Abstract
Study Design
Systematic review.
Objective
To determine the incidence, pathogenesis, and clinical outcomes related to neurogenic fevers following traumatic spinal cord injury (SCI).
Methods
A systematic review of the literature was performed on thermodysregulation secondary to acute traumatic SCI in adult patients. A literature search was performed using PubMed (MEDLINE), Cochrane Central Register of Controlled Trials, and Scopus. Using strict inclusion and exclusion criteria, seven relevant articles were obtained.
Results
The incidence of fever of all origins (both known and unknown) after SCI ranged from 22.5 to 71.7% with a mean incidence of 50.6% and a median incidence of 50.0%. The incidence of fever of unknown origin (neurogenic fever) ranged from 2.6 to 27.8% with a mean incidence of 8.0% and a median incidence of 4.7%. Cervical and thoracic spinal injuries were more commonly associated with fever than lumbar injuries. In addition, complete injuries had a higher incidence of fever than incomplete injuries. The pathogenesis of neurogenic fever after acute SCI is not thoroughly understood.
Conclusion
Neurogenic fevers are relatively common following an acute SCI; however, there is little in the scientific literature to help physicians prevent or treat this condition. The paucity of research underscored by this review demonstrates the need for further studies with larger sample sizes, focusing on incidence rate, clinical outcomes, and pathogenesis of neurogenic fever following acute traumatic SCI.
Keywords: autonomic dysreflexia, fever, spinal cord injuries, thermoregulation, neurogenic fever, acute spinal cord injury, spine trauma, neurologic intensive care
Introduction
With a reported incidence ranging from 38.5 to 71.7% and a mean incidence of 48.9%, the presence of a fever is common following an acute spinal cord injury (SCI).1 2 3 4 Patients with acute traumatic SCI are at increased risk of fever because of thermoregulatory abnormalities arising from dysfunction of the autonomic system. Febrile complications of SCI have the following etiologies: infections (urinary tract, pulmonary, upper respiratory tract, soft tissues, gastrointestinal, and spinal abscess), deep venous thrombosis, pulmonary embolism, colitis, heterotopic ossification, and drug fevers.1 2 3 4 5 The most common identifiable cause of fever is urinary tract infection.1 3 4 6 7 8 9
Despite the many documented known origins of fever, there are also cases with no identifiable origin. Many have argued that fevers of unknown origin may be classified as neurogenic and may be attributed to impairment of autonomic function.10 Neurogenic fever is currently a diagnosis of exclusion, assigned after all possible alternative etiologies have been ruled out.11 12 The purpose of this systematic review is to analyze and provide clinical guidelines to study the incidence, pathogenesis, clinical outcomes, and management options for patients with neurogenic fever and acute traumatic SCI.
Methods
Study Search
A systematic review of the literature on neurogenic fever and SCI was performed using PubMed (MEDLINE), Cochrane Central Register of Controlled Trials, and Scopus (EMBASE, MEDLINE, COMPENDEX). No restrictions were placed on publication date. Utilizing PubMed, the following Medical Subject Headings (MeSH)/Index terms were entered: “Spinal Cord Injuries”[Mesh] and “Fever”[Mesh]. Additionally, the following terms were searched in each database: “spinal cord,” “pyrexia,” and “hyperthermia.” After removing duplicates, 1,503 articles were collected.
Inclusion and Exclusion Criteria
The primary requirement for inclusion was the description of one or more cases of a febrile episode of unknown etiology following SCI. For the purposes of this review, we focused on the reports that defined a febrile episode as greater than or equal to 100°F (37.7°C). Cases that presented unidentifiable fever after SCI in addition to other associated events were included. Only seven results were found that met our inclusion criteria.
Studies describing only known causes of hyperthermia or pyrexia after SCI were excluded, as were all pediatric cases (patients younger than 19 years of age), editorials, and opinion articles.
Study Finalization
Details of the systematic review methodology are shown in Fig. 1. The 1,503 articles were screened first by title and second by abstract to remove irrelevant studies. The full texts of the remaining 131 articles were subsequently screened. Of those articles, 8 texts were not in the English language, and 120 were irrelevant. Finally, 4 additional studies not obtained in the original search were introduced. The final 7 studies (Table 1) were reviewed for the following clinical data: minimum temperature required for fever; recorded time of fever; number of patients presenting with fever; total number of febrile events in all patients with SCI; incidence percentage of fever; mean length of stay, acute or rehab; number of patients with fever of unknown origin; average duration of fever of unknown origin; American Spinal Injury Association Impairment Score; nature and frequency of unsuccessful treatment attempts; and cause of death. The incidence of fever of unknown origin was calculated by dividing the number of patients with fever of unidentifiable etiology by the total number of patients with SCI who presented with fever. The terms mentioned previously were then extracted into evidence tables.
Fig. 1.
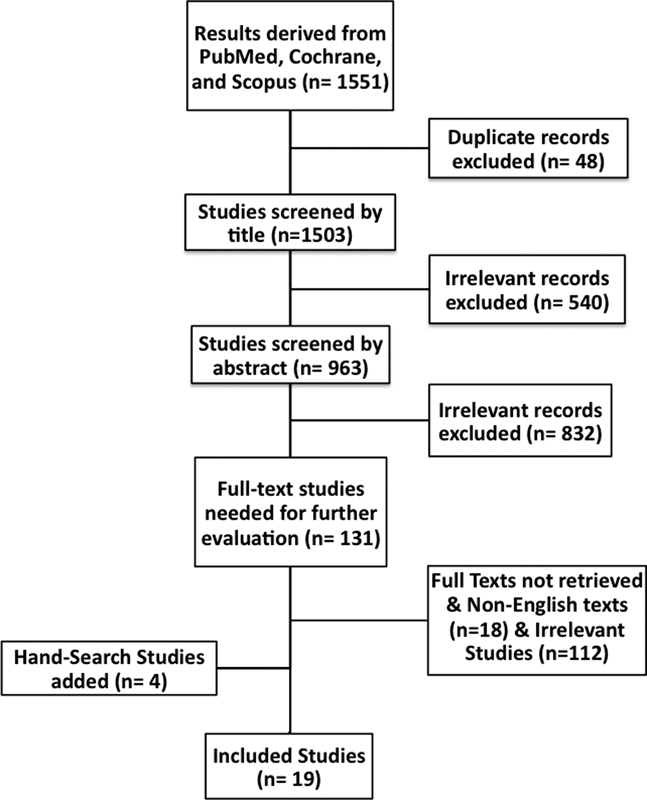
Flowchart illustrating the systematic review methodology and article selection process.
Table 1. Case series of fever following acute traumatic SCI.
| Primary author | Recorded time of fever | Prospective or retrospective | No. of SCI patients | Definition of fever | No. of patients presenting with fever | Incidence of fever (%) | Total no. of febrile events in all SCI patients | Mean LOS (d) | Mean age (y) | No. of patients with fever of unknown origin | Incidence of fever of unknown origin (%) | Mean duration of undiagnosed fever (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beraldo4 | NR | Prospective | 129 | Axillary temperature ≥ 38°C | 62 | 48.1 | 75 | NR | NR | 6 | 4.7 | NR |
| Colachis2 | NR | Retrospective | 156 | Core body temperature > 37.7°C | 60 | 38.5 | NR | 82.7 (rehab) | 31 | 4 | 2.6 | 3.8 |
| Commichau36 | NR | Prospective | 387 | First temperature ≥ 38.3°C | 87 | 22.5 | NR | 3.3 | 54 | 26 | 6.7 | NR |
| McKinley5 | Acute | Retrospective | 48 | Any recorded temperature > 37.7°C | 29 | 60.4 | 58 | NR | 41 | NR | NR | 2.2 |
| Rehab | Retrospective | 40 | 20 | 50.0 | 66 | NR | 42 | NR | NR | 1.4 | ||
| Sugarman1 | NR | Retrospective | 46 | NR | 33 | 71.7 | 106 | NR | 42 | 3 | 6.5 | NR |
| NR | Prospective | 70 | 46 | 65.7 | 71 | 67 (acute and rehab) | 41 | 3 | 4.3 | NR | ||
| Ulger10 | Acute | Retrospective | 18 | NR | 5 | NR | NR | 7.6 | 42 | 5 | 27.8 | 5.2 |
| Unsal-Delialioglu3 | Rehab | Retrospective | 392 | Elevation of body temperature > 37.7°C at least twice in 24 h | 187 | 47.7 | NR | 54.86 (rehab) | 37 | 13 | 3.3 | NR |
Abbreviations: LOS, length of stay; NR, not recorded; SCI, spinal cord injury.
The objective of this review was to answer the following questions:
What is the incidence of thermoregulatory dysfunction of known and unknown causes following acute traumatic SCI?
What are the clinical outcomes and consequences of fever of neurogenic origin in acute traumatic SCI?
What is the possible pathogenesis of neurogenic fever after acute traumatic SCI?
What does translational research demonstrate about thermoregulatory dysfunction following SCI?
Results
Incidence of Thermoregulatory Dysfunction of Known and Unknown Causes following Acute Traumatic Spinal Cord Injury
Acute traumatic SCI has an incidence rate in the United States of ∼12,000 cases per year.13 The mean length of hospital stay ranges from 55 to 83 days.1 2 3 Thermoregulatory dysfunction is defined as the impairment of temperature regulation and includes hyperthermia as well as hypothermia, with the former condition more common in traumatic SCI.14 Fever of various known and unknown origins may occur in SCI. Table 1 summarizes the results of all seven studies. The incidence of all fever and fever of unknown origin after SCI is depicted in Fig. 2. The incidence of fever of all (known and unknown) origins is reported to be as high as 71.7%, ranging from 22.5 to 71.7% with a mean incidence of 50.6% and a median incidence of 50.0%.1 2 3 4 The incidence of fever of unknown origin ranges from 2.6 to 27.8%, with a mean incidence of 8.0% and a median incidence of 4.7%.1 2 3 4 In patients with SCI, fever of unknown origin is often attributed to neurogenic sources, following extensive investigation of its possible causes.
Fig. 2.
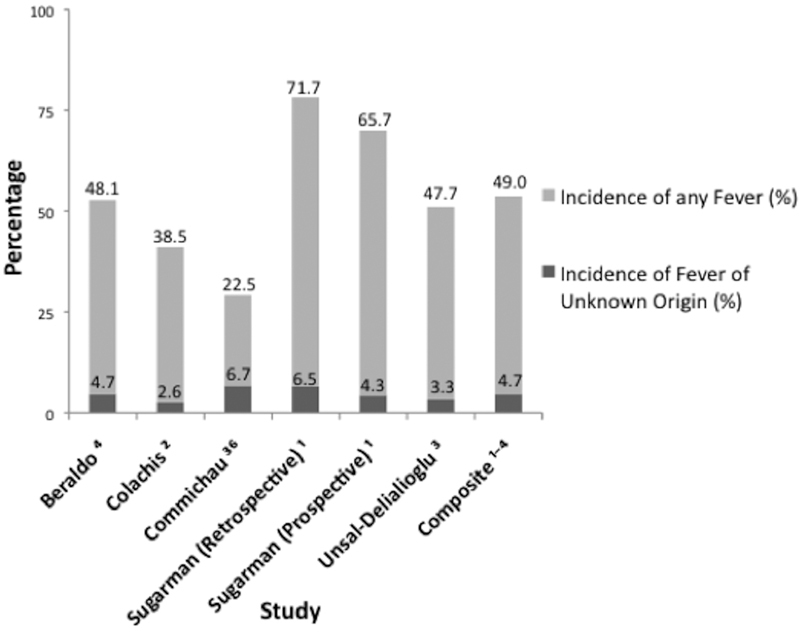
Incidence of fever of all known and unknown origins after traumatic spinal cord injury.
The relation of fever to the level and extent of SCI is represented in Table 2. In a report by Colachis and Otis exploring the topic of fever following spinal injury, complete injuries were more common (63) than incomplete (8) injuries. Cervical SCIs were the most common level (50 cases), followed by thoracic (21 cases) levels. No SCI cases were observed at the lumbar level. Of the 156 patients in this study, 71 were considered at risk for developing thermoregulatory dysfunction and 60 experienced one or more febrile episodes.2 In another study by Unsal-Delialioglu et al, thoracic lesions were most common (236 patients), followed by cervical lesions (80 cases) and lumbar lesions (76 cases).3 Two hundred five patients had complete lesions, and the other 187 patients' lesions were incomplete. Ulger et al reported five cases of fever of unknown origin, all at the cervical level (Table 3).10 Most sources have consistently indicated that the incidence of fever is significantly higher in patients with complete SCI compared with patients with incomplete SCI.2 3 4 14
Table 2. Relation of fever to level and extent of spinal cord injury.
| Primary author | |||
|---|---|---|---|
| Beraldo4 | Colachis2 | Unsal-Delialioglu3 | |
| Level of injury | |||
| No. of cervical | 44/129 (34.1%) | 50/71 (70.4%) | 80/392 (20.4%) |
| No. of thoracic | 85/129 (65.9%) | 21/71 (29.6%) | 236/392 (60.2%) |
| No. of lumbar | 0 | 0 | 76/392 (19.4%) |
| Extent of injury | |||
| No. of complete | 75/129 (58.1%) | 63/71 (88.7%) | 205/392 (52.3%) |
| No. of incomplete | 54/129 (41.9%) | 8/71 (11.3%) | 187/392 (47.7%) |
Table 3. Case reports of fatal undiagnosed fever following cervical spinal cord injury (Ulger et al10).
| No. | Sex | Age (y) | SCI levels | ASIA score | Duration of fever (d) | Failed treatments | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | M | 39 | C5, C6 | A | 5 | Dopamine | Cardiac arrest |
| 2 | M | 50 | C5, C6 | A | 6 | Dopamine | Cardiac arrest |
| 3 | M | 52 | C6 | A | 3 | Antipyretic and inotropic agents | No cause stated |
| 4 | M | 40 | C5 | A | 5 | Antipyretic agents, supplemental fluids, and cooling agents (ice packs) | Cardiac arrest |
| 5 | M | 29 | C1, C4, C5 | A | 7 | Dopamine, antipyretic agents, and cooling agents (ice packs) | Cardiac arrest |
Abbreviation: ASIA, American Spinal Injury Association; SCI, spinal cord injury.
Clinical Outcomes of Thermoregulation Dysfunction and Neurogenic Fever in Acute Traumatic Spinal Cord Injury
Behavioral characteristics of thermoregulatory dysfunction include altered posture or creating a thermal environment with insulating clothing. Some physical changes that may occur in the thermoregulatory-impaired patient include various temperature adjustments: vasomotor alterations, sweating, and both shivering and nonshivering measures of thermogenesis.15
Ulger et al described five subjects with thermodysregulation following traumatic SCI, all resulting in death (Table 3). None of the subjects had known traumatic brain injuries, although one may have experienced an anoxic brain injury from drowning. Four of the five died of cardiac arrest, and the fifth had no stated cause of death. The average duration of fever was 5.2 days.10 Although this small case series sounds a cautionary note regarding the prognosis of patients with acute traumatic SCI with fever of unknown etiology, it remains unknown whether such patients have a worse prognosis compared with patients with acute traumatic SCI with fever of known origin.1 2 3 4
According to Colachis and Otis, fever duration for patients with SCI during acute care was significantly longer (4.9 days) than during the rehabilitation period (1.9 days).2 McKinley et al observed average maximal temperatures for fevers of both identified and unidentified etiologies during both the acute and rehabilitation phases.5 During acute care, the maximum average unidentified fever temperature was 101.18°F, and the maximum average identified fever temperature was 102.58°F. During rehabilitation, however, the maximum average unidentified fever temperature was 101.78°F, and the maximum average identified fever temperature was 101.58°F. Thus, the average maximum temperature of patients with unidentified fever reported during rehabilitation was greater than the average maximum temperature reported during acute care. This investigation noted a trend toward higher temperatures with increased time from original injury. It also found that the duration of documented fever was shorter during rehab than during acute care (1.3 days in rehabilitation versus 2.2 days in acute care).5
Pathogenesis of Neurogenic Fever after Acute Traumatic Spinal Cord Injury
Exposure to extreme high or low temperature changes can result in either hyperthermia or hypothermia.16 Sugarman et al originated the term quadriplegic fever for prolonged periods of hyperthermia lasting up to several months following acute traumatic SCI.1 In a study conducted by Pollock et al, patients with cervical spinal cord injuries were exposed to a warm water bath (102.5°F) for 40 to 45 minutes.17 The authors then compared temperatures of their subjects with those of a control group of able-bodied individuals immersed in warm water of identical temperature for the same duration of time. Results demonstrated the patients with SCI experienced a core temperature increase of 3.6°F compared with 1.6°F for the control group. These findings are consistent with impaired thermoregulation in patients with SCI.17
It is generally recognized that patients who become paraplegic or tetraplegic following SCI are more likely to develop fever due to lack of temperature regulation. Their fever results from the derangement of thermoregulated functions such as vasoconstriction, vasodilatation, and sweating.15 After injury to the spinal cord, the afferent pathways from the hypothalamus (the thermoregulatory control center) may be interrupted, resulting in abnormal temperature regulation. Colachis and Otis reported that injuries above level T6 resulted in autonomic dysregulation.2 Guttman et al similarly found that patients with complete lesions above the thoracic sympathetic outflow were unable to regulate their body temperature.18 Thus, the level of the lesion affects the amount of loss of thermoregulatory control.19
Translational Research on Thermoregulatory Dysfunction Subsequent to Spinal Cord Injury
Several translational studies have examined the correlation between SCI and fever. In a trial by Yu et al, hyperthermia was induced in rats with injured spinal cords by placing the animals in a thermoregulated Plexiglas container (Arkema, Colombes, France).20 Thirty minutes after induced trauma to the rats' spinal cords, the core temperatures of the animals increased from a baseline of 37 to 39.5°C and held for a 4-hour period. When compared with normothermic (37°C) animals, traumatized rats that underwent an induced core temperature increase for the second time showed worse outcomes in both behavioral and histopathologic measures.20 The translational study by Basso et al followed the locomotor activity in hypothermic and normothermic spinal cord–injured rats.21 The authors found that with elevated temperatures, the hypothermic rats had significantly less locomotor recovery over the 44-day observation period compared with the normothermic SCI rats.21 These two studies suggest that febrile responses associated with SCI may result in worse outcomes.
A third translational study by Das et al used a rat model to show that hypothermia is a possible complication following SCI.22 They compared the neurologic recovery of rats with a 50 to 60% lesion of their spinal cord, an 80 to 90% lesion, and a 100% lesion (complete severing) of their spinal cord: 18% of the rats with a 50 to 60% lesion, 19% of the rats with an 80 to 90% lesion, and 28% of the rats with a 100% lesion experienced hypothermia after injury.22
Discussion
A neurogenic fever occurs in approximately 1 of every 20 to 25 patients who sustain an acute SCI; however, despite the relative frequency of this condition, there is little in the scientific literature to help physicians prevent or treat it. The paucity of research underscored by this review demonstrates the need for further studies with larger sample sizes, focusing on the incidence rate, clinical outcomes, and pathogenesis of neurogenic fever following acute traumatic SCI.
Febrile events that occur as a result of hyperthermia are most frequently characterized by an elevation in the core body temperature, at or above 37.7°C.2 3 5 When associated with SCI, these febrile events can occur at any point during the acute care phase, the inpatient rehabilitation period, or after discharge from the hospital.5
Colachis and Otis found that all cases of SCI above the major splanchnic sympathetic outflow (levels T4–T6) resulted in autonomic dysregulation.2 Accordingly, individuals with traumatic upper thoracic and cervical SCI are also more susceptible to core body temperature changes from alterations in the external environment.2 This finding is important for predicting and anticipating patients who may be at high risk for thermodysregulation. Future studies would benefit from closely examining patients with SCI with injuries above level T6 for possible thermodysregulation.
Translational studies demonstrate the importance of maintaining a normothermic temperature in SCI. Yu et al observed that animals kept at a higher temperature (39.5°C) experienced greater neurologic deficits than those maintained at normal rectal temperature (37°C).20 Even mild hyperthermia after SCI can potentially compromise functional outcome and exacerbate tissue damage. Accordingly, steps should be taken to prevent detrimental hyperthermia following SCI.20
Das et al demonstrated that up to 28% of rats with an SCI developed hypothermia; however, it is unclear if the autonomic dysregulation leads to increased mortality, because the rats were commonly sacrificed shortly after the induced injury.22
Studies examining temperature regulation in patients with traumatic brain injury (TBI) could potentially provide insight into understanding the mechanisms and treatment options for patients with neurogenic fever in SCI. Following TBI, patients exhibited high temperatures, bradycardia, lack of sweating, as well as plateaulike temperature curves that could persist for weeks and be resistant to antipyretic medications.23 24 25 26 It has also been reported that patients with TBI are at a greater risk for a secondary injury. Holtzclaw found that for every 1°C rise in body temperature, there is a 13% increase in the metabolic rate. This significant increase, resulting in increased stress on energy reserves, may contribute to secondary metabolic injuries.27
The mechanism by which fever occurs after SCI has not been fully elucidated. Because the hypothalamus is the key center in the brain involved in thermoregulation, any damage to this area often results in thermodysregulation, especially hyperthermia.7 When accompanied by damage to the focal centers in the pons, damage to the hypothalamic preoptic area (POA) may cause thermal increases in the central nervous system.28 The POA receives thermosensory information, which rises from the skin thermoreceptors through the spinal cord to this area in the hypothalamus. It also receives information from pyrogenic signals of prostaglandin E2 produced in response to infection.29 Guieu and Hardy studied the activity of the POA in rabbits while manually controlling the internal temperature of both the POA and the spinal cord through the use of a thermode. Their results demonstrated that temperature changes in the spinal cord affected the thermoregulatory neurons in the POA. The findings from this study suggest that temperature-sensitive neurons within the spinal cord can sense changes in temperature within the cord itself.30 Thus, nerve damage that occurs during a traumatic SCI may lead to the observed thermodysregulation seen in patients experiencing neurogenic fever independent of the sympathetic system. Other studies have hypothesized that the occurrence of blood within the cerebrospinal fluid, notably the intraventricular spaces, can result in increased central nervous system temperature, producing fever. Neurotransmitter release, accelerated free radical production, increased intracellular glutamate concentration, and sensitivity of neurons to excitotoxic injury can also contribute to increases in internal temperature.31 32 33 Further studies are needed to determine if laboratory analysis should be undertaken to examine neurotransmitter release, glutamate levels, inflammatory markers, and indicators of hypothalamic upregulation in patients with SCI with a fever of unknown origin. Quantification of these values may provide significant leads in developing further theories on the pathogenesis of neurogenic fever. It is anticipated that greater understanding of the mechanism behind neurogenic fever following SCI will lead to more effective treatment for this group of severely injured patients.
Thompson et al developed an algorithm to diagnose neurogenic fever following TBI.12 To date, no such systematic algorithm has been proposed or generally accepted for fever subsequent to traumatic SCI; the authors' proposed algorithm for the diagnosis of neurogenic fever after a SCI is shown in Fig. 3. Before a diagnosis of neurogenic fever can be made, an exhaustive systematic search for infection and other febrile causes should be undertaken. For example, all potential sources of infection must be evaluated and ruled out, both within the wound (abscesses, osteomyelitis, among others) and systemically (i.e., urinary, blood, and so on). In addition, noninfectious causes of fever such as deep vein thromboses, pulmonary emboli, and heterotrophic ossifications must be eliminated. Even after this extensive methodology is undertaken, neurogenic fever is likely to remain a diagnosis by exclusion.12 This approach for diagnosing neurogenic fever has several drawbacks because it requires a significant number of tests, numerous invasive procedures, greater pain, and possible inhibition of functional improvement due to the delayed diagnosis. Furthermore, the increased cost, time, and effort for both patient and physician results in prolonged acute care hospitalization and delayed transfer to rehabilitation.12 34
Fig. 3.
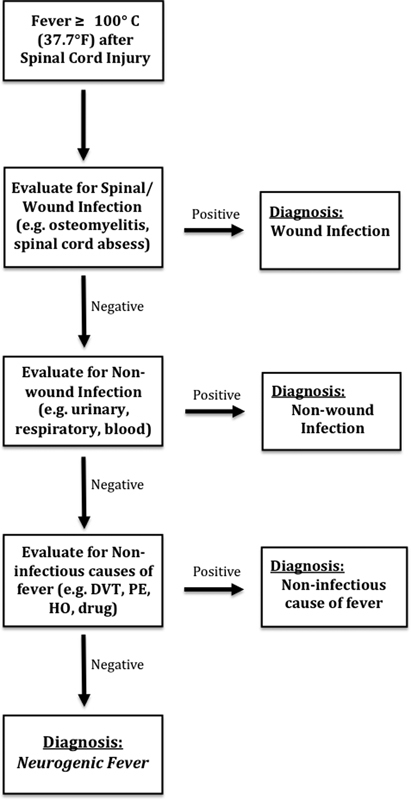
Algorithm for the diagnosis of neurogenic fever following traumatic spinal cord injury. Abbreviations: DVT, deep venous thrombosis; HO, heterotopic ossification; PE, pulmonary embolism.
A greater understanding of the possible mechanisms for fever following SCI is essential, because thermodysregulation can lead to worse patient outcomes with prolonged febrile events.30 Currently, there are no measures known to be effective in treating neurogenic fever. Because many documented cases have resulted in incomplete information obtained prior to patient demise, physicians are challenged to develop treatment options in the setting of unknown etiology.10 In the brain injury population, several cases have shown partial success with use of external-cooling methods until a proper cause of fever is identified. Additionally, the following drugs have been reported as successful in treating neurogenic fever in TBI patients: bromocriptine, amantadine, dantrolene, and propranolol.12
Our review was limited by the relatively low number of high-quality clinical investigations and by inconsistent definitions of fever, ranging from 100 to 101.5°F. Because of the limited number and heterogeneity of available studies, a quantitative analysis establishing the clinical outcomes associated with neurogenic fever after an SCI could not be completed. After a review of the available literature with a biostatistician, it was decided that systematically reporting the results of the individual studies was the best way to present the available data. Examples of the heterogeneous nature of the studies were case series including patients with a neurogenic fever after an SCI and others that included all patients with fevers but did not necessary identify the total number of patients with SCI. In addition, only three studies documented the number of complete versus incomplete lesions and the number of cervical, thoracic, and lumbar injuries (Table 2). Finally, although many articles did not mention the possibility of TBI in addition to SCI in patients, dual diagnoses represent an important but largely unknown confounding factor in these studies. Over the past 15 years, incidence rates of 24 to 59% have been given for presence of acute SCI with simultaneous TBI.35 However, recent reports suggested that this percentage may be underestimated, offering rates of 60 to 74% and even higher rates of TBI among those with cervical injuries.36 37
Although the inability to perform a quantitative systematic review is a significant limitation of this study, the results should spur further high-level research on neurogenic fevers after an SCI. Unfortunately, without further analysis a truly evidenced-based discussion with the patient and family is impossible. To our knowledge, this review provides the most comprehensive summation of the literature on this complex topic to date—providing physicians both with a foundation for patient care and future research.
Conclusion
The paucity of high-level studies available on neurogenic fevers after an acute SCI highlights the need for further research on the incidence, risk factors, clinical outcomes, and management of neurogenic fever. It is known that fever is a common complication of traumatic SCI. Most fevers have an identifiable cause, with urinary tract infection being the most common etiology. Based on the current literature, neurogenic fever occurs in 4 to 5% of patients with acute traumatic SCI. The mechanism of neurogenic fever following SCI is yet to be defined. Due to the small number of patients reported in published case series, the impact of neurogenic fever on mortality, length of stay, and neurologic recovery also remains unknown. Further studies are needed to better define the relationship between neurogenic fever and important clinical outcomes.
Footnotes
Disclosures Katherine E. Savage: none Christina V. Oleson: none Gregory D. Schroeder: Travel expenses (Medtronic, AOSpine) Gursukhman S. Sidhu: none Alexander R. Vaccaro: Board membership (AOSpine, Innovative Surgical Design, Association of Collaborative Spine Research, Spinicity); Consultant (DePuy, Medtronic, Stryker Spine, Globus, Stout Medical, Gerson Lehman Group, Medacorp, Innovative Surgical Design, Orthobullets, Ellipse, Vertex); Royalties (Medtronic, Stryker Spine, Biomet Spine, Aesculap, Thieme, Jaypee, Elsevier, Taylor and Francis); Stock options (Replication Medica, Globus, Paradigm Spine, Stout Medical, Spine Medica, Computational Biodynamics, Progressive Spinal Technologies, Spinology, Small Bone Innovations, Cross Current, In Vivo, Flagship Surgical, Advanced Spinal Intellectual Properties, Cytonics, Bonovo Orthopaedics, Electrocore, Gamma Spine, Location Based Intelligence, Flow Pharma, RSI, Rothman Institute and Related Properties, Innovative Surgical Design, Spinicity)
References
- 1.Sugarman B, Brown D, Musher D. Fever and infection in spinal cord injury patients. JAMA. 1982;248(1):66–70. [PubMed] [Google Scholar]
- 2.Colachis S C III, Otis S M. Occurrence of fever associated with thermoregulatory dysfunction after acute traumatic spinal cord injury. Am J Phys Med Rehabil. 1995;74(2):114–119. [PubMed] [Google Scholar]
- 3.Unsal-Delialioglu S, Kaya K, Sahin-Onat S, Kulakli F, Culha C, Ozel S. Fever during rehabilitation in patients with traumatic spinal cord injury: analysis of 392 cases from a national rehabilitation hospital in Turkey. J Spinal Cord Med. 2010;33(3):243–248. doi: 10.1080/10790268.2010.11689701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beraldo P S, Neves E G, Alves C M, Khan P, Cirilo A C, Alencar M R. Pyrexia in hospitalised spinal cord injury patients. Paraplegia. 1993;31(3):186–191. doi: 10.1038/sc.1993.35. [DOI] [PubMed] [Google Scholar]
- 5.McKinley W, McNamee S, Meade M, Kandra K, Abdul N. Incidence, etiology, and risk factors for fever following acute spinal cord injury. J Spinal Cord Med. 2006;29(5):501–506. doi: 10.1080/10790268.2006.11753899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman S B Yarkony G M Stiens S A Spinal cord injury rehabilitation. 2. Medical complications Arch Phys Med Rehabil 199778(3, Suppl):S53–S58. [DOI] [PubMed] [Google Scholar]
- 7.Badjatia N Hyperthermia and fever control in brain injury Crit Care Med 200937(7, Suppl):S250–S257. [DOI] [PubMed] [Google Scholar]
- 8.Tow A P, Kong K H. Prolonged fever and heterotopic ossification in a C4 tetraplegic patient. Case report. Paraplegia. 1995;33(3):170–174. doi: 10.1038/sc.1995.38. [DOI] [PubMed] [Google Scholar]
- 9.Weingarden D S, Weingarden S I, Belen J. Thromboembolic disease presenting as fever in spinal cord injury. Arch Phys Med Rehabil. 1987;68(3):176–177. [PubMed] [Google Scholar]
- 10.Ulger F, Dilek A, Karakaya D, Senel A, Sarihasan B. Fatal fever of unknown origin in acute cervical spinal cord injury: five cases. J Spinal Cord Med. 2009;32(3):343–348. doi: 10.1080/10790268.2009.11760788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A, Timothy J, Thapa A. Neurogenic fever. Singapore Med J. 2007;48(6):492–494. [PubMed] [Google Scholar]
- 12.Thompson H J, Pinto-Martin J, Bullock M R. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74(5):614–619. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NSCIS Center . Birmingham, AL: National Spinal Cord Injury Statistical Center; 2012. Spinal Cord Injury Facts and Figures at a Glance. [Google Scholar]
- 14.Lin V W, Cutter N C, New York, NY: Demos Medical Publishing; 2003. Spinal Cord Medicine: Principles and Practice. [Google Scholar]
- 15.Attia M, Engel P. Thermoregulatory set point in patients with spinal cord injuries (spinal man) Paraplegia. 1983;21(4):233–248. doi: 10.1038/sc.1983.37. [DOI] [PubMed] [Google Scholar]
- 16.Montgomerie J Z Infections in patients with spinal cord injuries Clin Infect Dis 19972561285–1290., quiz 1291–1292 [DOI] [PubMed] [Google Scholar]
- 17.Pollock L J, Boshes B, Chor H, Finkelman I, Arieff A J, Brown M. Defects in regulatory mechanisms of autonomic function in injuries to spinal cord. J Neurophysiol. 1951;14(2):85–93. doi: 10.1152/jn.1951.14.2.85. [DOI] [PubMed] [Google Scholar]
- 18.Guttmann L, Silver J, Wyndham C H. Thermoregulation in spinal man. J Physiol. 1958;142(3):406–419. doi: 10.1113/jphysiol.1958.sp006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers J H, Scheld W M. Fever in neurologic diseases. Infect Dis Clin North Am. 1996;10(1):45–66. doi: 10.1016/s0891-5520(05)70285-3. [DOI] [PubMed] [Google Scholar]
- 20.Yu C G Jagid J Ruenes G Dietrich W D Marcillo A E Yezierski R P Detrimental effects of systemic hyperthermia on locomotor function and histopathological outcome after traumatic spinal cord injury in the rat Neurosurgery 2001491152–158., discussion 158–159 [DOI] [PubMed] [Google Scholar]
- 21.Basso D M, Beattie M S, Bresnahan J C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 22.Das G D, Das K G, Brasko J, Riedl M, Rai P, Rajeswari V. Spinal traumas: some postoperative complications in experimental animals. Brain Res Bull. 1989;22(1):33–37. doi: 10.1016/0361-9230(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 23.Childers M K, Rupright J, Smith D W. Post-traumatic hyperthermia in acute brain injury rehabilitation. Brain Inj. 1994;8(4):335–343. doi: 10.3109/02699059409150984. [DOI] [PubMed] [Google Scholar]
- 24.Sazbon L, Groswasser Z. Outcome in 134 patients with prolonged posttraumatic unawareness. Part 1: Parameters determining late recovery of consciousness. J Neurosurg. 1990;72(1):75–80. doi: 10.3171/jns.1990.72.1.0075. [DOI] [PubMed] [Google Scholar]
- 25.Cunha B A, Tu R P. Fever in the neurosurgical patient. Heart Lung. 1988;17(6 Pt 1):608–611. [PubMed] [Google Scholar]
- 26.Segatore M. Fever after traumatic brain injury. J Neurosci Nurs. 1992;24(2):104–109. doi: 10.1097/01376517-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Holtzclaw B J. The febrile response in critical care: state of the science. Heart Lung. 1992;21(5):482–501. [PubMed] [Google Scholar]
- 28.Young A B, Ott L G, Beard D, Dempsey R J, Tibbs P A, McClain C J. The acute-phase response of the brain-injured patient. J Neurosurg. 1988;69(3):375–380. doi: 10.3171/jns.1988.69.3.0375. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 30.Guieu J D, Hardy J D. Effects of heating and cooling of the spinal cord on preoptic unit activity. J Appl Physiol. 1970;29(5):675–683. doi: 10.1152/jappl.1970.29.5.675. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich W D, Bramlett H M. Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- 32.Suehiro E, Fujisawa H, Ito H, Ishikawa T, Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. J Neurotrauma. 1999;16(4):285–297. doi: 10.1089/neu.1999.16.285. [DOI] [PubMed] [Google Scholar]
- 33.Ginsberg M D, Sternau L L, Globus M Y, Dietrich W D, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992;4(3):189–225. [PubMed] [Google Scholar]
- 34.Whyte J, Filion D T, Rose T R. Defective thermoregulation after traumatic brain injury. A single subject evaluation. Am J Phys Med Rehabil. 1993;72(5):281–285. doi: 10.1097/00002060-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Commichau C, Scarmeas N, Mayer S A. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60(5):837–841. doi: 10.1212/01.wnl.0000047344.28843.eb. [DOI] [PubMed] [Google Scholar]
- 36.Macciocchi S, Seel R T, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch Phys Med Rehabil. 2008;89(7):1350–1357. doi: 10.1016/j.apmr.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 37.Tolonen A, Turkka J, Salonen O, Ahoniemi E, Alaranta H. Traumatic brain injury is under-diagnosed in patients with spinal cord injury. J Rehabil Med. 2007;39(8):622–626. doi: 10.2340/16501977-0101. [DOI] [PubMed] [Google Scholar]


