ABSTRACT
The intestinal barrier is gaining increasing attention because it is related to intestinal homeostasis and disease. Different parameters have been used in the past to assess intestinal barrier functions in experimental studies; however most of them are poorly defined in healthy mice. Here, we compared a number of barrier markers in healthy mice, established normal values and correlations. In 48 mice (24 C57BL/6J, 24 BALB/cJ background), we measured mucus thickness, and expression of mucin-2, α-defensin-1 and -4, zonula occludens-1, occludin, junctional adhesion molecule-A, claudin-1, 2 and -5. We also analyzed claudin-3 and fatty acid binding protein-2 in urine and plasma, respectively. A higher expression of mucin-2 protein was found in the colon compared to the ileum. In contrast, the α-defensins-1 and -4 were expressed almost exclusively in the ileum. The protein expression of the tight junction molecules claudin-1, occludin and zonula occludens-1 did not differ between colon and ileum, although some differences occurred at the mRNA level. No age- or gender-related differences were found. Differences between C57BL/6J and BALB/cJ mice were found for α-defensin-1 and -4 mRNA expression, and for urine and plasma marker concentrations. The α-defensin-1 mRNA correlated with claudin-5 mRNA, whereas α-defensin-4 mRNA correlated with claudin-3 concentrations in urine. In conclusion, we identified a number of murine intestinal barrier markers requiring tissue analyses or measurable in urine or plasma. We provide normal values for these markers in mice of different genetic background. Such data might be helpful for future animal studies in which the intestinal barrier is of interest.
KEYWORDS: claudin, fatty acid binding protein, mucus, occludin, permeability
Introduction
An increasing number of gastrointestinal (GI) diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and celiac disease, as well as extra-intestinal diseases, such as asthma, arthritis, and the metabolic syndrome have been connected to an impairment of the intestinal barrier.1-3 Therefore, this barrier gains increasing attention and is now the subject of many studies. However, the tools used for studying the intestinal barrier vary extensively limiting the possibilities of comparing the results.3 Moreover, many intestinal barrier parameters have been poorly evaluated in both humans and animals used for experimental studies.
Mucus, epithelial tight junctions (TJ), and – on a functional level – defensins seem to be of particular importance for the intestinal barrier and have therefore been proposed as potential markers of mucosal permeability.4-10 They are regulated by various factors such as the intestinal microbiota,11 food,12,13 and intrinsic factors, including mucosal perfusion,14-16 nerves,17,18 and components of the immune system.19,20 Their usage is limited because the measurement requires intestinal tissue samples that cannot be easily obtained from healthy or diseased individuals. In mice, the availability of such samples is less problematic, but the measurements are hampered by a lack of standardization. For example, mucus measurements or the expression of tight junction molecules are poorly standardized and normal values in healthy mice are virtually lacking.
In the present study, we measured mucus thickness and the expression of a number of molecules related to the intestinal barrier in healthy mice of different age, sex and genetic background in order to propose parameters that might be suitable as biomarkers of the barrier, and to define normal values for them. Such data should be considered for future mouse studies on gut barrier function, and diseases associated with intestinal barrier dysfunction.
Results
In tissue samples, mucus thickness, Mucin-2 (Muc-2), claudin-1, occludin and zonula occludens-1 (ZO-1) protein expression, as well as mRNA expression of 6 tight junction molecules (ZO-1, occludin, junctional adhesion molecule-A (JAM-A), Claudin-1, 2, and 5) and 2 α-defensins-1 and 4 (Defa-1 and -4) were measured (for primers, see Table 1). Moreover, we measured claudin-3 in urine and intestinal fatty acid binding protein-2 (iFABP-2) in plasma and urine. No significant differences were found for all these parameters between male and female mice, or between young (10–20 weeks old) and old (6–12 months old) mice (data not shown). However, the genetic background (C57BL/6J or BALB/cJ) and in particular, the site of tissue collection (ileum or colon) mattered for some but not all parameters. Significant differences between C57BL/6J and BALB/cJ mice were only found for trefoil factor 3 (Tff3), Defa-1 and -4 expression, iFABP-2 concentrations in plasma, and for claudin-3 concentrations in urine, whereas significant differences between ileum and colon were found for Muc-2 protein expression, as well as for occludin, claudin-5, and Defa-1 and -4 mRNA expression (Table 2).
Table 1.
Primers used for real-time RT-PCR detection.
| Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|
| Claudin-1 | TCCTTGCTGAATCTGAACA | AGCCATCCACATCTTCTG |
| Claudin-2 | GTC ATC GCC CAT CAG AAG AT | ACT GTT GGA CAG GGA ACC AG |
| Claudin-5 | GCT CTC AGA GTC CGT TGA CC | CTG CCC TTT CAG GTT AGC AG |
| JAM-A | CACCTTCTCATCCAGTGGCATC | CTCCACAGCATCCATGT-GTGC |
| Muc-2 | GAT GGC ACC TAC CTC GTT GT | GTC CTG GCA CTT GTT GGA AT |
| Occludin | ACT CCT CCA ATG GAC AAG TG | CCC CAC CTG TCG TGT AGT CT |
| Tff3 | TCT GGC TAA TGC TGT TGG TG | CTC CTG CAG AGG TTT GAA GC |
| ZO-1 | CCA CCT CTG TCC AGC TCT TC | CAC CGG AGT GAT GGT TTT CT |
| 18s | GTA ACC CGT TGA ACC CCA TT | CCA TCC AAT CGG TAG TAG CG |
| Defa-1 | TCA AGA GGC TGC AAA GGA AGA GAA C | TGG TCT CCA TGT TCA GCG ACA GC |
| Defa-4 | CCA GGG GAA GAT GAC CAG GCT G | TGC AGC GAC GAT TTC TAC AAA GGC |
| ß-actin | GCT GAG AGG GAA ATC GTG CGT G | CCA GGG AGG AAG AGG ATG CGG |
For abbreviations, see text.
Table 2.
Median and 90% interval of parameters for assessment of the intestinal mucosal barrier function examined in healthy mice.
| C57BL/6J |
BALB/cJ |
|||||
|---|---|---|---|---|---|---|
| Loc. | N* | Median | 90% P | Median | 90% P | |
| Mucus thickness (µm) | Ileum | 24/24 | 10.6 | 7.90–14.2 | 10.9 | 9.25–13.0 |
| Mucus thickness (µm) | Colon | 24/24 | 10.7 | 9.03–14.2 | 10.9 | 8.97–15.5 |
| Muc-2 protein (% area) | Ileum | 23/24 | 6.58 | 0.48–23.6 | 8.37 | 3.06–20.0 |
| Muc-2 protein (% area) | Colon | 23/21 | 10.5 | 1.87–38.8 | 17.1 | 4.20–27.4 |
| Muc-2 mRNA (/18S) | Ileum | 23/23 | 2.43 | 1.00–14.3 | 2.58 | 1.00–5.15 |
| Muc-2 mRNA (/18S) | Colon | 24/24 | 2.06 | 1.00–12.6 | 1.94 | 1.00–6.22 |
| Tff3 mRNA (/18S) | Ileum | 24/24 | 1.53 | 1.00–2.88 | 2.44 | 1.00–7.38 |
| Tff3 mRNA (/18S) | Colon | 23/23 | 1.58 | 1.00–4.47 | 2.29 | 1.00–6.13 |
| Occludin mRNA (/18S) | Ileum | 24/24 | 1.80 | 1.00–3.92 | 1.67 | 1.00–3.41 |
| Occludin mRNA (/18S) | Colon | 24/24 | 2.44 | 1.00–7.38 | 2.06 | 1.00–4.65 |
| Occludin protein level | Ileum | 23/23 | 0.27 | 0.06–0.70 | 0.38 | 0.06–0.80 |
| Occludin protein level | Colon | 24/23 | 0.24 | 0.03–0.82 | 0.27 | 0.05–0.91 |
| ZO-1 mRNA (/18S) | Ileum | 23/24 | 1.89 | 1.00–5.46 | 2.07 | 1.00–4.38 |
| ZO-1 mRNA (/18S) | Colon | 23/24 | 2.30 | 1.00–6.40 | 2.30 | 1.00–7.61 |
| ZO-1 protein level | Ileum | 18/18 | 2.30 | 1.05–3.32 | 2.21 | 0.72–4.10 |
| ZO-1 protein level | Colon | 16/16 | 1.55 | 0.72–2.61 | 1.91 | 0.52–3.63 |
| Claudin-1 mRNA (/18S) | Ileum | 23/24 | 0.88 | 0.41–4.03 | 1.05 | 0.28–3.86 |
| Claudin-1 mRNA (/18S) | Colon | 17/20 | 0.42 | 0.16–4.47 | 0.50 | 0.14–5.95 |
| Claudin-1 protein level | Ileum | 23/24 | 0.35 | 0.07–0.56 | 0.29 | 0.04–0.57 |
| Claudin-1 protein level | Colon | 23/23 | 0.19 | 0.02–0.66 | 0.16 | 0.04–0.82 |
| Claudin-2 mRNA (/18S) | Ileum | 22/24 | 1.51 | 1.00–5.00 | 2.61 | 1.00–5.97 |
| Claudin-2 mRNA (/18S) | Colon | 23/24 | 1.67 | 1.00–5.27 | 2.47 | 1.00–5.35 |
| Claudin-5 mRNA (/18S) | Ileum | 23/24 | 1.83 | 1.00–9.15 | 1.44 | 1.00–4.06 |
| Claudin-5 mRNA (/18S) | Colon | 24/23 | 3.36 | 1.00–15.9 | 2.89 | 1.00–13.6 |
| JAM-A mRNA (/18S) | Ileum | 24/24 | 0.91 | 0.41–1.77 | 1.0 | 0.49–1.76 |
| JAM-A mRNA (/18S) | Colon | 20/20 | 0.43 | 0.15–6.26 | 0.52 | 0.10–6.87 |
| Defa-1 mRNA (/β-actin) | Ileum | 24/24 | 4.5×105 | 1.9×105 −8.7×105 | 1.5×105 | 6.8×104−5.9×105 |
| Defa-1 mRNA (/β-actin) | Colon | 24/24 | 2.1×103 | 1.2×102 −9.2×103 | 4.9×102 | 2.3×102 −1.1×104 |
| Defa-4 mRNA (/β-actin) | Ileum | 23/24 | 5.2×105 | 3.1×105 −7.5×105 | 9.28 | 1.23–100 |
| Defa-4 mRNA (/β-actin) | Colon | 24/24 | 18.6 | 4.19–132 | 0.00 | 0.0 –0.0 |
| Claudin3 protein (ng/ml) | Urine | 23/22 | 9.50 | 1.13–35.8 | 38.8 | 11.1–61.8 |
| iFABP-2 protein (ng/ml) | Urine | 23/22 | 0.36 | 0.13–5.67 | 0.38 | 0.15–1.53 |
| iFABP-2 protein (ng/ml) | Plasma | 23/22 | 34.2 | 6.75–107 | 18.1 | 3.48–32.9 |
Note. For each group, the median (50% percentile) and 90% percentile range (5–95% percentile) is shown. Abbreviations: P, percentile, others see text. *Number of animals for each mouse strain (C57BL/6J, BALB/cJ) for which data were available (extreme outliers were excluded). Statistics: Underlined results were significantly different between the 2 mouse strains; all P < 0.001. Results highlighted in gray were significantly different between tissue localization (ileum and colon); P < 0.001 for Defa-1 and Defa-4 in BALB/cJ mice, Defa-1 in C57BL/6J mice; all other P < 0.05.
Mucus thickness was about 10–12 µm in both ileum and colon, independent of the genetic background of the animals (Figs. 1A and 2). In contrast, a higher protein level of Muc-2 was found in the colon of C57BL/6J or BALB/cJ mice compared to the ileum, the differences being statistically significant (Figs. 1B and 2).
Figure 1.
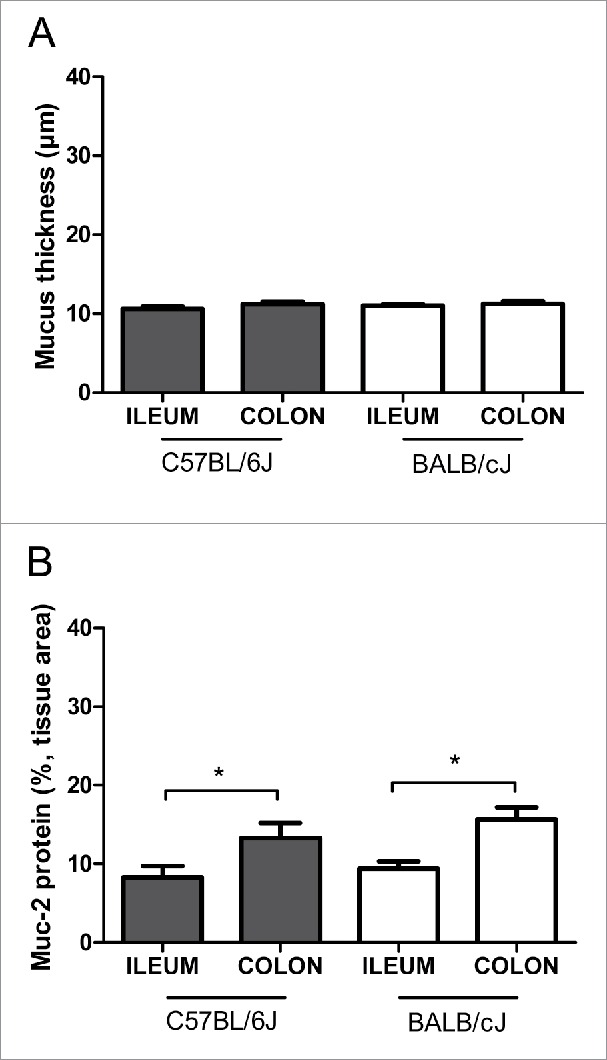
Intestinal mucus analysis in healthy mice. (A) Quantitative analysis of mucus thickness in ileum and colon using Alcian blue/periodic acid–Schiff's (AB/PAS) staining. (B) Densitometric analysis of Muc-2 protein expression in ileum and colon (expressed in % of tissue area colored brown, see Fig. 2). We analyzed healthy mice (female:male ratio 1:1) of 2 different strains (C57BL/6J or BALB/cJ mice) at different ages (10–20 weeks old or 6–12 months old). Neither age nor sex (not shown) nor strain differences were found. Statistics: mean ± SEM, N = 21–24 per group, *p < 0.05.
Figure 2.
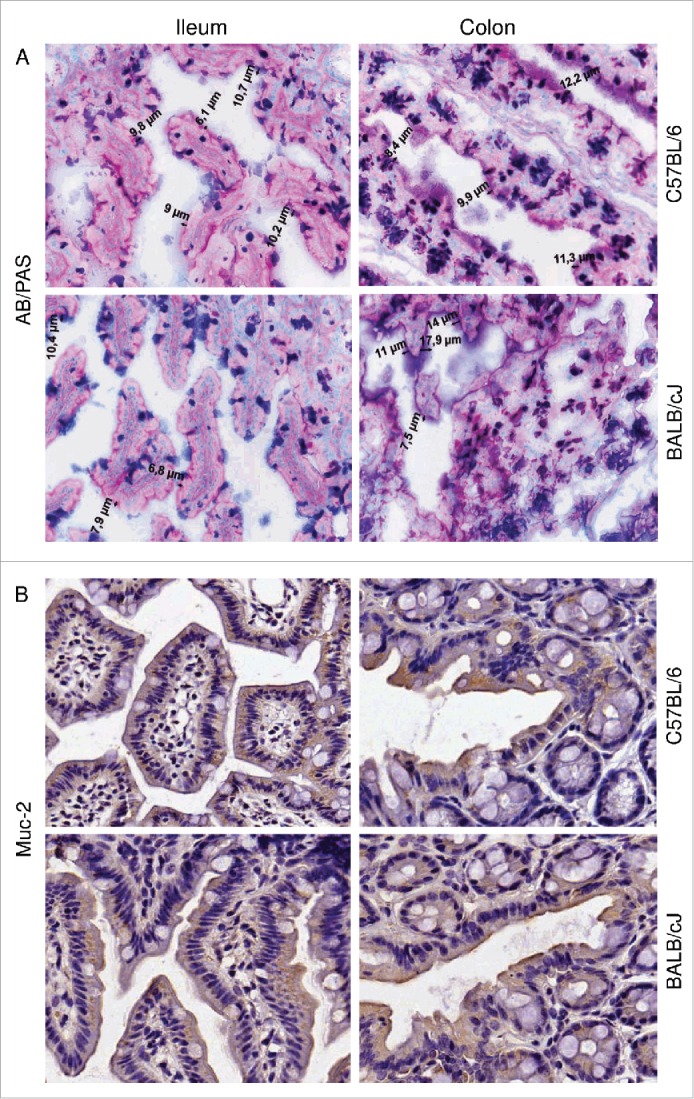
The total mucins and Muc-2 protein staining in healthy mice. (A) Representative photomicrographs showing mucus thickness (indicated in µm) in sections stained with Alcian blue/periodic acid–Schiff's (AB/PAS). (B) Immunohistochemical staining for Muc2. Results for the ileum (left panels) and colon (right panels) of C57BL/6J and BALB/cJ mice are shown (magnification 200x).
Tight junction mRNA expression was higher in the colon than in the ileum, as shown for occludin mRNA in C57BL/6J mice, and for claudin-5 mRNA in BALB/cJ mice (Fig. 3A and E). No significant differences between ileum and colon were found for ZO-1, claudin-1, -2 and JAM-A mRNA levels in both strains (Fig. 3B, C, D and F). The protein levels of the tight junction molecules occludin, ZO-1 and claudin-1, although differences were seen at the occludin mRNA level, did not differ between ileum and colon, neither in C57BL/6J nor in BALB/cJ mice (Fig. 4). When comparing mice of C57BL/6J and BALB/cJ background, no differences at all were found regarding all tight junction molecules analyzed. However, claudin-3 protein concentrations in urine were higher in BALB/cJ compared to C57BL/6J mice, and iFABP-2 concentrations in plasma (and by trend in urine) were higher in C57BL/6J mice compared to BALB/cJ mice (Fig. 5).
Figure 3.
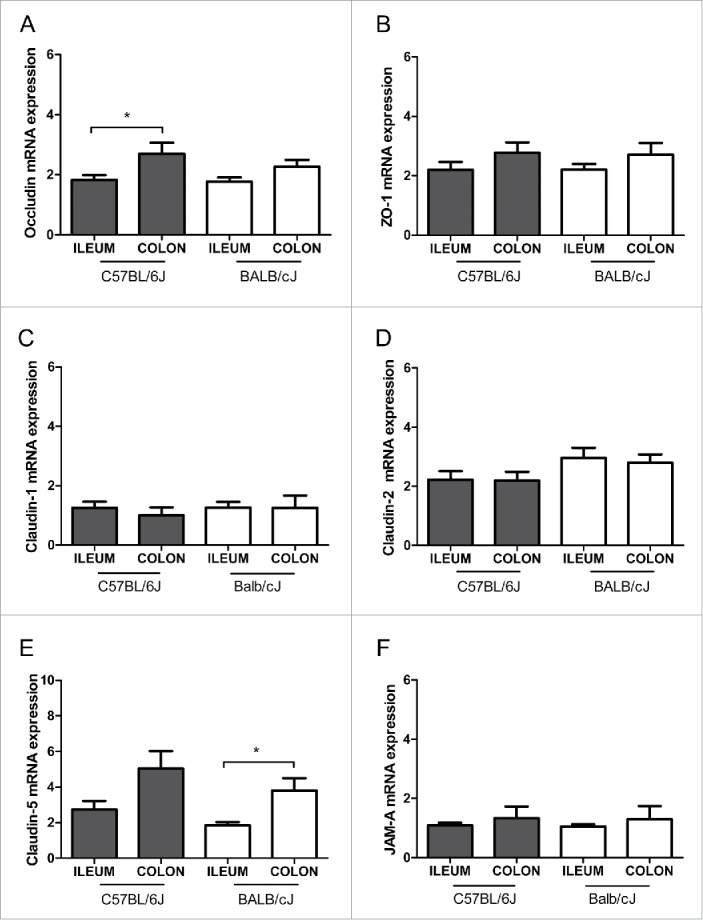
Tight junction mRNA expression in healthy mice. Relative mRNA expression of the tight junction molecules occludin (A), ZO-1 (B), claudin-1 (C), claudin-2 (D), claudin-5 (E) and junctional adhesion molecule-A (JAM-A) (F) are shown for the ileum and colon in C57BL/6J and BALB/cJ mice. The mRNA expression was normalized to 18S. Statistics: mean ± SEM, N = 17–24 per group, *p < 0.05.
Figure 4.
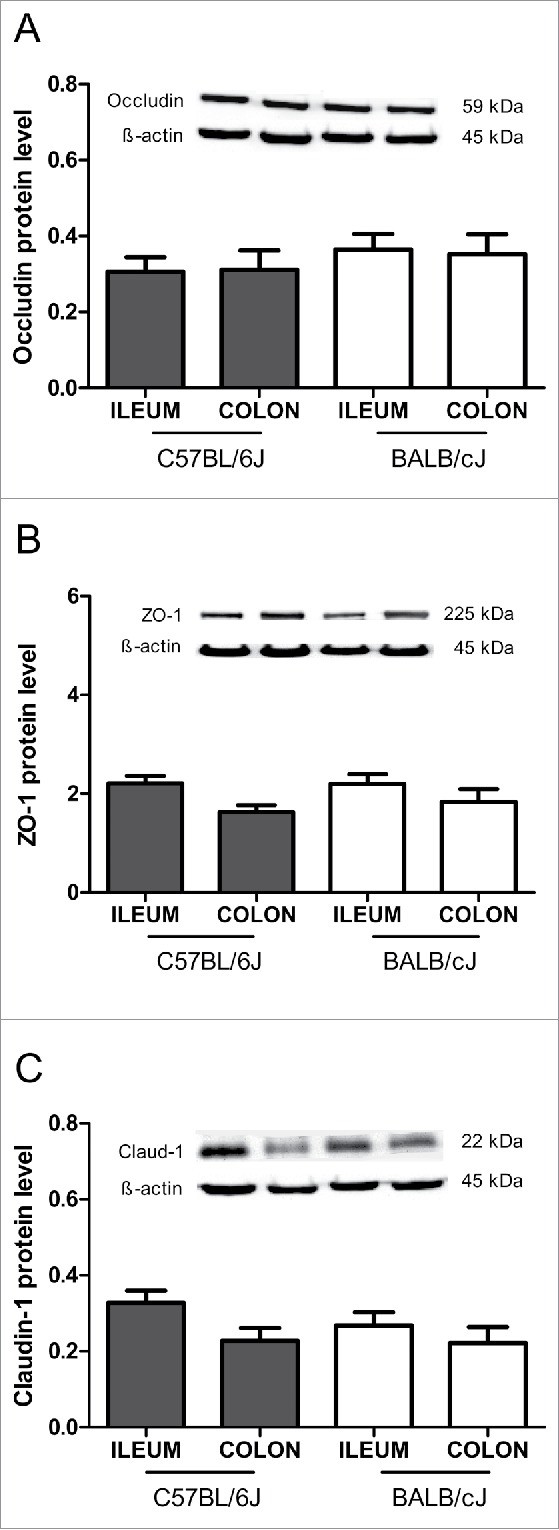
Tight junction protein levels in healthy mice. Immunoblot analysis of tight protein levels for occludin (A), zonula occludens-1 (ZO-1) (B) and claudin-1 (C) are shown for the ileum and colon in C57BL/6J and BALB/cJ mice. The protein levels were normalized to β-actin. Statistics: mean ± SEM, N = 16–24 per group.
Figure 5.
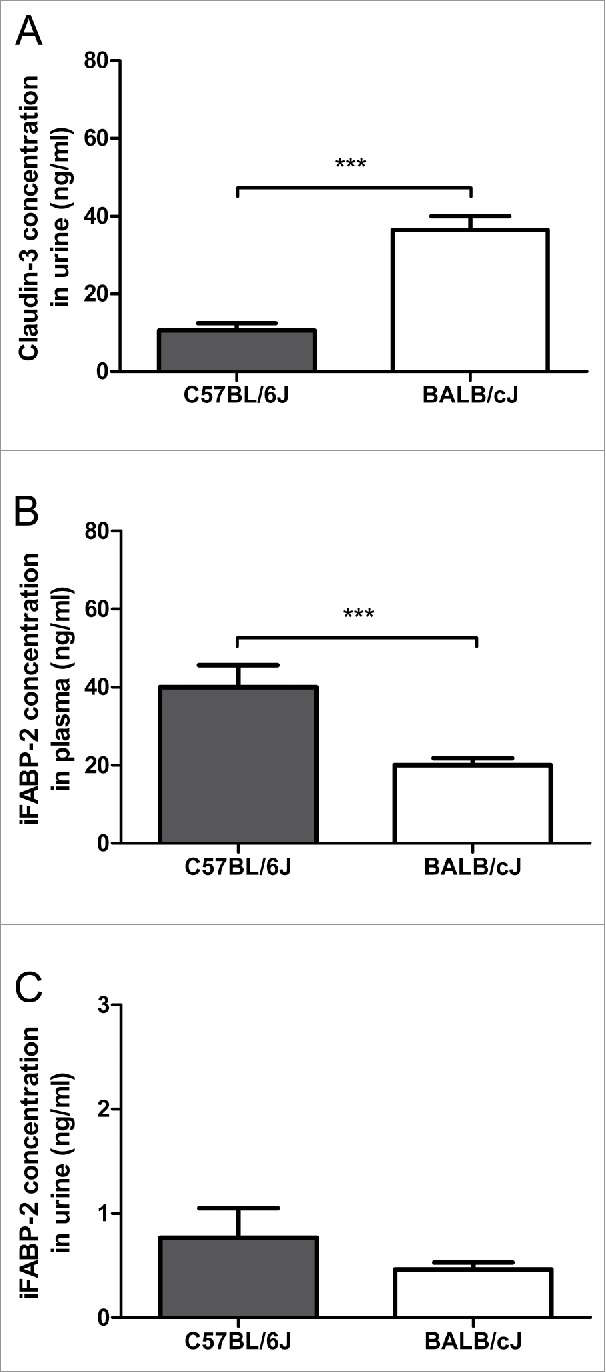
Systemic levels of intestinal barrier parameters. Levels of claudin-3 (A) and intestinal fatty acid binding protein (iFABP-2) (B and C) were measured in the urine (A, C) or plasma (B) of healthy mice of different strains (C57BL/6J and BALB/cJ). Statistics: mean ± SEM, N = 22–23 per group, ***p < 0.001.
We also measured α-defensins mRNA expression, because α-defensins have been proposed as being relevant for the intestinal barrier function. Defa-1 was found to be high expressed in the ileum of both C57BL/6J and BALB/cJ mice and less in the colon (Tab. 2). Defa-4 was distinctly higher expressed in the ileum (5.2 × 105 transcripts / 10 ng RNA) than in the colon (18.6 transcripts / 10 ng RNA) of C57BL/6J mice, but only in the ileum (at low levels 9.3 transcripts / 10 ng RNA) in BALB/cJ mice. Both defensins were less expressed in BALB/cJ mice compared to C57BL/6J mice (Fig. 6, Table 2).
Figure 6.
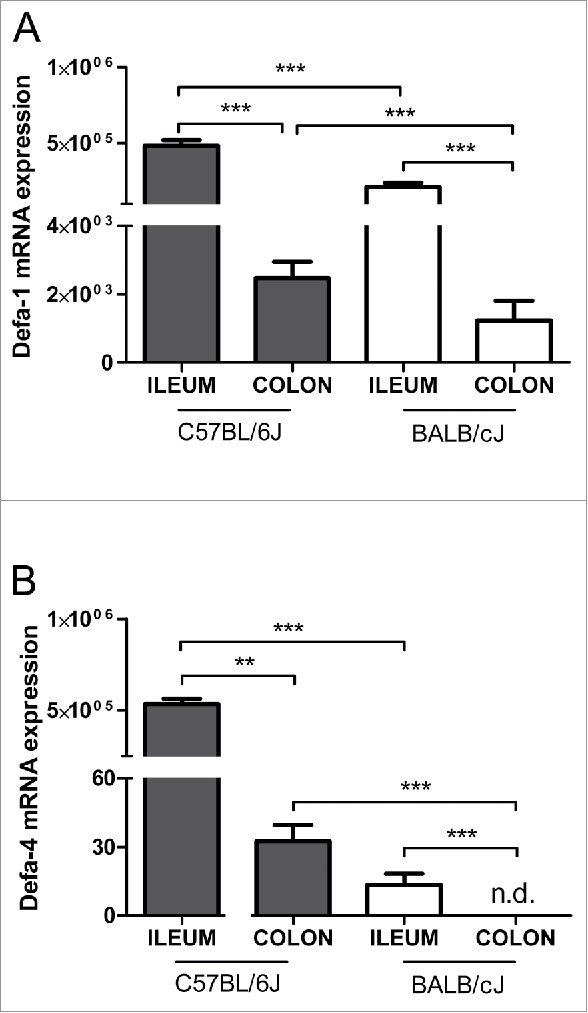
The mRNA Expression of α-defensins in the intestine of healthy mice. The relative mRNA levels for α-defensin-1 (Defa-1) (A) and α-defensin-4 (Defa-4) (B) transcripts normalized to β-actin are shown for the ileum and colon in C57BL/6J and BALB/cJ mice. Statistics: mean ± SEM, N = 23–24 per group, *p < 0.05, ***p < 0.001.
In a previous study, we examined functional barrier markers such as fluorescein isothiocyanate-dextran 4000 (FITC-D4000), ovalbumin (OVA) and lipopolysaccharide (LPS) in blood or lactulose/mannitol (Lac/Man) ratio, polyethylene glycol (PEG) 400/4000 recovery in urine.21 Although such markers do usually not allow to distinguish between the different intestinal sites, they might give a hint which of the barrier markers measured in the present study might have functional consequences for the global gut barrier situation. Interestingly, BALB/cJ mice, which expressed lower abundances of Defa-1, and especially of Defa-4, in both ileum and colon, compared to C57BL/6J mice, had higher mannitol recovery rates (23%, compared to 9% in C57BL/6J mice).
Analysis of correlations between the different parameters revealed that almost all tight junction mRNA expression levels correlated with each other in the colon, and also partly in the ileum (Table 3). The α-defensin Defa-1 mRNA correlated with claudin-5 mRNA, whereas Defa-4 mRNA correlated with claudin-3 concentrations in urine. Moreover, Defa-1 mRNA was closely correlated with Defa-4 both in the ileum and colon, and negatively correlated with Muc-2 protein expression in the colon. The iFABP-2 in plasma was the only marker that correlated negatively with mucus thickness in the colon (Table 3). Urine iFABP-2 values fit to plasma levels, however differences between mice of different genetic background were less pronounced and correlations with mucus thickness were not found. LPS measured in portal vein plasma correlated with Defa-1 mRNA expression in the ileum as well as in the colon, and with iFABP-2 concentrations in plasma. The Lac/Man ratio positively correlated with the mRNA expression of both Defa-1 and -4, and inversely with mucus thickness in small intestine (Table 3).
Table 3.
Correlation analysis for markers of intestinal permeability.
| Parameters | Ra | P | |
|---|---|---|---|
| ZO-1 mRNA (ileum) | Occludin mRNA (ileum) | 0.760 | <0.001 |
| ZO-1 mRNA (colon) | Occludin mRNA (colon) | 0.830 | <0.001 |
| ZO-1 protein (colon) | Occludin protein (colon) | 0.481 | <0.001 |
| ZO-1 mRNA (colon) | Claudin-2 mRNA (colon) | 0.803 | <0.001 |
| ZO-1 mRNA (ileum) | Claudin-5 mRNA (ileum) | 0.502 | <0.001 |
| ZO-1 mRNA (colon) | Claudin-5 mRNA (colon) | 0.588 | <0.001 |
| ZO-1 mRNA (ileum) | JAM-A mRNA (ileum) | 0.595 | <0.001 |
| ZO-1 protein (colon) | Claudin-1 protein (colon) | 0.739 | <0.001 |
| Claudin-1 mRNA (colon) | JAM-A mRNA (colon) | 0.887 | <0.001 |
| Claudin-1 mRNA (ileum) | JAM-A mRNA (ileum) | 0.507 | <0.001 |
| Occludin mRNA (ileum) | JAM-A mRNA (ileum) | 0.710 | <0.001 |
| Occludin mRNA (ileum) | Claudin-5 mRNA (ileum) | 0.546 | <0.001 |
| Occludin mRNA (colon) | Claudin-2 mRNA (colon) | 0.721 | <0.001 |
| Claudin-2 mRNA (colon) | Claudin-5 mRNA (colon) | 0.730 | <0.001 |
| Muc-2 mRNA (ileum) | ZO-1 mRNA (ileum) | 0.457 | 0.001 |
| Muc-2 mRNA (ileum) | JAM-A mRNA (ileum) | 0.470 | <0.001 |
| Tff3 mRNA (ileum) | Occludin mRNA (ileum) | 0.547 | <0.001 |
| Tff3 mRNA (colon) | Claudin-2 mRNA (colon) | 0.551 | <0.001 |
| Defa-1 mRNA (colon) | Claudin-5 mRNA (colon) | 0.338 | 0.009 |
| Defa-1 mRNA (ileum) | Defa-4 mRNA (ileum) | 0.707 | <0.001 |
| Defa-1 mRNA (colon) | Defa-4 mRNA (colon) | 0.564 | <0.001 |
| Defa-1 mRNA (colon) | Muc-2 protein (colon) | −0.455 | 0.001 |
| Defa-4 mRNA (colon) | Claudin-3 in urine | −0.611 | 0.001 |
| Defa-4 mRNA (ileum) | Claudin-3 in urine | −0.565 | 0.002 |
| Defa-4 mRNA (ileum) | iFABP-2 in plasma | 0.557 | 0.002 |
| iFABP-2 in plasma | ZO-1 mRNA (colon) | 0.427 | 0.017 |
| iFABP-2 in plasma | Claudin-1 protein (ileum) | 0.549 | 0.002 |
| iFABP-2 in plasma | Claudin-2 mRNA (colon) | 0.437 | 0.014 |
| iFABP-2 in plasma | Claudin-5 mRNA (colon) | 0.496 | 0.006 |
| iFABP-2 in plasma | Mucus thickness (colon) | −0.487 | 0.007 |
| LPS in portal vein plasma | Defa-1 mRNA (ileum) | 0.483 | 0.002 |
| LPS in portal vein plasma | Defa-1 mRNA (colon) | 0.417 | 0.006 |
| LPS in portal vein plasma | iFABP-2 in plasma | 0.425 | 0.017 |
| Lac/Man ratio (urine) | Defa-1 mRNA (ileum) | 0.379 | 0.004 |
| Lac/Man ratio (urine) | Defa-4 mRNA (ileum) | 0.474 | <0.001 |
| Lac/Man ratio (urine) | Defa-4 mRNA (colon) | 0.499 | <0.001 |
| Lac/Man ratio (urine) | Mucus thickness (ileum) | −0,351 | 0.007 |
Spearman's rank correlation coefficient R; N = 42–48.
Discussion
Our data show that several potential markers of the intestinal barrier can be assessed in mice, including mucus thickness, mRNA expression and protein levels of Muc-2 and junctional molecules, respectively, as well as mRNA expression of defensins and protein levels of soluble markers such as claudin-3 and iFABP-2. For the first time, we measured all these parameters in parallel, and provided normal values (medians and 90% percentile ranges) for healthy mice of different genetic backgrounds. We assessed the colon and the small intestine, namely the ileum, because these are the sites with pronounced bacterial load and thus the barrier has a particular protective function for the host. This does neither exclude other parts of the small intestine being of potential relevance nor claim that data from the ileum can be extrapolated to the jejunum or duodenum. Our data revealed that the different barrier measures do not necessarily relate to each other, suggesting that different aspects of the intestinal barrier are reflected by the different markers. According to our correlation analyses, one can separate at least 4 groups of intestinal barrier markers: tight junction molecules, defensins, mucin-related markers, and intestinal fatty acid binding protein. The mRNA expression of almost all tight junction molecules measured in the present study (JAM-A, ZO-1, occludin, claudin-1, -2 and -5) are closely correlated, independent of whether they were measured in the colon or in the ileum. Similar observations were made in a mouse study which showed that 1.25-OH2-vitamin D3 plays a protective role in mucosal barrier homeostasis by maintaining the integrity of junction complexes. In this study, ZO-1, occludin, and claudin-1 gene expression were comparably affected by vitamin D3 administration.22 Epithelial JAM-A plays a key role in maintaining intestinal barrier function and in protecting against inflammatory bowel disease through the regulation of epithelial cell barrier integrity both in man and in mice.23 Therefore, we and others examined JAM-A and a number of other transmembrane adhesion proteins, such as claudins, in mouse24 and rat25 intestine at different sites. In mice, most claudins were expressed in decreasing or increasing gradients or in more complex patterns along the longitudinal axis of the intestine, with up to 1000-fold differences in relative abundance, while expression of ZO-1, occludin, and JAM were unchanged.24 In rats, strongest expression of “tightening” claudins 1, 3, 4, 5, and 8, and lowest expression of claudins mediating permeability, namely claudin-2, -7, and -12, was detected in the colon and in the duodenum, compared to jejunum and ileum.25 Our data confirm, at least for claudin-5, that small and large intestine tight junction molecules are regulated in a different manner. This might have a functional impact since claudins have different functional properties. For example, claudin-7 obviously enforces tightening of the intestinal barrier, whereas other claudins such as claudin-2 and -15 rather promote “leakiness” thus allowing absorption of nutrients.26
In the present study, we measured α-defensins expressed in small intestinal Paneth cells and in intestinal epithelial cells in mice. The two α-defensins Defa-1 and -4 measured in our study were closely correlated with each other at the mRNA level, both in the ileum and colon. Nevertheless, only Defa-4 expression, independent of whether from the ileum or colon, was correlated with the soluble marker claudin-3 measured in urine, whereas Defa-1 was correlated with claudin-5 mRNA expression and negatively correlated with Muc-2 protein expression. The plasma marker iFABP-2 was also related to most tight junction markers and inversely correlated with mucus thickness. Interestingly, we found no correlation between plasma and urine concentrations of iFABP-2, possibly because of the short halftime of iFABP-2 of 11 min making measurements in urine samples collected over 6 hours difficult.
In a recent publication, we reported on functional markers of intestinal permeability such as Lac/Man ratio, PEG 400/4000 recovery in urine, FITC-dextran 4000 and ovalbumin concentration in plasma as well as portal vein plasma LPS levels.21 When comparing these data with the data of the present study, an inverse association between mucus thickness in the ileum and Lac/Man ratio, and between iFABP-2 in plasma with LPS in portal vein plasma was found (data not shown). No association between Lac/Man ratio or LPS levels and expression of TJ molecules was found. Why mucus thickness and Muc-2 protein expression in the colon were hardly related with any other marker, and if related, the correlation was an inverse one, remains unclear. These observations indicate that mucus is regulated differently to other epithelial products, like α-defensins and tight junction molecules.
Because of the multiple correlations we found between the mRNA expression of ZO-1 and other TJ molecules, one might assume that the analysis of only one tight junction molecule, such as ZO-1, might be sufficient and representative for the whole cluster of tight junction molecules. This assumption is supported by data from the literature suggesting ZO-1 as being a type of master player within the tight junction complex, because the cytoplasmatic protein ZO-1 is expressed ubiquitously in all tight junctions and binds directly to the cytoplasmatic tails of claudins27 and occludin.28,29 Coincidently, the level of ZO-1 expression is approximately proportional to the number of fibrils expressed in a junction.30 ZO-1 redistribution is an early cellular event preceding physiological cell shedding, as well as redistribution of other tight junction function along the lateral plasma membrane to sustain the epithelial barrier during cell shedding.31
Because expression of the 2 α-defensins correlated closely, the measurement of only one of them, e.g., Defa-1, might be sufficient. It has clearly been shown that TCF-1-mediated Wnt signaling regulating the expression of α-defensins in the small intestine is closely related to barrier dysfunction.32 The biological significance of the minor expressions of Defa-1 and -4 in the colon has to be questioned, and is therefore not discussed further. Interestingly, Defa-4 transcripts were found at much higher amounts in the ileum of C57BL/6J mice, compared to BALB/cJ mice, in which virtually no transcripts were detected, further emphasizing the influence of the genetic background on intestinal barrier regulation.33 The reason why Defa-1 and -4 mRNA expression in both ileum and colon correlated with LPS levels in portal vein and with the Lac/Man ratio, respectively, remains unclear at present.
In the present study, we tested two soluble barrier markers, iFABP-2 in plasma and urine and claudin-3 in urine, for usage in mice, after they have been successfully used in human studies (for details see methods), because they might substitute for cumbersome Western blot-based tissue examinations. Fatty acid binding proteins are small cytosolic, water-soluble proteins, which are released upon enterocyte membrane integrity loss. They are readily released into the circulation and rapidly cleared renally, which makes them useful as plasma and urine markers for enterocyte damage.34 Claudin-3 seems to be a suitable candidate marker for early non-invasive detection of intestinal tight junction integrity loss due to its small size,35 abundant endogenous intestinal expression and paracellular localization.30 Several studies showed a strong relationship between intestinal TJ loss and urinary claudin-3 levels in both a rat hemorrhagic shock model and in human studies.35,36
According to our present data, urine claudin-3 and plasma iFABP-2 levels can be measured also in mice. These markers, besides mucus and Muc-2 analysis, are worth measuring because they do reflect other features of the barrier than tight junction molecules and α-defensins do. We selected the major gel-forming mucin Muc-2 among the MUC molecules because, as opposed to the membrane-bound MUC1, MUC3, and MUC17, the MUC2 glycoprotein appears to be protective, and to correlate with mucus thickness.37,38 Muc2-deficient mice lack colonic mucus and are hypersensitive to DSS-induced colitis, whereas Muc1-deficient mice were protected,39 emphasizing the crucial role of MUC2 for a normal intestinal barrier function. However, we found no correlation between Muc-2 protein expression and mucus thickness in ileum or colon (data not shown). Our mucus data fit to some previously published data,40 but differ to others reporting thicknesses up to 40 µm at least in the colon.41 Such differences are probably related to the methodological approach used for mucus thickness measurements. It cannot be ignored that, with the methods we used, the adherent inner mucus layer was measured rather than the whole one.
Our data clearly indicate that age and sex are not relevant parameters in regard to influencing the intestinal barrier markers measured in the present study. In contrast, the genetic background of the mice might be relevant, at least for some markers (such as both α-defensins, and both soluble markers claudin-3 and iFAPB-2). The most relevant parameter is the measurement site of tissue markers, since we found significant differences between ileum and colon for the expression of the majority of markers including the Muc-2 protein concentration, and mRNA expression of occludin, claudin-5, and both α-defensins.
Conclusion
The intestinal mucosa is continuously challenged by toxins, antigens, and potentially harmful microorganisms. Therefore, the precise regulation of the intestinal barrier is mandatory for the maintenance of mucosal immune homeostasis and gut health.42 Alterations in the barrier can lead to the disruption of intestinal homeostasis, and subsequent inflammation and disease, as shown in many animal models of colitis, enterocolitis, and hepatitis, as well as in human diseases.43,44 To understand such important mechanisms, methods of barrier assessment need to be established, evaluated, and compared with each other. While much work has been done in animal models of disease, comparatively little efforts have been made to characterize and compare intestinal barrier markers and their normal values in healthy mice. In the present study, we identified a number of key intestinal barrier markers, such as Muc-2, ZO-1, and α-defensin-1 expression in tissue, and claudin-3 and iFABP-2 concentrations in urine and plasma, respectively, which might be suitable when assessing different barrier functions in the mouse model. Moreover, we provided normal values for the small and large intestine in mice of different genetic backgrounds. Such data might be helpful in future animal studies, not only for understanding pathophysiology, but also for therapeutic studies that select the intestinal barrier as a new target.3,45
Material and methods
Animals and treatments
Experiments were performed in 2 different mice strains (C57BL/6J and BALB/cJ) of both sexes (female, male) at 2 ages (either 10–20 weeks old or 6–12 months old). All animals were purchased at these ages from Janvier Labs (S.A.S., Le-Genes-St-Isle, France), and used for the experiments after 2 weeks of adaptation. The mice were housed in a controlled environment (inverted 12 h daylight cycles, lights-off at 07:00 in summer and at 06:00 in winter) with free access to food and water in a standard pathogen-free barrier facility at the University of Hohenheim. Animals were anesthetized with 100 mg ketamin/kg and 16 mg xylazin/kg body weight by i.p. injection. Blood was collected just prior to sacrifice. Portions of gut tissue were frozen immediately in liquid nitrogen, and then either fixed in neutral-buffered formalin, or frozen-fixed in OCT mounting media (Sakura / cat. number - 25608-930). All procedures were approved by the local Institutional Animal Care and Use Committee (Regional Council Stuttgart, permit number: V306/13EM). All experiments were conducted according to the recommendations of the Federation of European Laboratory Animal Science Associations.
Histochemical staining for the total mucins and assessment of the mucus thickness
Periodic Acid – Schiff's (PAS) and Alcian blue (AB) stainings were used to visualize the total (neutral and acid) mucins in ileum and colon as described.46-48 Briefly, frozen sections of gut tissue (3 µm) were dried at room temperature. Before staining with Alcian blue (Merck / cat. number - 1.01647) for 30 min, gut tissue sections were fixed in Carnoy's solution (Morphisto / cat. number - 10159.01000) for 30 min and washed in PBS (pH = 7,4) for 10 min. Alcian blue stains the acid mucins. After a wash step with tap water for 10 min, short rinsing in distilled water, and oxidation of neutral mucins with Periodic acid (Merck / cat. number - 1.01646./1) for 10 min, tissue sections were stained with Schiff's reagent (Carl Roth / cat. number - X900.2) for 15 min. After another wash step with tap water for 3 min and short rinsing in distilled water, tissue sections were dehydrated shortly in ethanol 95% and 100% (Carl Roth / cat. number - K928.2), and then for 20 min in 100% Roti-Histol (Carl Roth / cat. number - 6640.2), after which they were then mounted with entellan (Merck / cat. number - 1.07961.0500). Neutral mucins stained magenta, acidic mucins blue, and neutral/acidic mucins violet.46,47 For the quantitate examination, pictures from 8 to 10 different areas of each tissue section were analyzed using an inverted light microscope (Zeiss). The total mucus thickness was measured using the Axio Vision Rel. 4.8 software at the magnification 200x. The thickness of the adherent mucus layer was measured perpendicular to the mucosal surface from the edge of the epithelium to the outermost part of the mucus layer and expressed in µm. The means (± SEM) of 10 measurements were calculated.
Immunohistochemical staining for Muc-2 in colon and ileum
Paraffin-embedded gut tissue sections (3 µm) were cut and stained for Muc-2 using a polyclonal rabbit antibody as described in the manufactures protocol (abcam/cat. number – ab76774). To detect specific binding of primary antibody, tissue sections were incubated with a peroxidase-linked secondary anti-rabbit antibody (Dako / cat. number – K4002) and the Dako Liquid DAB + Substrate Chromogen System (Dako / cat. number – K3467). Using an image acquisition and analysis system incorporated in the microscope, the extent of staining in the gut tissue sections was defined as a percent of the field area within the default color range determined by analysis software (Axio Vision Release 4.8, Zeiss) at the magnification 200x. Means (± SEM) of data obtained from 10 different areas of each tissue section were then calculated.
Protein isolation and immunoblotting
To prepare protein samples, snap-frozen tissue specimen from the ileum or colon were homogenized in peqGOLD TriFast (PEQLAB / cat. number - 302020) and protein was isolated according to the manufacturer's instructions. Protein lysates (20 μg protein/well) were separated in a 8% SDS-polyacrylamide gel and transferred to Hybond™-P polyvinylidene difluoride membranes (Amersham Biosciences / cat. number - 10600023). The resulting blots were then probed with a primary antibody directed against occludin (1:500 in 5% skim milk overnight; abcam / cat. number - ab167161), claudin-1 (1:125, in 5% skim milk overnight; Invitrogen / cat. number - 374900) or zonula occludens-1 (ZO-1; 1:250, in 5% skim milk overnight; Invitrogen / cat. number - 402200). Appropriate secondary-horseradish peroxidase (HRP) antibody (Rabbit; 1:5000, in 5% skim milk for 1h; Cell Signaling / cat. number - 7074) was applied. The bands were visualized using a Super Signal Western Dura kit (Thermo scientific / cat. number - 34075). To ensure equal loading, all blots were stained with Ponceau red (Sigma / cat. number - P7170); signals of tight junction molecules were normalized to β-actin (1:750 in 2,5% bovine serum albumin overnight; Cell Signaling / cat. number – 4970). Protein bands were analyzed by densitometry using Fluorchem Software “AlphaEasyFC™” (Alpha Innotech, San Leandro, USA).
RNA isolation and real-time RT-PCR for mucins and tight junction molecules
Total RNA was extracted from gut tissue samples using peqGOLD TriFast™ (PEQLAB / cat. number - 302020). RNA concentrations were determined spectrophotometrically using the NanoDrop2000 device (Thermo Scientific). An amount of 1 µg total RNA was reversely transcribed using a Promega-Kit “Reverse Transcription System” and Random primers after a DNase digestion step (Promega / cat. number - A3500).
Polymerase chain reaction (PCR) primers for claudin-1, 2 and -5, JAM-A, occludin, Muc-2, Tff3, ZO-1 and 18S (Table 1) were designed using the Primer 3 software (Whitehead Institute for Biomedical Research). Eva®Green Universal PCR Master Mix (Bio-Rad / cat. number - 172-5204) was used to prepare the PCR mix. The amplification reactions were carried out in an iCycler (Bio-Rad Laboratories) with an initial hold step (95°C for 3 min) and 45 cycles of a 3-step PCR (95°C for 15 sec, 60°C for 15 sec, 72°C for 30 sec). The fluorescence intensity of each sample was measured at each temperature change to monitor amplification of the target gene. The comparative CT - method was used to determine the amount of target gene normalized to an endogenous reference (18S mRNA) and relative to a calibrator (2−ΔΔCt). The purity of PCR products was verified by melting curves and gel electrophoresis.
Real-time PCR for α-defensins
We utilized cDNA derived from 10 ng of total RNA as a template for the real-time PCR reaction. Expression levels were quantified using a LightCycler® 480 that applied plasmid standards for all products as previously described.32,49 Transcripts were all normalized with respect to the expression of β-actin. Oligonucleotide primers are listed in Table 1. Quantification of the single PCR products and analysis of the standard curves for each primer set (plasmid DNA from 1 to 10−7 ng in 1:10 dilution steps) was performed using the LightCycler Software (Roche Diagnostics Deutschland GmbH, Mannheim, Germany). The purity of PCR products was verified by melting curves and gel electrophoresis.
Urine and plasma measurements
The intestinal fatty acid binding protein-2 (iFABP-2) concentration in plasma and urine was determined analogously to the methods reported in previous human studies50 using a high-sensitive ELISA assay with a detection limit of 0.061 ng/ml (Hölzel / cat. number - SEA559Mu). Plasma samples (diluted 1:10), urine samples (undiluted), standards (from 10 to 0.3156 ng/ml), and blanks (PBS buffer) were transferred to a 96-well microplate and further treated according to the manufacturer's instructions.
Claudin-3 concentrations in urine were determined analogously to the methods reported in previous human studies35 using a high-sensitive ELISA assay with a detection limit of 13.8 pg/ml (Hölzel / cat. number – SEF293Mu). The diluted urine samples (1:30), standards (from 2,000 to 31.2 pg/ml), and blanks (PBS buffer) were transferred to a 96-well microplate and further treated according to the manufacturer's instructions.
Statistical analysis
Results are shown as medians (50% percentiles) and 90 percent intervals (ranging from 5% to 95% percentiles) or as means ± SEM if the data were normally distributed. Normal distribution was tested using the Kolmogorov-Smirnov test. Normally distributed data from multiple groups were compared by one-way ANOVA and the Bonferroni's multiple comparison test. All other data were compared by the Kruskal-Wallis test and Dunn's multiple comparison test. If only 2 groups were compared, either the unpaired t-test or – if not normally distributed – the Mann-Whitney U test was used. Outliers (max one out of 24 values per group) were identified using the Grubb's test and eliminated from analysis. Correlation analysis was performed using the Spearman rank correlation test. To test the null hypothesis, the significance level α was defined as 0.05. A P-value < α was considered as statistically significant. Statistical analysis was performed using SPSS, version 22 (IBM, Ehningen, Germany). For figure presentations we used Graph Pad Prism, version 6 (Graph Pad Inc., La Jolla, CA).
Abbreviations
- AB
alcian blue
- Defa-1 and -4
α-defensin-1 and -4
- FITC-D4000
Fluorescein Isothiocyanate-Dextran 4000
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- iFABP-2
intestinal fatty acid binding protein-2
- JAM-A
junctional adhesion molecule-A
- LPS
lipopolysaccharide
- Muc-2
mucin-2
- OVA
ovalbumin
- PAS
periodic acid – Schiff's reagent
- PCR
polymerase chain reaction
- PEG
polyethylene glycol
- Tff3
trefoil factor 3
- TJ
tight junction
- ZO-1
zonula occludens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The study was funded by the German Research Foundation (DFG) within the SPP 1656 Intestinal Microbiota, Project “Modulation of the intestinal barrier by the intestinal microbiota—Role of dietetic factors and the mucosal immune system” (BI 424/8-1) to SCB.
References
- [1].Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012; 24:503-12; PMID:22583600; http://dx.doi.org/ 10.1111/j.1365-2982.2012.01921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9:799-809; PMID:19855405; http://dx.doi.org/ 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- [3].Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol 2014; 14:189; PMID:25407511; http://dx.doi.org/ 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al.. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013; 342:447-53; PMID:24072822; http://dx.doi.org/ 10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dupont A, Heinbockel L, Brandenburg K, Hornef MW. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes 2014; 5:761-5; PMID:25483327; http://dx.doi.org/ 10.4161/19490976.2014.972238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johansson ME. Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis 2014; 20:2124-31; PMID:25025717; http://dx.doi.org/ 10.1097/MIB.0000000000000117 [DOI] [PubMed] [Google Scholar]
- [7].Fishman JE, Levy G, Alli V, Zheng X, Mole DJ, Deitch EA. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014; 42:264-70; PMID:24978882; http://dx.doi.org/ 10.1097/SHK.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev 2015; 14:479-89; PMID:25676324; http://dx.doi.org/ 10.1016/j.autrev.2015.01.009 [DOI] [PubMed] [Google Scholar]
- [9].Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol 2014; 36:166-76; PMID:25220018; http://dx.doi.org/ 10.1016/j.semcdb.2014.09.002 [DOI] [PubMed] [Google Scholar]
- [10].Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol 2008; 1 Suppl 1:S67-74; PMID:19079235; http://dx.doi.org/ 10.1038/mi.2008.48 [DOI] [PubMed] [Google Scholar]
- [11].Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al.. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 2013; 11:61; PMID:23692866; http://dx.doi.org/ 10.1186/1741-7007-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kawashima R, Kawakami F, Maekawa T, Yamamoto H, Koizumi W, Ichikawa T. Elemental diet moderates 5-fluorouracil-induced gastrointestinal mucositis through mucus barrier alteration. Cancer Chemother Pharmacol 2015; 76:269-77; PMID:26048344; http://dx.doi.org/ 10.1007/s00280-015-2790-z [DOI] [PubMed] [Google Scholar]
- [13].Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011; 141:769-76; PMID:21430248; http://dx.doi.org/ 10.3945/jn.110.135657 [DOI] [PubMed] [Google Scholar]
- [14].Fishman JE, Levy G, Alli V, Sheth S, Lu Q, Deitch EA. Oxidative modification of the intestinal mucus layer is a critical but unrecognized component of trauma hemorrhagic shock-induced gut barrier failure. Am J Physiol Gastrointest Liver Physiol 2013; 304:G57-63; PMID:23125158; http://dx.doi.org/ 10.1152/ajpgi.00170.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zuhl M, Schneider S, Lanphere K, Conn C, Dokladny K, Moseley P. Exercise regulation of intestinal tight junction proteins. Br J Sports Med 2014; 48:980-6; PMID:23134759; http://dx.doi.org/ 10.1136/bjsports-2012-091585 [DOI] [PubMed] [Google Scholar]
- [16].Liu KX, Chen SQ, Zhang H, Guo JY, Li YS, Huang WQ. Intestinal ischaemia/reperfusion upregulates beta-defensin-2 expression and causes acute lung injury in the rat. Injury 2009; 40:950-5; PMID:19486970; http://dx.doi.org/ 10.1016/j.injury.2009.01.103 [DOI] [PubMed] [Google Scholar]
- [17].Taylor Nordgård C, Draget KI. Dynamic responses in small intestinal mucus: relevance for the maintenance of an intact barrier. Eur J Pharm Biopharm 2015; 95:144-50; PMID:25657121; http://dx.doi.org/ 10.1016/j.ejpb.2015.01.024 [DOI] [PubMed] [Google Scholar]
- [18].Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubière M, Salvador-Cartier C, Gillet M, Ferrier L, Loubière P, Dague E, et al.. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol 2014; 307:G420-9; PMID:24970779; http://dx.doi.org/ 10.1152/ajpgi.00290.2013 [DOI] [PubMed] [Google Scholar]
- [19].Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, McDonald V, Florin TH, McGuckin MA. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013; 144:357-368; PMID:23123183; http://dx.doi.org/ 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- [20].Murphy SF, Rhee L, Grimm WA, Weber CR, Messer JS, Lodolce JP, Chang JE, Bartulis SJ, Nero T, Kukla RA, et al.. Intestinal epithelial expression of TNFAIP3 results in microbial invasion of the inner mucus layer and induces colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 2014; 307:G871-82; PMID:25234043; http://dx.doi.org/ 10.1152/ajpgi.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Volynets V, Reichold A, Bárdos G, Rings A, Bleich A, Bischoff SC. Assessment of the Intestinal Barrier with Five Different Permeability Tests in Healthy C57BL/6J and BALB/cJ Mice. Dig Dis Sci 2016; 61:737-46; PMID:26520109; http://dx.doi.org/ 10.1007/s10620-015-3935-y [DOI] [PubMed] [Google Scholar]
- [22].Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 2012 May 30; 12:57; PMID:22647055; http://dx.doi.org/ 10.1186/1471-230X-12-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, et al.. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 2008; 135:173-84; PMID:18514073; http://dx.doi.org/ 10.1053/j.gastro.2008.04.002 [DOI] [PubMed] [Google Scholar]
- [24].Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 2006; 6:581-8; PMID:16458081; http://dx.doi.org/ 10.1016/j.modgep.2005.12.001 [DOI] [PubMed] [Google Scholar]
- [25].Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B 2010; 180:591-8; PMID:20049600; http://dx.doi.org/ 10.1007/s00360-009-0440-7 [DOI] [PubMed] [Google Scholar]
- [26].Lu Z, Ding L, Lu Q, Chen YH. Claudins in intestines: distribution and functional significance in health and diseases. Tissue Barriers 2013 Jul 1; 1(3):e24978; PMID:24478939; http://dx.doi.org/ 10.4161/tisb.24978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 1999; 147:1351-63; PMID:10601346; http://dx.doi.org/ 10.1083/jcb.147.6.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 1994; 127:1617-26; PMID:7798316; http://dx.doi.org/ 10.1083/jcb.127.6.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kimura M, Sawada N, Kimura H, Isomura H, Hirata K, Mori M. Comparison between the distribution of 7H6 tight junction-associated antigen and occludin during the development of chick intestine. Cell Struct Funct 1996; 21:91-6; PMID:8726478; http://dx.doi.org/ 10.1247/csf.21.91 [DOI] [PubMed] [Google Scholar]
- [30].Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001; 120:411-22; PMID:11159882; http://dx.doi.org/ 10.1053/gast.2001.21736 [DOI] [PubMed] [Google Scholar]
- [31].Guan Y, Watson AJ, Marchiando AM, Bradford E, Shen L, Turner JR, Montrose MH. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am J Physiol Cell Physiol 2011; 300:C1404-14; PMID:21346149; http://dx.doi.org/ 10.1152/ajpcell.00270.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Beisner J, Teltschik Z, Ostaff MJ, Tiemessen MM, Staal FJ, Wang G, Gersemann M, Perminow G, Vatn MH, Schwab M, et al.. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: implications for Crohn's disease. Am J Physiol Gastrointest Liver Physiol 2014; 307:G487-498; PMID:24994854; http://dx.doi.org/ 10.1152/ajpgi.00347.2013 [DOI] [PubMed] [Google Scholar]
- [33].Shanahan MT, Tanabe H, Ouellette AJ. Strain-specific polymorphisms in Paneth cell α defensins of C57BL/6 mice and evidence of vestigial myeloid α-defensin pseudogenes. Infect Immun 2011; 79:459-73; PMID:21041494; http://dx.doi.org/ 10.1128/IAI.00996-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, van Heurn LW, Heineman E, Buurman WA. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol 2009; 43:727-33; PMID:19359998; http://dx.doi.org/ 10.1097/MCG.0b013e31819194b0 [DOI] [PubMed] [Google Scholar]
- [35].Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruïne A, Masclee AA, Heineman E, Buurman WA. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol 2010; 44:e14-9; PMID:19525861; http://dx.doi.org/ 10.1097/MCG.0b013e31819f5652 [DOI] [PubMed] [Google Scholar]
- [36].Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock 2009; 31:164-9; PMID:18650780; http://dx.doi.org/ 10.1097/SHK.0b013e31817fc310 [DOI] [PubMed] [Google Scholar]
- [37].Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010; 12:319-30; PMID:20703838; http://dx.doi.org/ 10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, et al.. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 2014; 260:8-20; PMID:24942678; http://dx.doi.org/ 10.1111/imr.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 2011; 300:G327-33; PMID:21109593; http://dx.doi.org/ 10.1152/ajpgi.00422.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res 2009; 58:111-9; PMID:18198997 [DOI] [PubMed] [Google Scholar]
- [41].Gustafsson JK, Ermund A, Johansson ME, Schütte A, Hansson GC, Sjövall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol 2012; 302:G430-8; PMID:22159279; http://dx.doi.org/ 10.1152/ajpgi.00405.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bischoff SC. 'Gut health': a new objective in medicine? BMC Med 2011; 9:24; PMID:21401922; http://dx.doi.org/ 10.1186/1741-7015-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol 2013; 4:280; PMID:24062746; http://dx.doi.org/ 10.3389/fimmu.2013.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao TY, Su LP, Ma CY, Zhai XH, Duan ZJ, Zhu Y, Zhao G, Li CY, Wang LX, Yang D. IGF-1 decreases portal vein endotoxin via regulating intestinal tight junctions and plays a role in attenuating portal hypertension of cirrhotic rats. BMC Gastroenterol 2015; 15:77; PMID:26152281; http://dx.doi.org/ 10.1186/s12876-015-0311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 2013; 11:1075-83; PMID:23851019; http://dx.doi.org/ 10.1016/j.cgh.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jordan N, Newton J, Pearson J, Allen A. A novel method for the visualization of the in situ mucus layer in rat and man. Clinical Science 1998; 95:97-106; PMID:9662491; http://dx.doi.org/ 10.1042/cs0950097 [DOI] [PubMed] [Google Scholar]
- [47].Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc 2003; 62:237-43; PMID:12756973; http://dx.doi.org/ 10.1079/PNS2002205 [DOI] [PubMed] [Google Scholar]
- [48].Cohen M, Varki NM, Jankowski MD, Gagneux P. Using unfixed, frozen tissues to study natural mucin distribution. J Vis Exp 2012; 67:3928; PMID:23023050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wehkamp J, Wang G, Kubler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, et al.. The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol 2007; 179:3109-3118; PMID:17709525; http://dx.doi.org/ 10.4049/jimmunol.179.5.3109 [DOI] [PubMed] [Google Scholar]
- [50].Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, Dejong CH, Buurman WA, Poeze M. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg 2011; 253:303-8; PMID:21245670; http://dx.doi.org/ 10.1097/SLA.0b013e318207a767 [DOI] [PubMed] [Google Scholar]


