Abstract
BACKGROUND
In a phase 3 study, the authors assessed the effects of dasatinib at doses of 140 mg once daily and 70 mg twice daily in patients who had either chronic myeloid leukemia (CML) in advanced phases or Philadelphia chromosome-positive acute lymphoblastic leukemia and were resistant or intolerant to imatinib. In the current report, the results for patients with CML in blast phase after 2 years of follow-up are reported.
METHODS
Patients were stratified according to whether they had CML in myeloid blast phase (MBP-CML) or in lymphoid blast phase (LBP-CML) and were randomized (1:1) within each stratum to receive either oral dasatinib 140 mg once daily or 70 mg twice daily.
RESULTS
In patients with MBP-CML, the major hematologic response rate was 28% for both regimens; and, in patients with LBP-CML, the major hematologic response rate was 42% for once-daily dasatinib and 32% for twice-daily dasatinib. The major cytogenetic response rates were 25% for once-daily dasatinib and 28% for twice-daily dasatinib in patients with MBP-CML, and the respective rates in patients with LBP-CML were 50% and 40%. The overall survival rate at 24 months was 24% for once-daily dasatinib and 28% for twice-daily dasatinib in patients with MBP-CML, and the respective values in patients with LBP-CML were 21% and 16%. Adverse events indicated a trend toward improved tolerability for the once-daily regimen.
CONCLUSIONS
The current results suggested that dasatinib 140 mg once daily had similar efficacy and improved tolerability relative to the 70-mg twice-daily regimen in patients with imatinib-resistant, blast phase CML.
Keywords: dasatinib, imatinib, drug resistance, blast crisis, chronic myeloid leukemia
Patients with chronic myeloid leukemia (CML) who progress from the chronic phase (CML-CP) to the blast phase (CML-BP) have a poor prognosis.1 In these patients, conventional chemotherapy regimens are of limited value, and 2-year survival rates after allogeneic stem cell transplantation (SCT) do not exceed 20%.1 CML is characterized by the presence of the Philadelphia chromosome (Ph), which encodes the oncogenic breakpoint cluster region-Abelson murine leukemia viral fusion protein (BCR-ABL) tyrosine kinase.2 Although BCR-ABL gene expression is sufficient for the initiation and sustenance of CML-CP, blast crisis often is characterized by additional molecular and cytogenetic defects. It is believed that these defects are orchestrated through both BCR-ABL-dependent and BCR-ABL-independent mechanisms.2 Imatinib (Glivec/Gleevec; Novartis, Basel, Switzerland) is a BCR-ABL tyrosine kinase inhibitor (TKI) used in the treatment of CML.3,4 Whereas this drug has dramatically improved the prognosis for patients with CML-CP, hematologic remission with imatinib in CML-BP are low (remission rate, 7.9%–26%) and of short duration (median, 6–10 months).4–6
The development of primary and acquired imatinib resistance involves multiple mechanisms, including BCR-ABL mutations and activation of the SRC family of kinases (SFK).7,8 Dasatinib (Sprycel; Bristol-Myers Squibb, New York, NY), a multikinase inhibitor that has an in vitro potency against unmutated BCR-ABL 325 times greater than that of imatinib, inhibits most known BCR-ABL mutants.9,10 Dasatinib also inhibits other tyrosine kinases, including the SFK.9 Orally administered dasatinib has produced consistent clinical benefit in patients with CML-CP, CML in accelerated phase (CML-AP), CML-BP, and Ph-positive acute lymphoblastic leukemia (ALL) who are resistant or intolerant to imatinib and is approved for use at a dosing regimen of 70 mg twice daily.11–15 A phase 3 dose-optimization study in patients with imatinib-resistant or intolerant CML-CP demonstrated that dasatinib 100 mg once daily had similar efficacy and improved tolerability relative to 70 mg twice daily16; consequently, the currently recommended initial dasatinib dose for these patients is 100 mg once daily.
Because once-daily regimens had not been evaluated for advanced-phase CML, a phase 3 trial was designed to assess the efficacy and tolerability of dasatinib 140 mg once daily versus 70 mg twice daily in patients with CML-AP, CML-BP, or Ph-positive ALL who had hematologic resistance or intolerance to imatinib. Data from a 2-year follow-up of that study are available now, and the results from the CML-BP subset are reported here.
MATERIALS AND METHODS
Patients and Treatment
This was a randomized, multicenter, open-label, phase 3 study. Written informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki and was approved by the appropriate ethics committees/institutional review boards (National Clinical Trials [NCT] identifier, NCT00123487).
Key inclusion and exclusion criteria that we used for patients with CML-BP who had resistance or intolerance to imatinib were described previously.14 Imatinib resistance was defined as no hematologic response to imatinib after 4 weeks, or an increase ≥50% in peripheral blood blasts after 2 weeks of treatment with imatinib ≥600 mg daily, or losing a hematologic response while receiving imatinib ≥600 mg daily. Imatinib intolerance was defined as grade ≥3 nonhematologic toxicity or grade 4 hematologic toxicity that lasted >2 weeks on imatinib ≥600 mg daily and led to imatinib discontinuation or dose reduction to ::;400 mg daily with loss of hematologic response.
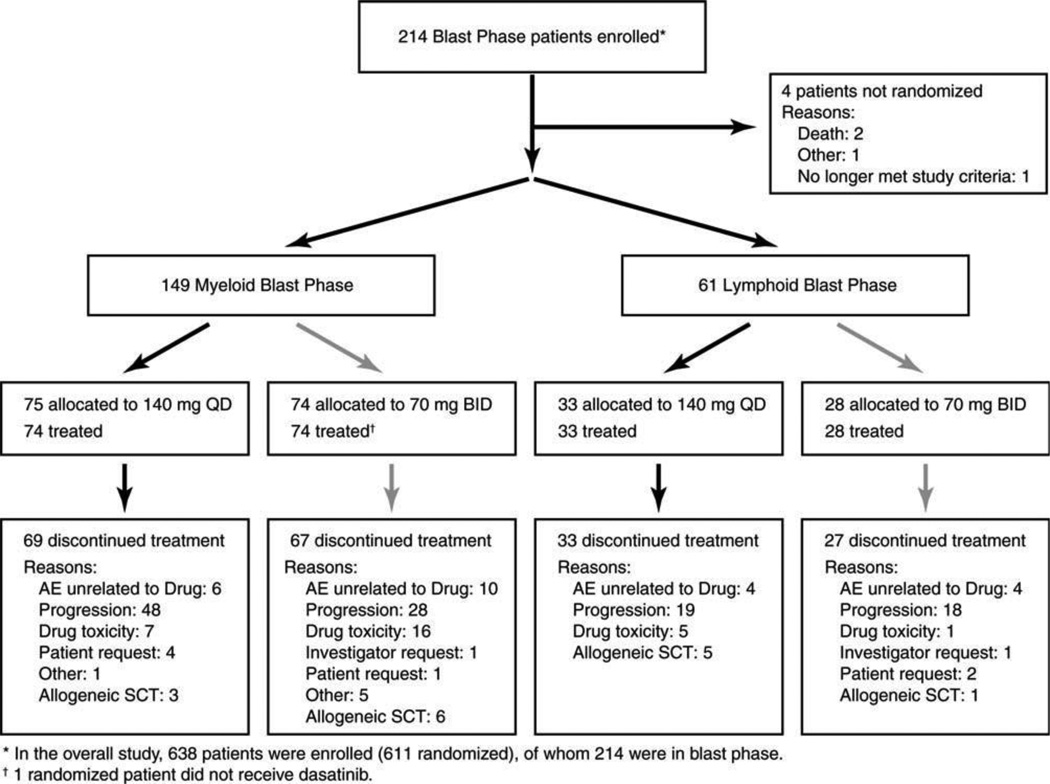
Patients who had CML-BP were stratified according to disease subtype: namely, myeloid blast phase CML (MBP-CML) and lymphoid blast phase CML (LBP-CML). Patients within each stratum were randomized 1:1 using a permuted block procedure to receive oral dasatinib at either 140 mg once daily or 70 mg twice daily (Fig. 1).
Figure 1.
This is a Consolidated Standards of Reporting Trials (CONSORT) diagram for patients with chronic myeloid leukemia in blast phase. QD indicates once-daily dasatinib; BID, twice-daily dasatinib; AE, adverse event; SCT, stem cell transplantation.
For inadequate responses, dasatinib doses could be escalated to 180 mg once daily or 90 mg twice daily within the respective treatment groups. The dose also could be reduced to 100 mg once daily and then 80 mg once daily for the once-daily regimen and to 50 mg twice daily and then 40 mg twice daily for the twice-daily regimen for toxicity. The criteria that we used for dose escalations, reductions, and interruptions were described previously.14
Efficacy Assessment
Hematologic responses, including complete hematologic response (CHR), no evidence of leukemia (NEL), and minor hematologic response (MiHR), were defined as described previously.14 Although the CHR definition incorrectly included a peripheral blood basophil count <20%, a review of patients who had a confirmed CHR indicated that the peripheral blood basophil count <2% in all 37 patients except 1 patient with MBP-CML who had a peripheral blood basophil count of 6% and 1 patient with CMP-LBP who had a peripheral blood basophil count of 3.3%. A major hematologic response (MHR), the primary efficacy endpoint, was defined as achieving a confirmed CHR or NEL. A confirmed hematologic response had to meet all criteria for at least 28 consecutive days.
Secondary endpoints included major cytogenetic response (MCyR), times to and durations of MHR and MCyR, progression-free survival (PFS), overall survival (OS), and safety. The definitions that we used for cytogenetic responses, including complete cytogenetic response (CCyR) and partial cytogenetic response (PCyR), were described previously.14 An MCyR was defined as either a CCyR or a PCyR.
Complete blood counts were monitored weekly until Week 20, then monthly up to 1 year, and every 3 months thereafter. Differential and cytogenetic assessments of bone marrow were conducted at the end of Months 1, 2, 3, 6, 9, and 12 and every 6 months thereafter.
Disease progression was defined as the loss of MHR or MiHR in responding patients over 2 consecutive weeks after starting the maximum dasatinib dose, or no reduction from baseline percentage blasts in peripheral blood or bone marrow over 4 weeks after the start of therapy, or an increase ≥50% in the peripheral blood blast count over 2 weeks, or death for any reason. PFS was calculated as the time from randomization to either disease progression, death, or discontinuation because of progression. Patients who neither progressed nor died were censored on the date of last cytogenetic or hematologic assessment. OS was calculated as the time from randomization to death, and patients who had not died or who were lost to follow-up were censored on the last date they were known to have been alive.
Leukocytes were prepared from blood samples that were collected from patients at baseline. Total leukocyte RNA was extracted, and BCR-ABL messenger RNA was amplified using reverse transcriptase-polymerase chain reaction. BCR-ABL point mutations were assessed by direct sequencing.
Safety Assessment
Adverse events were assessed continuously and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0; National Cancer Institute, Bethesda, Md).
Statistical Analysis
The study was powered to evaluate noninferiority by analyzing the difference of MHR rates between the dasatinib once-daily and twice-daily schedules in the combined CML-AP, CML-BP, and Ph-positive ALL populations. Because this report focuses only on the CML-BP subset, formal comparisons between the 2 dosing regimens cannot be made and are descriptive. Efficacy analyses included all randomized patients, and safety analyses included all treated patients. Durations of MHR, MCyR, and CCyR were calculated using patients who achieved these responses. Response rates with 2-sided, exact 95% confidence intervals (CIs) were calculated using the Clopper and Pearson method. The Kaplan-Meier product-limit method was used to estimate PFS, OS, and times to and durations of MHR, MCyR, and CCyR. Median values were provided along with 95% CIs using the Brookmeyer and Crowley method.
RESULTS
Patient Characteristics and Disposition
Of 210 patients with CML-BP from 62 sites who were randomized to receive either dasatinib 140 mg once daily or 70 mg twice daily, 149 patients had MBP-CMP, and 61 patients had LBP-CML (Fig. 1). At the 2-year follow-up assessment, 12 patients with MBP-CML (once-daily regimen, 5 patients; twice-daily regimen, 7 patients) and 1 patient with LBP-CML (twice-daily regimen) were still on the study. The remaining patients discontinued treatment for multiple reasons, with disease progression the most common cause for discontinuing treatment on once-daily and twice-daily regimens in both patient cohorts (Fig. 1). Nine patients with MBP-CML and 6 patients with LBP-CML left the study to pursue stem cell transplantation (SCT).
Baseline disease characteristics and demographics were similar between the once-daily and twice-daily regimens for both patient cohorts (Table 1). Both cohorts had prolonged periods of previous CML and advanced disease. Patients in both cohorts were pretreated extensively with imatinib and other therapies and mostly were imatinib resistant.
Table 1.
Baseline Patient Characteristics
| CML in Myeloid Blast Phase | CML in Lymphoid Blast Phase | |||
|---|---|---|---|---|
| Characteristic | Dasatinib 140 mg QD, n575 |
Dasatinib 70 mg BID, n574 |
Dasatinib 140 mg QD, n533 |
Dasatinib 70 mg BID, n528 |
| Median age [range], y | 48 [16–78] | 52 [18–76] | 49 (21–76) | 56 (21–78) |
| Median CML duration [range], mo | 41 [1–461] | 40 [1–212] | 31 [5–172] | 23 [4–137] |
| Median blast phase duration [range], mo | 5.2 [0.03–103.5] | 4.7 [0.1–85.8] | 3.6 [0.2–41.0] | 7.1 [0.3–61.7] |
| Highest previous imatinib dose, n (%)a | ||||
| 400–600 mg | 42 (56) | 40 (54) | 18 (55) | 17 (61) |
| >600 mg | 33 (44) | 34 (46) | 15 (45) | 10 (36) |
| Previous imatinib therapy duration, n (%) | ||||
| <1 y | 28 (37) | 23 (31) | 17 (52) | 13 (46) |
| 1–3 y | 25 (33) | 26 (35) | 9 (27) | 12 (43) |
| >3 y | 22 (29) | 25 (34) | 7 (21) | 3 (11) |
| Imatinib resistance, n (%) | 60 (80) | 64 (86) | 27 (82) | 26 (93) |
| Imatinib intolerance, n (%) | 15 (20) | 10 (14) | 6 (18) | 2 (7) |
| Other previous treatment, n (%) | ||||
| Interferon-a | 28 (37) | 28 (38) | 13 (39) | 7 (25) |
| Chemotherapy | 46 (61) | 37 (50) | 22 (67) | 21 (75) |
| Stem cell transplantation | 12 (16) | 9 (12) | 6 (18) | 6 (21) |
| Radiotherapy | 7 (9) | 6 (8) | 5 (15) | 4 (14) |
| Median platelet count/nL [range] | 46 [2–1495] | 49 [7–3993] | 47 [2–331] | 41 [6–611] |
| Platelets <100/nL | 48 (64) | 48 (65) | 25 (76) | 19 (68) |
| Median WBC count/nL [range] | 22 (0.4–223) | 21 [0.1–258] | 12 [1–300] | 7 [1–127] |
| WBC count ‡20/nL | 42 (56) | 38 (51) | 15 (45) | 9 (32) |
| Median bone marrow blasts [range], % | 42 [0–99] | 41 [0–94] | 70 [0–98] | 81 [1–100] |
CML indicates chronic myeloid leukemia; QD, once daily; BID, twice daily; WBC, white blood cell.
No data were available for 1 patient with CML in lymphoid blast phase who was randomized to receive dasatinib 70 mg twice daily.
Hematologic Response
In patients with MBP-CML, the rate of confirmed MHR was 28% for both regimens, including a 17% CHR rate for the once-daily regimen and an 18% CHR rate for the twice-daily regimen (Table 2). The median time to MHR was 2 months for the once-daily regimen and 3 months for the twice-daily regimen, and the respective median MHR duration was 8 months and 9 months (Table 2).
Table 2.
Best Hematologic and Cytogenetic Responses
| CML in Myeloid Blast Phase |
CML in Lymphoid Blast Phase |
|||
|---|---|---|---|---|
| Response | Dasatinib 140 mg QD |
Dasatinib 70 mg BID |
Dasatinib 140 mg QD |
Dasatinib 70 mg BID |
| Hematologic response | ||||
| No. of patients | n¼75 | n¼74 | n¼33 | n¼28 |
| MHR, n (%) | 21 (28) | 21 (28) | 14 (42) | 9 (32) |
| 95% CI | 18–40 | 19–40 | 26–61 | 16–52 |
| Median time to MHR [95%CI], moa | 2 [1.8–2.8] | 3 [1.5–2.8] | 2 [1.0–1.8] | 2 [1.2–3.0] |
| Median duration of MHR [95% CI], moa | 8 [5.6–20.7] | 9 [7.1-NA] | 5 [4.0–8.1] | 8 [7.1–9.9] |
| Any BCR-ABL mutation, n/total (%) | 9/32 (28) | 9/28 (32) | 7/20 (35) | 4/14 (29) |
| No mutation, n/total (%) | 10/33 (30) | 10/36 (28) | 3/9 (33) | 2/7 (29) |
| Complete hematologic response, n (%) | 13 (17) | 13 (18) | 7 (21) | 4 (14) |
| No evidence of leukemia, n (%) | 8 (11) | 8 (11) | 7 (21) | 5 (18) |
| Cytogenetic responseb | ||||
| No. of patients | n¼72 | n¼71 | n¼32 | n¼25 |
| MCyR, n (%) | 18 (25) | 20 (28) | 16 (50) | 10 (40) |
| 95% CI | 16–37 | 18–40 | 32–68 | 21–61 |
| Median time to MCyR [95%CI], moa | 1.9 [1.4–2.8] | 1.6 [1.0–2.8] | 1.4 [1.0–1.9] | 1.4 [1.1–1.9] |
| Median duration of MCyR [95%CI], moa | 7.7 [4.7–13.1] | 10.1 [7.3-NA] | 4.0 [3.0–6.3] | 7.0 [2.0–10.5] |
| CCyR, n (%) | 10 (14) | 15 (21) | 12 (38) | 9 (36) |
| Median time to CCyR [95% CI], moa | 2.1 [1.8–2.8] | 2.8 [1.7–3.6] | 1.8 [1.0–1.9] | 1.4 [1.1–1.9] |
| Median duration of CCyR [95% CI], moa | NR [3.7-NA] | 7.4 [7.1-NA] | 4.9 [3.9–6.3] | 7.9 [3.7–10.5] |
CML indicates chronic myeloid leukemia; QD, once daily; BID, twice daily; MHR, major hematologic response; CI, confidence interval; BCR-ABL, breakpoint cluster region-Abelson murine leukemia viral oncogene fusion protein; NA, not available; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; NR, not reached.
Computed from Kaplan-Meier analysis in patients who achieved this response.
The analysis excluded 10 Philadelphia chromosome-negative patients. Cytogenetic response could not be determined among patients with CML in myeloid blast phase for 22 patients in the QD arm and 23 patients in the BID arm or among patients with CML in lymphoid blast phase for 8 patients in the QD arm and 9 patients in the BID arm.
In patients with LBP-CML, the rate of MHR was 42% for the once-daily regimen and 32% for the twice-daily regimen (Table 2). The median time to MHR was 2 months for both regimens, and the median MHR duration was 5 months for the once-daily regimen and 8 months for the twice-daily regimen (Table 2).
In both patient cohorts, 2 regimens produced similar rates of MHR in patients with or without baseline mutations (Table 2). None of the patients who had the threonine-to-isolucine mutation at codon 315 (T315I) BCR-ABL mutation (2 patients with MBP-CML and 4 patients with LBP-CML) achieved an MHR with either dosing schedule.
Cytogenetic Response
For the analysis of cytogenetic response, Ph-negative patients were excluded in both the MBP-CML cohort and the LBP-CML cohort. In patients with MBP-CML, the MCyR rates were 25% for the once-daily regimen and 28% for the twice-daily regimen, including CCyR rates of 14% for the once-daily regimen and 21% for the twice-daily regimen (Table 2). The median time to and duration of MCyR with the once-daily regimen (1.9 months and 7.7 months, respectively) were similar to those with the twice-daily regimen (1.6 months and 10.1 months, respectively) (Table 2). The median time to and duration of CCyR with the once-daily and twice-daily regimens generally were similar (Table 2).
In patients with LBP-CML, the MCyR rates generally were similar between the once-daily (50%) and twice-daily (40%) regimens, and the CCyR rates also were similar (38% vs 36%, respectively) (Table 2). The median times to MCyR and CCyR ranging from 1.4 months to 1.8 months were similar between 2 regimens. The median durations of MCyR and CCyR in the once-daily arm were 4.0 months and 4.9 months, respectively, and those in the twice-daily arm were 7.0 months and 7.9 months, respectively (Table 2).
Of the 15 patients who discontinued the study treatment to pursue SCT, 12 patients achieved a cytogenetic response (CCyR, PCyR, or minimal cytogenetic response), and only 5 patients achieved a hematologic response (CHR or NEL) (Table 3).
Table 3.
Best Response Rates During Dasatinib Treatment for Patients Who Discontinued Dasatinib to Undergo Allogeneic Stem Cell Transplantation
| Dasatinib Regimen | Best CyRa | Duration of MCyR, db |
Best Hematologic Responsec |
Duration of MHR, db |
|---|---|---|---|---|
| CML in myeloid blast phase | ||||
| Dasatinib 140 mg QD | ||||
| Patient 1 | Complete | 1 | Complete | 62 |
| Patient 2 | Minimal | NA | NEL | 292 |
| Patient 3 | Partial | 1 | No response | NA |
| Dasatinib 70 mg BID | ||||
| Patient 4 | Complete | 1 | NEL | 56 |
| Patient 5 | Partial | 1 | No response | NA |
| Patient 6 | No response | NA | No response | NA |
| Patient 7 | Complete | 1 | No response | NA |
| Patient 8 | Partial | 1 | No response | NA |
| Patient 9 | No response | NA | No response | NA |
| CML in lymphoid blast phase | ||||
| Dasatinib 140 mg QD | ||||
| Patient 10 | Complete | 96 | No response | NA |
| Patient 11 | Complete | 118 | NEL | 144 |
| Patient 12 | Complete | 138 | Complete | 208 |
| Patient 13 | Minimal | NA | No response | NA |
| Patient 14 | Unable to determine | NA | No response | NA |
| Dasatinib 70 mg BID | ||||
| Patient 15 | Complete | 57 | No response | NA |
CyR indicates cytogenetic response; MCyR, major cytogenetic response; MHR, major hematologic response; CML, chronic myeloid leukemia; QD, once daily; NA, not applicable; NEL, no evidence of leukemia; BID, twice daily.
An MCyR was defined to include either a complete or partial cytogenetic response.
Censored at the last hematologic or cytogenetic assessment.
An MHR was defined to include either a complete hematologic response or NEL.
Progression-Free Survival
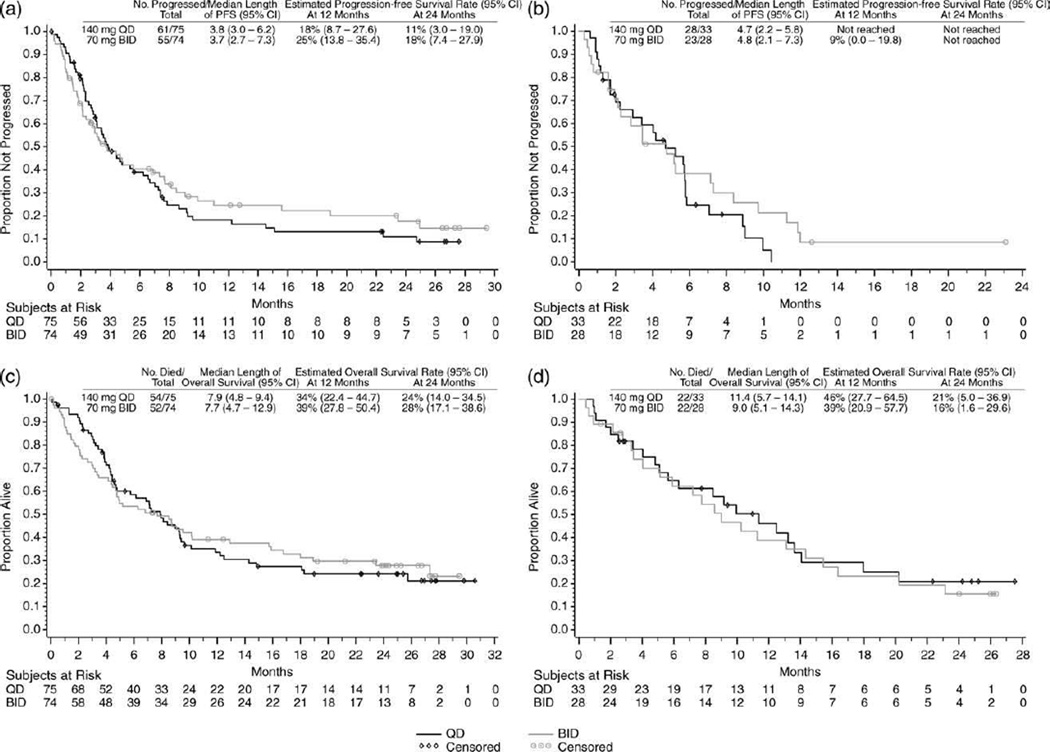
Among the patients with MBP-CML, the median PFS was similar between the once-daily arm (3.8 months) and the twice-daily arm (3.7 months). The 24-month PFS rate was 11% for the once-daily regimen and 18% for the twice-daily regimen (Fig. 2a). Among the patients with LBP-CML, the median PFS was similar between the once-daily arm (4.7 months) and the twice-daily arm (4.8 months) (Fig. 2b).
Figure 2.
These Kaplan-Meier curves illustrate (a,b) progression-free survival (PFS) in patients who had chronic myeloid leukemia (CML) in (a) myeloid blast phase and (b) lymphoid blast phase and (c,d) overall survival in patients who had CML in (c) myeloid blast phase and (d) lymphoid blast phase. For PFS analysis, patients who neither progressed nor died were censored on the date of their last cytogenetic or hematologic assessment. For overall survival analysis, patients who had not died or were lost to follow-up were censored on the last date they were known alive. QD indicates once-daily dasatinib; BID, twice-daily dasatinib; CI, confidence interval.
Overall Survival
Among the patients with MBP-CML, the median OS was similar between the once-daily arm (7.9 months) and the twice-daily arm (7.7 months), and the 24-month survival rates also were similar (24% vs 28%, respectively) (Fig. 2c). The majority of deaths were considered to be from CML in both the once-daily arm (54%) and the twice-daily arm (41%). Among the patients with LBP-CML, the median OS also was similar between the once-daily arm (11.4 months) and the twice-daily arm (9.0 months). The 24-month OS rate was 21% for the once-daily arm and 16% for the twice-daily arm(Fig. 2d). The majority of deaths were considered to be from CML in both the once-daily arm (30%) and the twice-daily arm (43%).
Safety
Treatment-related nonhematologic adverse events generally were grade 1 or 2 (Table 4). The more common events were fluid retention, diarrhea, headache, bleeding, nausea, fatigue, and rash. Rates of nonhematologic grade 3/4 events were similar between the 2 regimens in both the MBP-CML cohort and the LBP-CML cohort. The rates of pleural effusion (all grades) in patients with MBP-CML were similar between the once-daily arm (20%) and the twice daily arm (18%), whereas there was a trend favoring the once-daily regimen (21%) over the twice-daily regimen (36%) in patients with LBP-CML. The occurrence of grade 3/4 pleural effusion was ::;6% in both treatment arms, and there was only 1 grade 4 event. Cytopenias were common with similar incidence between the 2 regimens in both patient cohorts (Table 4). Twenty-three patients in the MBP-CML cohort (once-daily arm, 7 patients; twice-daily arm, 16 patients) and 6 patients in the LBP-CML cohort (once-daily arm, 5 patients; twice-daily arm, 1 patient) discontinued because of drug toxicity.
Table 4.
Selected Adverse Events Associated With Dasatinib
| No. of Patients (%) | ||||||||
| CML in Myeloid Blast Phase | CML in Lymphoid Blast Phase | |||||||
| Dasatinib 140 mg | Dasatinib 70 mg BID | Dasatinib 140 mg | Dasatinib 70 mg BID | |||||
| Adverse Events Nonhematologic |
All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
| No. of patients | 74 | 74 | 33 | 28 | ||||
| Fluid retention | 25 (34) | 4 (5) | 23 (31) | 8 (11) | 7 (21) | 2 (6) | 11 (39) | 2 (7) |
| Pleural effusion | 15 (20) | 4 (5) | 13 (18) | 4 (5) | 7 (21) | 2 (6) | 10 (36) | 1 (4) |
| Superficial edema | 10 (14) | 0 (0) | 10 (14) | 2 (3) | 1 (3) | 0 (0) | 7 (25) | 0 (0) |
| Other | 7 (9) | 1 (1) | 7 (9) | 4 (5) | 0 (0) | 0 (0) | 3 (11) | 1 (4) |
| Diarrhea | 15 (20) | 4 (5) | 15 (20) | 2 (3) | 6 (18) | 0 (0) | 11 (39) | 2 (7) |
| Headache | 13 (18) | 1 (1) | 9 (12) | 1 (1) | 5 (15) | 1 (3) | 7 (25) | 2 (7) |
| Bleeding | 14 (19) | 7 (9) | 20 (27) | 7 (9) | 8 (24) | 3 (9) | 6 (21) | 1 (4) |
| Other | 9 (12) | 2 (3) | 10 (14) | 1 (1) | 4 (12) | 1 (3) | 4 (14) | 0 (0) |
| GI bleeding | 7 (9) | 5 (7) | 11 (15) | 6 (8) | 3 (9) | 1 (3) | 1 (4) | 0 (0) |
| CNS bleeding | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 1 (3) | 1 (3) | 1 (4) | 1 (4) |
| Nausea | 17 (23) | 1 (1) | 9 (12) | 0 (0) | 7 (21) | 1 (3) | 7 (25) | 1 (4) |
| Fatigue | 15 (20) | 1 (1) | 8 (11) | 0 (0) | 3 (9) | 1 (3) | 4 (14) | 2 (7) |
| Rash | 11 (15) | 1 (1) | 9 (12) | 0 (0) | 7 (21) | 0 (0) | 3 (11) | 0 (0) |
| Febrile neutropenia | 9 (12) | 9 (12) | 5 (7) | 5 (7) | 4 (12) | 4 (12) | 3 (11) | 3 (11) |
| No. of Patients (%) | ||||||||
| CML in Myeloid Blast Phase | CML in Lymphoid Blast Phase | |||||||
| Dasatinib 140 mg | Dasatinib 70 mg BID | Dasatinib 140 mg | Dasatinib 70 mg BID | |||||
| Cytopenias | All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
All Grades |
Grade 3/4 |
| No. of patients | 72 | 73 | 33 | 28 | ||||
| Neutropenia | 65 (90) | 57 (79) | 65 (89) | 54 (74) | 31 (94) | 26 (79) | 26 (93) | 24 (86) |
| Thrombocytopenia | 67 (93) | 58 (81) | 67 (92) | 58 (80) | 32 (97) | 28 (85) | 28 (100) | 24 (86) |
| Anemia | 70 (97) | 55 (76) | 73 (100) | 57 (78) | 33 (100) | 17 (52) | 28 (100) | 14 (50) |
| Leukocytopenia | 60 (83) | 44 (61) | 61 (84) | 44 (60) | 30 (91) | 26 (79) | 26 (93) | 18 (64) |
CML indicates chronic myeloid leukemia; QD, once daily; BID, twice daily; GI, gastrointestinal; CNS, central nervous system.
Exposure and Dose Modifications
Patients with MBP-CML received similar median average daily doses of dasatinib on the once-daily (140 mg) and twice-daily (138 mg) regimens, and the median treatment duration was approximately 3 months for both regimens (Table 5). In patients with LBP-CML, the median average daily doses were 140 mg on the once-daily regimen and 123 mg on the twice-daily regimen, and the corresponding median treatment durations were 3.4 months and 3.6 months, respectively (Table 5). The rates of dose interruption in the MBP-CML cohort were similar between 2 regimens; whereas, in the LBP-CML cohort, there were fewer interruptions in the once-daily arm compared with the twice-daily arm (Table 5). In both the MBP-CML cohort and the LBP-CML cohort, the once-daily regimen (18% and 27%, respectively) necessitated fewer dose reductions than the twice-daily regimen (32% and 39%, respectively), and the majority of reductions were because of nonhematologic toxicities (Table 5). Dose escalation in the MBP-CML cohort was necessary for more patients who received once-daily dosing (49%) compared with twice-daily dosing (22%), whereas the escalation rates in the LBP-CML cohort were similar between the 2 treatment arms (Table 5).
Table 5.
Dasatinib Exposure and Dose Modifications
| CML in Myeloid Blast Phase | CML in Lymphoid Blast Phase | |||
|---|---|---|---|---|
| Variable | Dasatinib 140 mg QD, n574 |
Dasatinib 70 mg BID, n574 |
Dasatinib 140 mg QD, n533 |
Dasatinib 70 mg BID, n528 |
| Median average daily dasatinib dose [range], mg/d Treatment duration median [range], mo |
140 [49–177] 3.3 [0.0–27.7] |
138 [40–175] 3.1 [0.0–27.7] |
140 [60–170] 3.4 [0.1–10.4] |
123 [83–171] 3.6 [0.2–22.1] |
| Dose interruption, n (%) | 32 (43) | 34 (46) | 11 (33) | 16 (57) |
| Hematologic toxicitya | 10 (14) | 14 (19) | 3 (9) | 6 (21) |
| Nonhematologic toxicitya | 21 (28) | 17 (23) | 8 (24) | 9 (32) |
| Dose reduction, n (%) | 13 (18) | 24 (32) | 9 (27) | 11 (39) |
| Hematologic toxicitya | 3 (4) | 7 (10) | 2 (6) | 3 (11) |
| Nonhematologic toxicitya | 10 (14) | 13 (18) | 7 (21) | 7 (25) |
| Dose escalation, n (%) | 36 (49) | 16 (22) | 10 (30) | 8 (29) |
| Loss or lack of responsea | 17 (23) | 8 (11) | 5 (15) | 4 (14) |
| Rising percentage blastsa,b | 18 (24) | 7 (10) | 5 (15) | 4 (14) |
CML indicates chronic myeloid leukemia; QD, once daily; BID, twice daily.
Reasons for dose interruption, reduction, or escalation.
Rising percentage blasts on 2 consecutive hematologic assessments at least 2 weeks apart.
DISCUSSION
The 2-year follow-up results reported here demonstrate that hematologic and cytogenetic response rates and the time to and duration of these responses generally were similar between the dasatinib 140 mg once-daily regimen and the 70 mg twice-daily regimen in both the MBP-CML cohort and the LBP-CML cohort and that the durations and rates of PFS and OS also were similar. Although the most clinically relevant adverse events generally were similar between the 2 regimens, in patients with LBP-CML, pleural effusions occurred less frequently on the once-daily regimen (21%) than on the twice-daily regimen (36%). In addition, in both patient cohorts, the once-daily arm had fewer dose reductions or interruptions because of toxicity compared with the twice-daily arm. Although the subset analyses presented here were not powered adequately to detect small differences, similar efficacy and improved tolerability between the schedules were observed consistently in patients with CML-AP or Ph-positive ALL, 2 other subsets that were studied in this phase 3 trial.17,18 In addition, an earlier phase 3 trial in patients with CML-CP demonstrated similar efficacy for 4 different dasatinib schedules, including 140 mg once daily, 70 mg twice daily, 100 mg once daily, and 50 mg twice daily, and the 100 mg once-daily regimen had a superior safety profile.16 Taken together, these observations lead to the conclusion that dasatinib 140 mg once daily may have efficacy and improved safety similar to those of the 70 mg twice-daily regimen in patients with CML-BP.
Our efficacy and safety results are consistent with the pharmacodynamics and pharmacology of dasatinib. Consistent with a plasma terminal half-life of 3 to 4 hours of oral dasatinib, the twice-daily dose produced a more sustained inhibition of the BCR-ABL kinase, as monitored by the phosphorylation of the substrate, than the once-daily dosing.19 In patients with CML-CP, the dasatinib trough concentration (Cmin) was correlated with tolerability, suggesting that increased toxicity with the twice-daily dose may be a reflection of persistent target inhibition.19 Because it was demonstrated that transient exposure of CML lines to dasatinib in vitro caused an inhibition of apoptosis that lasted for 48 hours, it has been hypothesized that peak plasma levels cause the persistent inhibition of cell proliferation.20,21 The improved peak plasma level and reduced Cmin of dasatinib after once-daily oral administration provides a biochemical rationale for similar efficacy and improved tolerability of 140 mg once daily relative to 70 mg twice daily.
In addition to supporting the view that the once-daily regimen of dasatinib has similar efficacy and is likely to be tolerated better than the twice-daily regimen, even in the most advanced phases of the disease, the current results also reinforce the notion that dasatinib is highly efficacious in these conditions. Before entering our study, patients who had suffered from CML for a prolonged period (median, 23–41 months) were in blast phase for a median of at least 4 months, underwent extensive treatment, and failed on imatinib. Given the dismal clinical status of these patients and their lack of effective therapeutic options, the current data on dasatinib are important. Dasatinib treatment induced high response rates, both hematologic and cytogenetic, with good durability in some patients. The median OS duration and the 24-month OS rates across both schedules were reasonable in the MBP-CML cohort (median OS duration, 7.7–7.9 months; 24-month OS rate, 24%–28%) and the LBP-CML cohort (median OS duration, 9.0–11.4 months; 24-month OS rate, 16%–21%). On the basis of these results, a lower incidence of recurrence and improved clinical benefit probably could be expected with dasatinib treatment earlier in the course of CML-BP, when patients have received less pretreatment. Patients with CML-BP at diagnosis probably also could benefit from dasatinib as first-line treatment rather than as second-line treatment after failure on imatinib.
Short response duration because of disease recurrence or progression is a clinical challenge for these patients. Allogeneic SCT after a TKI-induced remission offers a highly desirable option to sustain a remission in these patients, because this approach may eradicate the Ph-positive clone. The use of dasatinib after SCT also could prove beneficial in maintaining a remission. Data are limited, however, on the outcomes of SCT after TKI treatment in patients with CML after imatinib resistance or failure22 and are particularly limited on the use of TKIs after patients have undergone SCT.23 Only a minority of patients with CML-BP (15 of 210 patients) in the current study discontinued dasatinib to undergo SCT, and these included patients who had not achieved a cytogenetic response during dasatinib therapy (Table 3), underscoring the very poor clinical status of the patients with CML-BP who were investigated. The encouraging efficacy of dasatinib in this study indicates the potential utility of dasatinib for improving the outcome of allogeneic SCT in patients with CML-BP.
In conclusion, the current analysis of 210 patients confirms and extends the results from 2 previous phase 2 studies that evaluated the efficacy and safety of dasatinib 70 mg twice daily in patients with CML-BP who were resistant or intolerant to imatinib.13,14 The analysis further demonstrates that dasatinib 140 mg once daily generally has similar efficacy and a trend toward improved tolerability compared with the 70 mg twice-daily regimen in these patients. Our data support the use of 140 mg once daily as a starting dose for the treatment of patients with CML-BP who are resistant or intolerant to imatinib.
Acknowledgments
This study was funded by Bristol-Myers Squibb (BMS). Dr. Saglio advised and received honoraria from BMS and Novartis (NVS); Dr. Hochhaus received research funding from BMS, NVS, and Wyeth; Dr. Goh advised BMS, received honoraria from BMS and Celgene, and received research funding from BMS, NVS, and Wyeth; Dr. Pasquini received honoraria from BMS and NVS; Dr. Maloisel advised NVS and Amgen and received honoraria from NVS and Aventis-Sanofi; Dr. Cortes received research funding from BMS and NVS; Dr. Paquette advised and received honoraria from BMS; Dr. Bradley-Garelik and Dr. Zhu are employed by BMS;
Footnotes
Presented at the 50th Annual Meeting of the American Society of Hematology, San Francisco, California, December 6–9, 2008.
Writing and editorial assistance were provided by Elizabeth Dolgos and Motasim Billah, employees of Bristol-Myers Squibb.
CONFLICT OF INTEREST DISCLOSURES
Dr. Masszi, Dr. Erben, and Dr. Dombret declared no competing interests.
REFERENCES
- 1.Giles FJ, Cortes JE, Kantarjian HM, O’Brien SM. Accelerated and blastic phases of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:753–774. doi: 10.1016/j.hoc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Cortes J, O’Brien S, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myelogenous leukemia in blast phase. Blood. 2002;99:3547–3553. doi: 10.1182/blood.v99.10.3547. [DOI] [PubMed] [Google Scholar]
- 5.Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 6.Palandri F, Castagnetti F, Testoni N, et al. Chronic myeloid leukemia in blast crisis treated with imatinib 600 mg: outcome of the patients alive after a 6-year follow-up. Haematologica. 2008;93:1792–1796. doi: 10.3324/haematol.13068. [DOI] [PubMed] [Google Scholar]
- 7.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Meng F, Kong LY, et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J Natl Cancer Inst. 2008;100:926–939. doi: 10.1093/jnci/djn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 10.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 11.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 12.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 13.Cortes J, Kim DW, Raffoux E, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22:2176–2183. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 15.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 16.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and - intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–6329. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson RA, Ottmann O, Shah NP, et al. Dasatinib 140 MG ONCE Daily (QD) has equivalent efficacy and improved safety compared with 70 mg twice daily (BID) in patients with imatinib-resistant or -intolerant Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL-2-year data from CA180-035 [abstract] Blood. 2008;112 Abstract 186. [Google Scholar]
- 19.Wang X, Hochhaus A, Kantarjian HM, et al. Dasatinib pharmacokinetics and exposure-response (E-R): relationship to safety and efficacy in patients (pts) with chronic myeloid leukemia (CML) [abstract] J Clin Oncol. 2008;26:175s. Abstract 3590. [Google Scholar]
- 20.Shah NP, Kasap C, Weier C, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Snead JL, O’Hare T, Adrian LT, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–3463. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Cortes J, Kantarjian H, et al. Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: no evidence for increased transplant-related toxicity. Cancer. 2007;110:340–344. doi: 10.1002/cncr.22778. [DOI] [PubMed] [Google Scholar]
- 23.Atallah E, Kantarjian H, de Lima M, et al. The role of dasatinib in patients with Philadelphia (Ph) positive acute lymphocytic leukemia (ALL) and chronic myeloid leukemia (CML) relapsing after stem cell transplantation (SCT) [abstract] Blood (ASH Annual Meeting Abstracts) 2006;108:4520. [Google Scholar]