Abstract
The immunological synapse controls T lymphocyte function by polarizing effector responses toward the antigen-presenting cell. In this review, I will discuss the molecular pathways required for synapse assembly, focusing on the central roles played by lipid second messenger signaling.
Keywords: T cell, signal transduction, cell polarity, PKC, actin, microtubules
T cell receptor (TCR) engagement of cognate peptide-major histocompatibility complex (pMHC) on the surface of an antigen-presenting cell (APC) induces the formation of a stereotyped, radially symmetric cell-cell junction known as an immunological synapse (IS)[1]. This process is accompanied by dramatic and polarized reorganization of the T cell cytoskeleton [2, 3]. Within minutes of TCR stimulation, the centrosome, which serves as a focal point for microtubules, moves to a position just beneath the center of the IS. Concomitantly, cortical filamentous actin (F-actin) becomes enriched in the periphery of the IS and depleted from the center, forming a characteristic annular structure.
These two cytoskeletal remodeling events serve as the foundation for IS structure and the basis for its function. Retrograde flow within the F-actin ring drives clustering of the αLβ2 integrin LFA-1 [4], thereby promoting adhesion to the APC. It also controls the trafficking of activated TCR complexes to the center of the IS, where they are internalized [5–7]. Centrosome reorientation, for its part, plays a critical role in shaping T cell secretory responses [8, 9]. The centrosome is closely associated with the Golgi apparatus and other vesicular organelles, and its reorientation brings these structures into close apposition with the actin depleted zone at the center of the IS, where they have unfettered access to the plasma membrane. This promotes the directional release of soluble factors toward the APC, which is thought to enhance the specificity and potency of both cytokine-mediated communication by CD4+ T cells and target cell killing by CD8+ cytotoxic T lymphocytes (CTLs). Directional secretion enables CTLs, for instance, to destroy APCs with secreted perforin and granzyme without harming innocent bystander cells.
Although the cytoskeletal architecture of the IS has been known for many years, the mechanisms controlling its assembly have been difficult to investigate because T cells, being very small and highly dynamic, represent a rather challenging cell biology system. In recent years, however, advances in imaging methodology have enabled progress in this area, which I will summarize below. This minireview will focus on the molecular pathways controlling centrosome reorientation and F-actin ring formation at the T cell IS, highlighting the importance of lipid second messenger signaling for both remodeling events. Space constraints prevent me from covering all aspects of cytoskeletal regulation in T cells, and I apologize to those whose work I have omitted here. I refer the reader to several excellent and more comprehensive recent reviews [2, 3, 10].
Diacylglycerol signaling and centrosome reorientation
In T cells, the position of the centrosome is tightly coupled to the site of TCR stimulation [11]. Indeed, the polarization response can distinguish between competing surfaces containing different densities of agonist pMHC, and almost always settles at the site of higher TCR stimulation [12, 13]. Not surprisingly, a number of key receptor-proximal signaling proteins are known to be essential for centrosome reorientation to the IS, including the Src-family kinase Lck, the Syk-family kinase Zap70, and the scaffolding molecules LAT and Slp76 [14, 15]. These molecules are required for almost every aspect of the T cell activation, however. Hence, their involvement in synaptic centrosomal polarity provides only limited insight into how the TCR signaling network specifically coordinates the process.
Our exploration of this pathway has been greatly facilitated by a photoactivation and imaging system we developed to study localized TCR signaling dynamics [13]. Primary CD4+ T cells expressing the 5C.C7 TCR are attached to glass surfaces coated with a photocaged form of their cognate ligand, a peptide derived from moth cytochrome c (MCC, amino acids 88-103) bound to the class II MHC molecule I-Ek. This pMHC complex bears a bulky, photocleavable group on a key lysine in the center of the MCC peptide that sterically disrupts TCR binding until it is exposed to UV light. Hence, we can activate polarized TCR signaling in an individual T cell simply by irradiating a micron sized region of the surface beneath it. Localized photoactivation of the TCR in this manner induces the reorientation of the centrosome to the region of TCR stimulation in approximately three minutes [13, 16], essentially mirroring the polarization kinetics seen in more traditional T cell-APC conjugate assays. Our system provides superior spatiotemporal control of centrosome movement and also allows us to monitor associated events at the plasma membrane using high-resolution total internal reflection fluorescence (TIRF) microscopy. These features have greatly facilitated our studies by enabling the identification of TCR-induced processes that are closely correlated with centrosome dynamics.
Using this approach, we discovered that centrosome reorientation is invariably associated with the accumulation of the lipid second-messenger diacylglycerol (DAG) at the site of TCR stimulation (Figure 1) [16]. DAG, which is generated by phospholipase-Cγ (PLCγ) downstream of the TCR, was known to form a plasma membrane gradient centered at the IS [17]. Our photoactivation experiments demonstrated that this event almost always occurred ~15 seconds before centrosome reorientation, strongly suggestive of a causal relationship. Consistent with this interpretation, application of a PLCγ inhibitor completely abrogated polarization responses. We observed similar effects after treating T cells with the phorbol ester PMA, a DAG proxy that masks the effects of endogenous DAG by stimulating unpolarized signaling. Surprisingly, and in contrast with previous work [14, 18], we found that Ca2+ influx was not required for centrosome recruitment. The discrepancy between our results and other studies likely reflects the fact that the photoactivation system separates centrosome polarization from adhesion to the stimulatory surface, whereas in more traditional approaches these events are convolved.
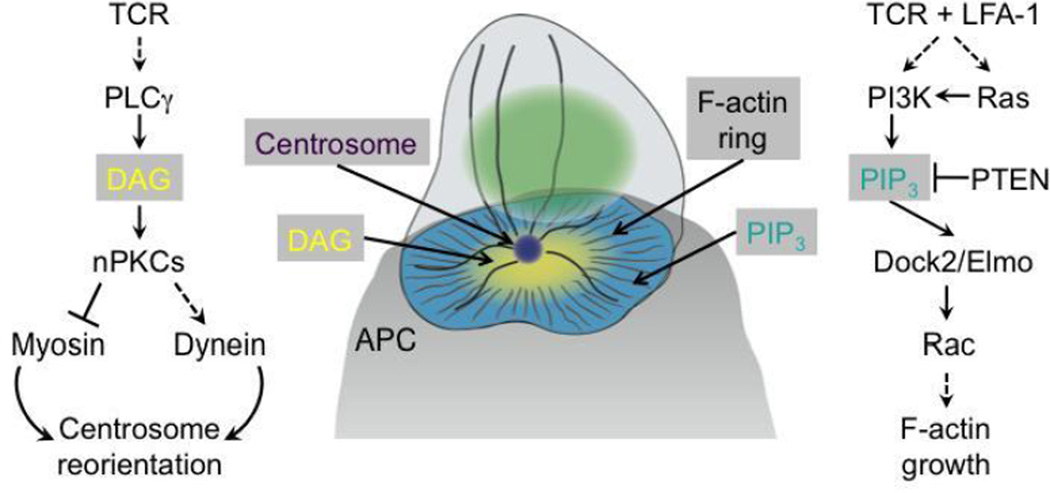
Figure 1. Lipid second messenger signaling at the immunological synapse.
Pathways coupling TCR engagement to centrosome reorientation and F-actin ring formation are shown to the left and right, respectively. Center, a schematic diagram depicting the DAG and PIP3 gradients that shape cytoskeletal architecture at the IS. F-actin and microtubules are shown as thin and thick black lines, respectively. The centrosome is colored purple and the nucleus green. APC = antigen-presenting cell.
DAG transduces signals by recruiting proteins that contain DAG-binding C1 domains. Of these, members of the protein kinase C family were of particular interest to us because of their established roles as cytoskeletal regulators [19]. In addition, the lymphocyte specific isoform PKCθ was known to be a key mediator of TCR signaling to the nucleus [20, 21]. PKCθ had also been observed to accumulate at the center of the IS [22], implying a possible role in the induction of cell polarity. The PKC family can be subdivided into three classes based on their domain structure and regulatory properties [23]. Classical PKCs (cPKCs) contain tandem typical C1 domains and a C2 domain, and require both DAG and Ca2+ for their activation. Novel PKCs (nPKCs) contain tandem C1 domains but divergent C2 domains, and require DAG, but not Ca2+, for their activation. Finally, atypical PKCs (aPKCs) contain an atypical C1 domain that does not bind DAG and they completely lack C2 domains. They are instead regulated by protein-protein interactions.
Using our photoactivation and imaging system, we found that three nPKC isoforms, PKCθ, PKCε, and PKCη, were recruited to the irradiated region before the centrosome and in a DAG dependent manner [24]. This result was quite surprising because, at the time, PKCε and PKCη were not thought to be involved in TCR signaling. Importantly, cPKCs like PKCα and aPKCs such as PKCζ did not display this localization behavior. Hence, the signaling requirements for centrosome polarization (i.e. localized DAG signaling but not Ca2+) matched the regulatory properties of the PKCs involved. Quantitative analyses of the imaging data revealed that nPKC recruitment occurred in two steps. PKCε and PKCη arrived at the IS first, ~15 seconds before centrosome reorientation, and occupied the entire synaptic membrane. Approximately 10 seconds later (~5 seconds before centrosome reorientation), PKCθ accumulated in a more constrained zone at the center of the IS. We and others have recently shown that this differential localization behavior is mediated by the V3 linker that connects the C1 domain region to the kinase domain [25, 26]. A PKCε chimera containing the PKCθ-V3 behaves like PKCθ, and vice versa. Although the precise mechanism by which the V3 linker controls PKC localization remains poorly defined, it is clear from this work and other studies that the recruitment tendencies of the tandem C1 domains can be shaped or even overridden by other determinants within the nPKC protein [27, 28]. Indeed, PKCδ, the fourth nPKC isoform, does not accumulate at the IS [24], despite the fact that it contains a DAG-responsive C1 domain.
The two-step recruitment behavior exhibited by PKCθ, PKCε, and PKCη suggested that PKCε and PKCη might function upstream of PKCθ in the centrosome polarization pathway. Consistent with this model, we found that simultaneous siRNA knockdown of PKCε and PKCη inhibited both PKCθ accumulation and centrosome reorientation [24]. By contrast, suppression of PKCθ impaired reorientation without affecting PKCη recruitment. Interestingly, knockdown of PKCε or PKCη alone had no effect on polarization responses, indicating that these two isozymes function redundantly in this context. This could explain why PKCε−/− and PKCη−/− mice have such subtle T cell phenotypes [29, 30].
We have recently shown that localized DAG-nPKC activity controls centrosome motility at least in part by coordinating the distribution of two molecular motor proteins, cytoplasmic dynein and nonmuscle myosin II (NMII) [31]. Dynein is a microtubule motor required for almost all intracellular minus end-directed traffic [32], while NMII is an actin-based motor that promotes contractile forces at the cell cortex [33]. Simultaneous suppression of both proteins was required to completely block centrosome reorientation [31], indicating that they function collaboratively in this context. Consistent with this interpretation, we found that dynein and NMII display complementary localization dynamics in photoactivation experiments. Whereas dynein accumulated at the site of TCR stimulation, NMII was depleted from this region and instead formed clusters in the cortex behind the advancing centrosome. These results suggest a model whereby dynein “pulls” on microtubules to reorient the centrosome from the front, while NMII dependent contractile forces “push” on the microtubule cytoskeleton from behind. Importantly, the reciprocal redistribution of dynein and NMII required nPKC activity. Indeed, we demonstrated that nPKCs induce NMII remodeling through direct phosphorylation of the myosin regulatory light chain. nPKCs do not appear to phosphorylate dynein in this context, however, implying a more complex regulatory relationship with this motor. We are currently performing proteomic screens to identify TCR-induced PKC phosphorylation events in an unbiased manner, which could shed light on this issue.
Phosphoinositol signaling and F-actin ring formation
IS growth is powered by a radially symmetric lamellipodium that spreads outward over the surface of the APC and then resolves into the peripheral F-actin ring [1, 2]. Actin polymerization within lamellipodial structures is mediated by the Arp2/3 complex, which generates branched F-actin arrays by nucleating filament growth off of the sides of existing filaments [34]. Arp2/3 activity is controlled in space and time by nucleation promoting factors (NPFs), which compose an evolutionarily conserved family of cytoskeletal regulators [35]. WASp and WAVE2, the predominant NPFs in T cells, both couple Arp2/3 dependent actin polymerization to upstream signals from Rho-family GTPases. WASp is activated by GTP-bound Cdc42, while WAVE2 operates downstream of Rac. Although both WASp and WAVE2 have been implicated in the TCR signaling cascade [2, 36], WAVE2 plays the more important role in promoting IS growth and F-actin ring formation [37, 38]. This is largely consistent with foundational work in other cell types showing that Rac stimulates lamellipodia formation, while Cdc42 induces filopodial structures [39].
Building upon these observations, we directly assessed the importance of Rac for synaptic F-actin growth and organization [40], making use of an established imaging system in which T cells are activated on supported lipid bilayers containing agonist pMHC and ICAM-1, an LFA-1 ligand [41]. On these bilayers, T cells form stable, radially symmetric synapses that contain prominent peripheral F-actin rings, which are easily visualized and scored using TIRF microscopy. Simultaneous engagement of the TCR and LFA-1 is required for F-actin ring formation in this context, highlighting the importance of integrin signaling for this aspect of synaptic architecture [40]. Using this system, we found that suppression of Rac1 and Rac2, the two isoforms expressed in T cells, dramatically reduced IS size and disrupted F-actin organization, consistent with a central role for the Rac-WAVE2 module.
Previous biochemical experiments had suggested that TCR-induced Rac activation depends on Dock2, an atypical Rac specific GEF of the CDM family [42]. Consistent with this data, we found that Dock2−/− T cells formed miniaturized synapses both on stimulatory bilayers and in conjugates with APCs [40]. Remarkably, both Dock2 and its constitutive binding partner, the adaptor protein Elmo1, localized to the periphery of the IS in a cortical region directly overlying the F-actin ring. This result implied that Rac dependent actin polymerization occurs preferentially in the periphery of the IS at least in part because the Dock2/Elmo1 complex is localized to that specific region.
Most CDM GEFs, including Dock2, contain a conserved DHR-1 domain that recognizes phosphatidylinositol trisphosphate (PIP3), the lipid second-messenger generated by phosphoinositide 3-kinase (PI3K) [43]. TCR signaling induces robust PIP3 production, and it has been known for some time that a substantial fraction of this lipid accumulates at the IS [44–46]. Whether this synaptic pool of PIP3 contributes to IS assembly and architecture, however, has only recently been addressed. Using TIRF microscopy in concert with a fluorescent PIP3 biosensor, we demonstrated that PIP3 accumulates in the periphery of the IS [40], immediately suggesting that it might recruit the Dock2/Elmo1 complex to this same membrane domain. Indeed, blocking PI3K activity or deleting the Dock2 DHR-1 domain disrupted peripheral localization of Dock2 and Elmo1. PI3K inhibitors also reduced IS growth and impaired F-actin ring formation, similar to the effects of Rac suppression. Conversely, shRNA-mediated suppression of PTEN, a lipid phosphatase that antagonizes PI3K signaling, led to a robust increase in IS size. Hence, PIP3 dependent signaling in the periphery of the IS controls the scope and organization of synaptic F-actin (Figure 1).
PIP3 production at the plasma membrane is primarily mediated by the class I PI3K family, which can be divided into two subgroups [47]. Class IA isoforms contain regulatory subunits of the p85/p55 family and signal downstream of receptor tyrosine kinases, while class IB isoforms associate with p87/p101 regulatory subunits and participate in G-protein coupled receptor cascades. Using isoform specific small molecular inhibitors and shRNA-mediated suppression, we demonstrated that synaptic PIP3 production and F-actin ring formation required class IA, but not class IB isoforms, with PI3Kδ playing the predominant role [40]. This result was consistent with several previous studies implicating class IA proteins in the TCR signaling cascade [48, 49]. We also found that the GTPase Ras, which activates class I PI3K family members by binding directly to their catalytic p110 subunits [50, 51], was required for synaptic PI3K signaling and F-actin architecture [40]. Taken together, these results established that the Ras-PI3K module, best known in T cells for its role in proliferative, transcription, and survival responses, is also an important cytoskeletal regulator.
Previous work has demonstrated that Ras activation at the T cell plasma membrane requires simultaneous engagement of the TCR and LFA-1 [52]. Remarkably, synaptic F-actin ring formation exhibits essentially the same stimulus criteria. These results suggest that Ras may couple LFA-1 signaling to F-actin ring formation at the IS, a possibility that is currently under investigation.
Lipid second messenger signaling and cell polarity
Over the past five years, it has become clear that lipid second-messenger signaling plays a critical role in shaping the IS. A central gradient of DAG control centrosome polarity, while an annular gradient of PIP3 specifies F-actin architecture (Figure 1). By rapidly diffusing into the local cellular neighborhood, lipids provide an efficient way to translate nanometer scale signals from receptor complexes into micron-scale structures like the IS. Of course, in order to keep the scope of their effects constrained, signaling lipids must be either destroyed or metabolized before they access inappropriate regions on the cell surface. A number of candidate enzymes exist that could constrain the scope of DAG and PIP3 diffusion in this manner, including diacylglycerol kinases and a number of phosphatidylinositol phosphatases [53, 54], which are currently under investigation.
These lipid dependent pathways can be distinguished from the more robust, long-lived polarity mechanisms utilized by epithelial cells, neurons, and astrocytes, which rely on the formation of protein complexes at the cell cortex [55]. In epithelial cells, for example, apical and basal surfaces are specified by the Par/aPKC complex and the Scribble/Dlg complex, respectively, which mutually antagonize each other’s function. Once assembled, these systems are highly stable and very difficult to undo. For instance, it takes hours to days for persistent TGFβ signaling to reverse Par complex function and induce epithelial to mesenchymal transition [56, 57]. Although this stability is well suited to the function of terminally polarized cell types, it is in general not appropriate for leukocytes, which rely on structural plasticity for their migration and effector function. CTLs, for instance, must build and dissolve cytolytic synapses quickly in order to kill multiple infected target cells in an efficient manner. Lipid gradients can be assembled and disassembled on the second to minute time-scale, and are the ideal tool for building transiently polarized cell-cell interfaces.
In certain situations, of course, the IS can persist for a considerable length of time. During naïve T cell priming, for instance, T cells are thought to remain tightly associated with the same dendritic cell for hours, if not days [58]. Polarity components such as Par/aPKC, Scribble, and Numb have been observed to distribute anisotropically in T cells like this, and it has been proposed that these proteins contribute to subsequent asymmetric T cell division on the surface of the dendritic cell [59–61]. Moving forward, it may be useful to think of lipid second messenger signaling as the first step along a polarity progression, a metastable choice point that enables the T cell to tailor the nascent cell-cell interface to its functional needs. Future studies will no doubt explore how T cells transition between molecularly distinct polarized states, and the factors that influence this decision.
Acknowledgments
Funding
Our own work was supported by the U.S. National Institutes of Health (R01-AI087644).
References
- 1.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can't have one without the other. Advances in immunology. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 3.Huse M, Le Floc'h A, Liu X. From lipid second messengers to molecular motors: microtubule-organizing center reorientation in T cells. Immunol Rev. 2013;256:95–106. doi: 10.1111/imr.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi J, Wu XS, Crites T, Hammer JA., 3rd Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol Biol Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huse M, Quann EJ, Davis MM. Shouts, whispers, and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annual review of cell and developmental biology. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 11.Sedwick CE, Morgan MM, Jusino L, Cannon JL, Miller J, Burkhardt JK. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J Immunol. 1999;162:1367–1375. [PubMed] [Google Scholar]
- 12.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, Davis MM. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Kuhne MR, Lin J, Yablonski D, Mollenauer MN, Ehrlich LI, Huppa J, Davis MM, Weiss A. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 15.Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 17.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Kupfer A, Dennert G, Singer SJ. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. The Journal of molecular and cellular immunology : JMCI. 1985;2:37–49. [PubMed] [Google Scholar]
- 19.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cellular signalling. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-theta in T cells. Cellular & molecular immunology. 2006;3:263–270. [PubMed] [Google Scholar]
- 22.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 23.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu R, Chen Y, Quann EJ, Huse M. The Variable Hinge Region of Novel PKCs Determines Localization to Distinct Regions of the Immunological Synapse. PloS one. 2014;9:e95531. doi: 10.1371/journal.pone.0095531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartwright NG, Kashyap AK, Schaefer BC. An active kinase domain is required for retention of PKCtheta at the T cell immunological synapse. Mol Biol Cell. 2011;22:3491–3497. doi: 10.1091/mbc.E10-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merino E, Abeyweera TP, Firth MA, Zawislak CL, Basu R, Liu X, Sun JC, Huse M. Protein kinase C-theta clustering at immunological synapses amplifies effector responses in NK cells. J Immunol. 2012;189:4859–4869. doi: 10.4049/jimmunol.1200825. [DOI] [PubMed] [Google Scholar]
- 29.Fu G, Hu J, Niederberger-Magnenat N, Rybakin V, Casas J, Yachi PP, Feldstein S, Ma B, Hoerter JA, Ampudia J, Rigaud S, Lambolez F, Gavin AL, Sauer K, Cheroutre H, Gascoigne NR. Protein kinase C eta is required for T cell activation and homeostatic proliferation. Sci Signal. 2011;4:ra84. doi: 10.1126/scisignal.2002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber T, Thuille N, Hermann-Kleiter N, Leitges M, Baier G. Protein kinase Cepsilon is dispensable for TCR/CD3-signaling. Mol Immunol. 2005;42:305–310. doi: 10.1016/j.molimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Kapoor TM, Chen JK, Huse M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc Natl Acad Sci U S A. 2013;110:11976–11981. doi: 10.1073/pnas.1306180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 35.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 36.Matalon O, Reicher B, Barda-Saad M. Wiskott-Aldrich syndrome protein--dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol Rev. 2013;256:10–29. doi: 10.1111/imr.12112. [DOI] [PubMed] [Google Scholar]
- 37.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 40.Le Floc'h A, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, Huse M. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med. 2013;210:2721–2737. doi: 10.1084/jem.20131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dustin ML. Insights into function of the immunological synapse from studies with supported planar bilayers. Curr Top Microbiol Immunol. 2010;340:1–24. doi: 10.1007/978-3-642-03858-7_1. [DOI] [PubMed] [Google Scholar]
- 42.Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–129. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 43.Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- 45.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 46.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 47.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 48.Garcon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 49.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. Embo J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 52.Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- 53.Huang YH, Sauer K. Lipid signaling in T-cell development and function. Cold Spring Harb Perspect Biol. 2010;2:a002428. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, Mao H, Luo N, Dong X, Yu X. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochimica et biophysica acta. 2008;1782:51–59. doi: 10.1016/j.bbadis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 59.Chang JT, Ciocca ML, Kinjyo I, Palanivel VR, McClurkin CE, Dejong CS, Mooney EC, Kim JS, Steinel NC, Oliaro J, Yin CC, Florea BI, Overkleeft HS, Berg LJ, Russell SM, Koretzky GA, Jordan MS, Reiner SL. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 61.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO, Russell SM. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]