org/ 10.1369/jhc.6A6944.2006 [DOI] [PubMed] [Google Scholar]
- [67].Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A 2015; 112:4340-5; PMID:25831548; http://dx.doi.org/ 10.1073/pnas.1421441112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A 2010; 107:18010-5; PMID:20921420; http://dx.doi.org/ 10.1073/pnas.1009399107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 2006; 291:F1288-99; PMID:16804102; http://dx.doi.org/ 10.1152/ajprenal.00138.2006 [DOI] [PubMed] [Google Scholar]
- [70].Inai T, Sengoku A, Guan X, Hirose E, Iida H, Shibata Y. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch Histol Cytol 2005; 68:349-60; PMID:16505581; http://dx.doi.org/ 10.1679/aohc.68.349 [DOI] [PubMed] [Google Scholar]
- [71].Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 1999; 145:579-88; PMID:10225958; http://dx.doi.org/ 10.1083/jcb.145.3.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99:649-59; PMID:10612400; http://dx.doi.org/ 10.1016/S0092-8674(00)81553-6 [DOI] [PubMed] [Google Scholar]
- [73].Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci 2004; 24:7051-62; PMID:15306639; http://dx.doi.org/ 10.1523/JNEUROSCI.1640-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 2004; 287:F305-18; PMID:15068973; http://dx.doi.org/ 10.1152/ajprenal.00341.2003 [DOI] [PubMed] [Google Scholar]
- [75].Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 2008; 19:1912-21; PMID:18287530; http://dx.doi.org/ 10.1091/mbc.E07-09-0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Khairallah H, El Andalousi J, Simard A, Haddad N, Chen YH, Hou J, Ryan AK, Gupta IR. Claudin-7, -16, and -19 during mouse kidney development. Tissue Barriers 2014; 2:e964547; PMID:25610756; http://dx.doi.org/ 10.4161/21688362.2014.964547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Markov AG, Kruglova NM, Fomina YA, Fromm M, Amasheh S. Altered expression of tight junction proteins in mammary epithelium after discontinued suckling in mice. Pflugers Arch 2012; 463:391-8; PMID:21975594; http://dx.doi.org/ 10.1007/s00424-011-1034-2 [DOI] [PubMed] [Google Scholar]
- [78].Bardet C, Courson F, Wu Y, Khaddam M, Salmon B, Ribes S, Thumfart J, Yamaguti PM, Rochefort GY, Figueres ML, et al. Claudin-16 Deficiency Impairs Tight Junction Function in Ameloblasts, Leading to Abnormal Enamel Formation. J Bone Miner Res 2016; 31(3):498–513; http://dx.doi.org 10.1002/jbmr.2726 [DOI] [PubMed] [Google Scholar]
- [79].Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Suláková T, Kuwertz-Bröking E, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 2001; 12:1872-81; PMID:11518780 [DOI] [PubMed] [Google Scholar]
- [80].Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 2007; 293:F166-77; PMID:17389678; http://dx.doi.org/ 10.1152/ajprenal.00087.2007 [DOI] [PubMed] [Google Scholar]
- [81].Kriegs JO, Homann V, Kinne-Saffran E, Kinne RK. Identification and subcellular localization of paracellin-1 (claudin-16) in human salivary glands. Histochem Cell Biol 2007; 128:45-53; PMID:17551748; http://dx.doi.org/ 10.1007/s00418-007-0291-9 [DOI] [PubMed] [Google Scholar]
- [82].Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777-88; PMID:8276896; http://dx.doi.org/ 10.1083/jcb.123.6.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ghassemifar MR, Sheth B, Papenbrock T, Leese HJ, Houghton FD, Fleming TP. Occludin TM4(-): an isoform of the tight junction protein present in primates lacking the fourth transmembrane domain. J Cell Sci 2002; 115:3171-80; PMID:12118072 [DOI] [PubMed] [Google Scholar]
- [84].McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 1996; 109 (Pt 9):2287-98; PMID:8886979 [DOI] [PubMed] [Google Scholar]
- [85].Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 2011; 193:565-82; PMID:21536752; http://dx.doi.org/ 10.1083/jcb.201010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev 2005; 57:883-917; PMID:15820558; http://dx.doi.org/ 10.1016/j.addr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- [87].Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta 2005; 1669:34-42; PMID:15842997; http://dx.doi.org/ 10.1016/j.bbamem.2005.01.008 [DOI] [PubMed] [Google Scholar]
- [88].Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor perspectives in biology 2009; 1:a002584; PMID:20066090; http://dx.doi.org/ 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 2011; 300:G1054-64; PMID:21415414; http://dx.doi.org/ 10.1152/ajpgi.00055.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Beeman N, Webb PG, Baumgartner HK. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell death & disease 2012; 3:e273; PMID:22361748; http://dx.doi.org/ 10.1038/cddis.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Michikawa H, Fujita-Yoshigaki J, Sugiya H. Enhancement of barrier function by overexpression of claudin-4 in tight junctions of submandibular gland cells. Cell Tissue Res 2008; 334:255-64; PMID:18855016; http://dx.doi.org/ 10.1007/s00441-008-0689-2 [DOI] [PubMed] [Google Scholar]
- [92].Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11:4131-42; PMID:11102513; http://dx.doi.org/ 10.1091/mbc.11.12.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 2000; 148:791-800; PMID:10684259; http://dx.doi.org/ 10.1083/jcb.148.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci 1999; 112 ( Pt 12):1879-88; PMID:10341207 [DOI] [PubMed] [Google Scholar]
- [95].Severson EA, Parkos CA. Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann N Y Acad Sci 2009; 1165:10-8; PMID:19538282; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04034.x [DOI] [PubMed] [Google Scholar]
- [96].Kostrewa D, Brockhaus M, D'Arcy A, Dale GE, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J 2001; 20:4391-8; PMID:11500366; http://dx.doi.org/ 10.1093/emboj/20.16.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A,, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998; 142:117-27; PMID:9660867; http://dx.doi.org/ 10.1083/jcb.142.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Monteiro AC, Parkos CA. Intracellular mediators of JAM-A-dependent epithelial barrier function. Ann N Y Acad Sci 2012; 1257:115-24; PMID:22671597; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lai CM, Boehme KW, Pruijssers AJ, Parekh VV, Van Kaer L, Parkos CA, Dermody TS. Endothelial JAM-A promotes reovirus viremia and bloodstream dissemination. J Infect Dis 2015; 211:383-93; PMID:25149763; http://dx.doi.org/ 10.1093/infdis/jiu476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci 2013; 126:1565-75; PMID:23418357; http://dx.doi.org/ 10.1242/jcs.113399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta 2008; 1778:601-13; PMID:18339298; http://dx.doi.org/ 10.1016/j.bbamem.2007.09.032 [DOI] [PubMed] [Google Scholar]
- [102].Garbett D, Bretscher A. The surprising dynamics of scaffolding proteins. Mol Biol Cell 2014; 25:2315-9; PMID:25122925; http://dx.doi.org/ 10.1091/mbc.E14-04-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem 2005; 74:219-45; PMID:15952887; http://dx.doi.org/ 10.1146/annurev.biochem.74.082803.133339 [DOI] [PubMed] [Google Scholar]
- [104].Ebnet K. Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 2008; 130:1-20; PMID:18365233; http://dx.doi.org/ 10.1007/s00418-008-0418-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006; 126:741-54; PMID:16923393; http://dx.doi.org/ 10.1016/j.cell.2006.06.043 [DOI] [PubMed] [Google Scholar]
- [106].Xu J, Lim SB, Ng MY, Ali SM, Kausalya JP, Limviphuvadh V, Maurer-Stroh S, Hunziker W. ZO-1 regulates Erk, Smad1/5/8, Smad2, and RhoA activities to modulate self-renewal and differentiation of mouse embryonic stem cells. Stem Cells 2012; 30:1885-900; PMID:22782886; http://dx.doi.org/ 10.1002/stem.1172 [DOI] [PubMed] [Google Scholar]
- [107].Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem 2003; 278:2692-700; PMID:12403786; http://dx.doi.org/ 10.1074/jbc.M206821200 [DOI] [PubMed] [Google Scholar]
- [108].Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol 2010; 2010:402593; PMID:20224657; http://dx.doi.org/ 10.1155/2010/402593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell 2008; 19:2465-75; PMID:18353970; http://dx.doi.org/ 10.1091/mbc.E07-12-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Maruyama CL, Leigh NJ, Nelson JW, McCall AD, Mellas RE, Lei P, Andreadis ST, Baker OJ. Stem Cell-Soluble Signals Enhance Multilumen Formation in SMG Cell Clusters. J Dent Res 2015; 94:1610-7; PMID:26285810; http://dx.doi.org/ 10.1177/0022034515600157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nelson J, Manzella K, Baker OJ. Current cell models for bioengineering a salivary gland: a mini-review of emerging technologies. Oral Dis 2013; 19:236-44; PMID:22805753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Baker OJ, Schulz DJ, Camden JM, Liao Z, Peterson TS, Seye CI, Petris MJ, Weisman GA. Rat Parotid Gland Cell Differentiation in Three-Dimensional Culture. Tissue Eng Part C Methods 2010;16(5):1135–44; PMID:20121592; http://dx.doi.org/ 10.1089/ten.TEC.2009.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].He X, Kuijpers GA, Goping G, Kulakusky JA, Zheng C, Delporte C, Tse CM, Redman RS, Donowitz M, Pollard HB, et al. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Arch 1998; 435:375-81; PMID:9426293; http://dx.doi.org/ 10.1007/s004240050526 [DOI] [PubMed] [Google Scholar]
- [114].Yamada A, Futagi M, Fukumoto E, Saito K, Yoshizaki K, Ishikawa M, Arakaki M, Hino R, Sugawara Y, Ishikawa M, et al. Connexin 43 Is Necessary for Salivary Gland Branching Morphogenesis and FGF10-induced ERK1/2 Phosphorylation. J Biol Chem 2016; 291:904-12; PMID:26565022; http://dx.doi.org/ 10.1074/jbc.M115.674663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Maria OM, Maria O, Liu Y, Komarova SV, Tran SD. Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol 2011; 43:622-31; PMID:21216302; http://dx.doi.org/ 10.1016/j.biocel.2011.01.001 [DOI] [PubMed] [Google Scholar]
- [116].Leigh NJ, Nelson JW, Mellas RE, McCall AD, Baker OJ. Three-dimensional cultures of mouse submandibular and parotid glands: a comparative study. J Tissue Eng Regen Med 2014; http://dx.doi.org/ 10.1002/term.1952. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Maria OM, Tran SD. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev 2011; 20:959-67; PMID:21187001; http://dx.doi.org/ 10.1089/scd.2010.0214 [DOI] [PubMed] [Google Scholar]
- [118].Maria OM, Zeitouni A, Gologan O, Tran SD. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A 2011; 17:1229-38; PMID:21189069; http://dx.doi.org/ 10.1089/ten.tea.2010.0297 [DOI] [PubMed] [Google Scholar]
- [119].Pradhan S, Liu C, Zhang C, Jia X, Farach-Carson MC, Witt RL. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryngol Head Neck Surg 2010; 142:191-5; PMID:20115973; http://dx.doi.org/ 10.1016/j.otohns.2009.10.039 [DOI] [PubMed] [Google Scholar]
- [120].Maslinska M, Przygodzka M, Kwiatkowska B, Sikorska-Siudek K. Sjogren's syndrome: still not fully understood disease. Rheumatol Int 2015; 35:233-41; PMID:24985362; http://dx.doi.org/ 10.1007/s00296-014-3072-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol 1994; 152:5532-9; PMID:8189070 [PubMed] [Google Scholar]


