Abstract
Objective
To evaluate a possible physiological mechanism underlying links between low childhood socioeconomic status (SES) and poor adult health by a) testing whether childhood SES is prospectively related to cardiovascular responses to laboratory stress in adulthood, and b) by determining whether psychological resources buffer cardiovascular reactivity and promote better recovery from stress.
Methods
Participants (n= 246; 55% Black; mean age= 32 years) were from a population-based sample of men in Pittsburgh, PA. Childhood SES was measured via the Hollingshead index (parental education and occupation) across 10 waves between the ages of 6 and 16. In adulthood, cardiovascular measures, including systolic and diastolic blood pressure (SBP, DBP), heart rate (HR), and high-frequency heart rate variability (HF-HRV), were taken during and following standardized laboratory psychological stressors. Participants completed measures of optimism, purpose in life, self-esteem, positive affect, and self-mastery, which were combined into a psychological resource factor.
Results
Lower childhood SES predicted higher HR and SBP at recovery, independent of age, race, BMI, current smoking, task demand, and current SES (HR: β = −.14, p = .04; SBP: β = −.14, p = .04). Psychological resources moderated the association between childhood SES and SBP (β =.14, p = .03). Lower childhood SES predicted SBP recovery only among men with fewer psychological resources.
Conclusions
Psychological resources may buffer the relation between low childhood SES and cardiovascular recovery from stress. This buffering may improve adult health to the extent that cardiovascular recovery contributes to the risk of low childhood SES for subsequent disease.
Keywords: socioeconomic status, childhood socioeconomic status, cardiovascular reactivity, cardiovascular recovery, psychological resources
Exposure to low socioeconomic status (SES) in childhood predicts increased cardiovascular morbidity and mortality decades later (reviewed in Cohen, Janicki-Deverts, Chen, & Matthews, 2010; Galobardes, Lynch, & Smith, 2008; Galobardes, Lynch, & Davey Smith, 2004; Galobardes, Smith, & Lynch, 2006; Pollitt et al., 2007). A myriad of physical, behavioral, and psychosocial pathways might explain why the socioeconomic environment in early life has a prolonged and pronounced impact on health. One of the key biological pathways underlying the link between childhood SES and adverse adult health is prolonged or repeated activation of the autonomic nervous system in response to stress (Cohen et al., 2010; Everson-Rose & Lewis, 2005). Exaggerated cardiovascular reactivity to psychological stress and prolonged recovery from stress predicts subclinical cardiovascular disease, morbidity, and mortality as summarized in several meta-analyses (Chida & Steptoe, 2010; Panaite, Salomon, Jin, & Rottenberg, 2015). Decreases in heart rate variability (HRV) during acute stress are also related to subclinical heart disease and prospective increases in blood pressure (BP; Gianaros et al., 2005; Matthews, Salomon, Brady, & Allen, 2003; Steptoe & Marmot, 2005).
The literature on childhood SES and cardiovascular reactivity to stress is mixed. Individuals with lower early life SES exhibit greater cardiovascular reactivity to acute psychological stressors in childhood and adolescence in some studies (Evans, Exner-Cortens, Kim, & Bartholomew, 2013; Gump, Matthews, & Räikkönen, 1999; Quas et al., 2014; Williams et al., 2008; Wilson, Kliewer, Plybon, & Sica, 2000), while others report null or inverse associations (Goosby, Malone, Richardson, Cheadle, & Williams, 2015; Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012; Lovallo, Farag, Sorocco, Cohoon, & Vincent, 2012; Lovallo, 2013; McLaughlin et al., 2015; Musante et al., 2000). We are not aware of any studies examining cardiovascular recovery from stress in association with childhood SES. In adulthood, SES has been linked with exaggerated cardiovascular reactivity and prolonged recovery (i.e., elevated BP and heart rate (HR) following stressor cessation or increased duration in returning to baseline BP and HR) (Everson et al., 2001; Merritt, Bennett, Williams, Sollers, John J, & Thayer, 2004; Steptoe & Marmot, 2006; Steptoe, Kunz-Ebrecht, et al., 2003), although there are inconsistencies in the adult literature as well (cf., Shaffer et al., 2012; Suchday, Krantz, & Gottdiener, 2005). Few studies have tracked whether cardiovascular responses to stress in adulthood vary as a function of the early socioeconomic environment (cf., Lynch et al., 1998). In the current study, we addressed these gaps in the literature by examining associations between childhood SES measured via repeated, prospective assessments, and cardiovascular reactivity and recovery to laboratory stress in adulthood.
An important consideration for policy and clinical implications in the literature on childhood SES and health is that of developmental timing. Hypotheses about health effects related to the timing and duration of SES in early life can be drawn from several conceptual models (reviewed in Cohen et al., 2010). For example, specific health outcomes may follow different sensitive periods throughout childhood and adolescence, and therefore, changes in SES at different time points may or may not alleviate health risks. However, many studies only utilize a single aggregated assessment of childhood SES, which precludes hypothesis testing across developmental periods. In the current study, SES was measured prospectively and annually over a decade of childhood and adolescence. We are therefore in a unique position to compare associations between childhood SES at several developmental periods with cardiovascular responses to stress in adulthood. Such evidence will help identify ages or developmental periods that are most sensitive regarding the cardiovascular implications of SES in early life.
Identifying factors that mitigate associations between low SES and poor health is a top public health priority, and psychological resources may function as such a mitigating factor (Gallo & Matthews, 2003). Psychological resources, such as purpose in life, self-esteem, optimism, and positive affect, are associated with salubrious health outcomes, including lower rates of disease and increased longevity as well as more favorable profiles of biological risk factors (Boehm & Kubzansky, 2012; Boylan & Ryff, 2015; Chida & Steptoe, 2008; Kim, Park, Sun, Smith, & Peterson, 2014; Kim, Strecher, & Ryff, 2014; Kim, Sun, Park, Kubzansky, & Peterson, 2012; Kim, Sun, Park, & Peterson, 2013; Pressman & Cohen, 2005). Self-rated health among low SES individuals with a high sense of control, specifically personal mastery, was comparable to the self-rated health of high income individuals, i.e. psychological resources mitigated the influence of low SES (Lachman & Weaver, 1998). Sense of control also predicted lower mortality risk for those with low, but not high, educational attainment (Turiano, Chapman, Agrigoroaei, Infurna, & Lachman, 2014). Morozink and colleagues (2010) further demonstrated that several dimensions of psychological well-being, including purpose in life, environmental mastery, and positive affect, predicted lower inflammatory cytokine interleukin-6 among those with less education. Furthermore, educational gradients in IL-6 were absent among individuals with high well-being. Less is known about associations between psychological resources and cardiovascular responses to stress, although available evidence supports reduced cardiovascular reactivity and enhanced recovery as a function of positive emotions and other psychological strengths (Brummett, Boyle, Kuhn, Siegler, & Williams, 2009; Fredrickson & Levenson, 1998; Fredrickson, Mancuso, Branigan, & Tugade, 2000; Ong & Allaire, 2005; Papousek et al., 2010; Steptoe, Gibson, Hamer, & Wardle, 2007).
Current Study
Bringing the above literatures together, the current study had two aims. First, we examined associations between childhood SES and both cardiovascular reactivity and recovery to stress in adulthood. We hypothesized that exposure to low SES in childhood would be associated with higher blood pressure and heart rate and lower HF-HRV during the tasks relative to baseline (reactivity) as well as during the post-task period (recovery). We explored whether the influence of SES in childhood varied by developmental stage. The second aim examined psychological resources as main effect as well as a moderator (i.e., buffer) of the association between low SES in childhood and cardiovascular responses to stress. We examined several psychological resources both independently and in aggregate in order to determine whether these variables are overlapping or independent in their association with cardiovascular risk. We hypothesized that men with greater resources would exhibit lower blood pressure and heart rate and higher HF-HRV during task and recovery periods. For psychological resources, we were more confident of our predictions related to recovery because nearly all prior studies on positive emotions and psychological strengths have demonstrated protective effects in the recovery domain (Fredrickson & Levenson, 1998; Fredrickson et al., 2000; Ong & Allaire, 2005; Papousek et al., 2010; Steptoe et al., 2007). Finally, because the sample included both Black and White men, we explored whether the respective impact of childhood SES and psychological resources on cardiovascular recovery varied by race.
Methods
Sample
The sample consisted of Black and White men from the population-based Pittsburgh Youth Study (PYS), a longitudinal study of boys who were initially recruited from Pittsburgh Public Schools in 1987–1988 (N =503). Further detail about the PYS is available elsewhere (Loeber et al., 2008). Only boys were included in the PYS given a primary interest in antisocial behavior and juvenile delinquency, which are more prevalent in boys than in girls (Sickmund & Puzzanchera, 2014). Men in the PYS were contacted to participate in the current study focused on cardiovascular health and risk for cardiovascular disease in adulthood (Mean age = 32 years; range 30–34 years). At the time of the current study, 18 men were deceased, 44 had previously dropped out of the PYS, 4 were severely mentally disabled, and 42 were incarcerated. Of the remaining 395 men, 312 participated (79%). Among those eligible but who did not participate, 27% (n=22) declined participation, 23% (n=19) failed to respond to contact or missed appointments, and 51% (n=42) could not be located. The laboratory protocol was completed by 267 men (86%). The sample completing the laboratory protocol did not differ from the initial PYS sample on race, childhood SES, or number of health problems in childhood, ps > .05. This study was approved by the Institutional Review Board at the University of Pittsburgh, and all men provided written, informed consent. Men who reported eating, drinking, smoking, or strenuously exercising within three hours of the laboratory study (n = 5), who were taking medication for high BP or a beta-blocker (n = 14), or both (n= 2) were excluded from analysis. The final analytic sample included 246 men (55% Black).
Measures
Childhood SES was measured yearly during the PYS via the two-factor Hollingshead index (1975), which incorporates parental educational attainment and occupational status as reported by the boy’s family. As an index of average childhood SES, we took the mean Hollingshead SES across 10 occasions between ages 6 and 16. In order to examine childhood SES in distinct developmental periods, we also created indices of SES by averaging Hollingshead from ages 7–9 (middle childhood), 10–12 (late childhood), and 13–16 (adolescence), respectively. We further explored whether changes in SES across developmental periods were related to cardiovascular parameters.
Psychological resource measures were completed online at the laboratory visit. Positive affect was assessed via the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988). Participants rated how they felt “in general” to ten positive adjectives on a 5-point scale. Purpose in life was measured with the 6-tem Life Engagement Test (Scheier et al., 2006); participants rate the extent of their agreement to statements such as, “I have lots of reasons for living,” on a 4-point scale. Optimism was evaluated via the Life Orientation Test-Revised (Scheier, Carver, & Bridges, 1994). This scale has six items (e.g., “In uncertain times, I usually expect the best”), and ratings are made on a 4-point scale. The Pearlin Mastery Scale was used to measure self-concept and assessed the extent to which individuals perceive they are in control of forces that significantly affect their lives (Pearlin, Lieberman, Menaghan, & Mullan, 1981). The Pearlin Mastery Scale has seven items (e.g., “I can do just about anything I really set my mind to do.”), and participants rate the extent to which they agree with the items on a 5-point scale. Finally, self-esteem was evaluated using the Rosenberg Self-Esteem Scale (Rosenberg, 1965). Participants rated the extent to which they agreed with ten items (e.g., “I feel that I have a number of good qualities”) on a 4-pont scale. For all measures, higher scores reflect greater psychological resources.
Principal components analysis was conducted on the scale scores from the five aforementioned psychological resource measures. Using an eigenvalue greater than 1 criterion and via inspection of the scree plot, one factor was extracted, explaining 60.8% of the variance. The median factor loading was 0.82 and ranged from 0.64 to 0.85. Thus, we created a composite of the psychological resources by averaging standardized (i.e., z-scored), unit-weighted variables for the five resource scales.
Laboratory Procedure
Participants were instructed to abstain from caffeinated beverages, tobacco, and exercise for at least three hours prior to the laboratory session. All tasks were performed with participants sitting upright in a comfortable lounge chair in a separate, quiet room. After a 10-minute baseline of watching a nature video, participants completed a mental arithmetic (3 minutes) and mirror tracing (3 minutes) task in counterbalanced order, and an anger recall speech task (3 minute preparation, 4 minute speech). Participants were informed of the tasks’ end by an experimenter, who remained in the room with the participant in order to facilitate the stress tasks and add a dimension of social evaluation. Each task was followed by a 5-minute recovery period, where participants watched a nature video. Experimenters were not present during the baseline or post-task periods. The speech task was followed by a 10-minute final recovery period watching a nature video. At the beginning of each recovery period, participants rated task demand and stressfulness on a 5-point Likert-type scale (1 = not at all; 5 = very).
Systolic BP (SBP) and diastolic BP (DBP) were monitored during the laboratory protocol using a CARESCAPE Dinamap V100 Vital Signs Monitor (GE Medical Systems Information Technologies, Inc.) with a standard occluding cuff placed on the participant’s non-dominant arm. Two resting measures were taken at the start of the protocol and tested for calibration against simultaneous BP taken using an auscultatory method with a second cuff placed on the dominant arm. The auscultatory cuff was removed before the baseline rest period. BP collection occurred every two minutes during the 10-minute baseline and 10-minute final rest periods, twice during each task, and twice during the 5-minute between-task rest intervals. HR and high frequency (HF) (.12–.4 Hz) HRV were collected from electrocardiogram (ECG) signals using a modified lead II configuration, and collection followed procedures stipulated in the guidelines for this measure (Jennings et al., 1981). This and the signal from a respiratory belt were transduced by Biopac System (Goleta, CA) modules (ECG and RSP modules of 100C series).
Physiological data processing
ECG signals were digitized (1000 Hz sampling, 16 bit analog to digital converter) and stored for analysis by Mindware Technology (Gahanna, OH) software. Measures of mean HR and HF-HRV were derived using the HRV module (Mindware, HRV 3.0.21). Measures were calculated over each entire experimental epoch, e.g. star tracing, recovery period after mental arithmetic. Trained staff examined each record and edited as necessary, deleting artifactual data points, routinely less than 5% of any data file. Inter-rater reliability among staff (4–5 individuals) was computed based on a randomly selected 10% of the data using the index from Bartko (1966). Intraclass correlations (ICC) were calculated using mixed effects models with random effects for the subject and rater. ICCs were high, with a median of .90 and range of .72–.998.
Covariates
Age, race (coded as White = 1, Black = 0), marital status, body mass index, current smoking (coded as 1), current SES, and average task demand were included as covariates. Marital status was dichotomously coded as married or in a marital-like relationship (coded as 1) versus all other (coded as 0). Body mass index was calculated based on measurements taken by staff at the laboratory visit (weight in kg/height in m2). Current SES was self reported and was measured using the two-factor Hollingshead Index. Average task demand was calculated as the mean of post-task ratings of how demanding the task was.
Statistical Analysis
HF-HRV was natural log transformed before use in analysis in order to achieve a normal distribution due to positive skew in raw values. Baseline levels of SBP, DBP, HR, and HF-HRV were calculated as the mean during the initial rest period. Reactivity was calculated separately for each cardiovascular parameter by regressing task levels on initial rest levels and saving the standardized residuals. These residuals were then averaged across task to create a reactivity index for SBP, DBP, HR, and HF-HRV, respectively. Aggregating across stress tasks is recommended for increasing reliability and generalizability of estimates (Kamarck, Jennings, & Manuck, 1993; Kamarck & Lovallo, 2003). Further, correlations among cardiovascular parameters across individual tasks were high. For reactivity, the median correlation across cardiovascular measures and tasks was 0.81 (range: .69–.93). For recovery, the median correlation across measures and tasks was 0.90 (range: .85–.98). Similarly, recovery was calculated separately for each cardiovascular parameter by regressing post-task levels on both task and initial rest levels and saving the standardized residuals; residuals were then averaged across all tasks (Mezick, Matthews, Hall, Jennings, & Kamarck, 2014). HF-HRV was additionally regressed on respiration rate during baseline, task, and post-task, respectively, to remove variance due to breathing. Thus, outcomes included one baseline index, one reactivity index, and one recovery index for each of the cardiovascular parameters.
Associations between childhood SES and cardiovascular responses to stress were examined in linear regression models. Separate models were run for cardiovascular parameters at baseline, reactivity, and recovery, respectively. All continuous predictor variables were mean centered in regression models. Initial analyses were designed to be sensitive to the possibility of periods during development during which SES might have a particularly strong influence on later cardiovascular reactivity and recovery. Absence of clear differences between developmental periods, however, led to a primary analysis where childhood SES was considered as an overall average from ages 6–16. The developmental period analyses are presented in Supplemental Table 1 and in the Exploratory Analysis section of the Results. Race differences in the association between childhood SES and cardiovascular stress response were examined via an interaction between race and childhood SES. Psychological resources were examined both as a main effect as well as a buffer of the association between childhood SES and cardiovascular responses to stress. Hypotheses regarding moderating effects of psychological resources were tested via an interaction between childhood SES and psychological resources in the prediction of cardiovascular responses to stress.
Results
Table 1 presents sample characteristics as well as the unadjusted cardiovascular measures at baseline, task, and post-task levels. Both in childhood and concurrently, participants were of lower to middle SES. As expected, SBP, DBP, and HR increased during the stress tasks and subsequently decreased during the recovery window, whereas HF-HRV decreased during reactivity and subsequently increased in the recovery window. On average, participants rated the stress tasks as moderately demanding.
Table 1.
Sample Characteristics (n=246)
| M (SD) or % | Range | |
|---|---|---|
| Age (years) | 32.1 (0.9) | 30–34 |
| Race (% Black) | 55.7% | |
| Marital status (% married or living like married) | 56.1% | |
| BMI (kg/m2) | 29.0 (6.7) | 17.6–63.1 |
| Current smoking (% yes) | 58.1% | |
| Current SES | 31.2 (14.6) | 6–66 |
| Child SES | 37.4 (9.3) | 9–66 |
| Task Demand (avg across tasks) | 3.1 (0.9) | 1–5 |
| Baseline SBP (mmHg) | 121.0 (10.6) | 99–160 |
| Task SBP (mmHg) | 133.2 (14.2) | 103–188 |
| Post-task SBP (mmHg) | 124.0 (10.6) | 102–161 |
| Baseline DBP (mmHg) | 71.6 (8.8) | 52–100 |
| Task DBP (mmHg) | 82.0 (10.0) | 58–116 |
| Post-task DBP (mmHg) | 72.3 (9.1) | 54–101 |
| Baseline HR (beats per minute) | 68.0 (11.1) | 41–106 |
| Task HR (beats per minute) | 75.1 (11.3) | 49–113 |
| Post-task HR (beats per minute) | 69.6 (10.9) | 44–107 |
| Baseline HF-HRV (ln units) | 6.3 (1.2) | 1.9–9.3 |
| Task HF-HRV (ln units) | 5.9 (1.3) | 0.9–8.7 |
| Post-task HF-HRV (ln units) | 6.1 (1.3) | 1.5–8.9 |
Note. SES was measured using the two-factor Hollingshead index. Task and post-task cardiovascular measures are presented in raw units and reflect an average across all tasks and post-task periods, respectively.
Linear regression models examined associations between childhood SES and psychological resources with the cardiovascular parameters at baseline, reactivity, and recovery, respectively. Results are presented in Table 2. Childhood SES was not significantly associated with any of the cardiovascular factors at baseline or reactivity. However, childhood SES was inversely associated with SBP and HR at recovery, adjusting for age, race, marital status, BMI, current smoking, current SES, and average task demand (SBP: β= −.14, t(233) = 2.11, p = .036; HR: β= −.14, t(230) = 2.05, p = .042). The psychological resource composite was not associated with baseline or reactivity levels of the cardiovascular factors (p’s > .10), but psychological resources were inversely associated with SBP at recovery, net of covariates (β= −.16, t(233) = 2.32, p = .021). Higher current SES was associated with lower HR at baseline (β = −.16, t(230) = 2.41, p = .02) and lower HF-HRV at recovery (β = −.16, t(230) = 2.12, p = .04).
Table 2.
Childhood SES, psychological resources, their interaction, and covariates predict cardiovascular factors at baseline, reactivity, and recovery.
| Baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | HR | HF-HRV (ln) | |||||
| β | p | β | p | β | p | β | p | |
| Model 1 | ||||||||
| Child SES | .08 | .17 | .01 | .88 | .08 | .23 | −.04 | .49 |
| Psychological Resources | .01 | .93 | .02 | .82 | .09 | .13 | −.06 | .35 |
| Current SES | .09 | .16 | .09 | .21 | −.16 | .02 | −.02 | .76 |
| BMI (ln) | .46 | <.001 | .28 | <.001 | .36 | <.001 | −.35 | <.001 |
| Race (1=White) | −.23 | <.001 | −.19 | <.01 | .18 | .01 | −.23 | <.001 |
| Marital Status (1=married) | .14 | .01 | .14 | .03 | −.02 | .77 | .10 | .10 |
| Age (years) | .01 | .84 | .07 | .30 | −.05 | .39 | −.05 | .45 |
| Current smoking (1= yes) | −.04 | .58 | −.02 | .79 | −.07 | .34 | .04 | .61 |
| Task Demand | .04 | .51 | .06 | .31 | .01 | .88 | −.07 | .24 |
| Model 2 | ||||||||
| Child SES X Psych Resources | −.04 | .52 | −.06 | .36 | −.05 | .39 | .05 | .43 |
| Reactivity | ||||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | HR | HF-HRV (ln) | |||||
| β | p | β | p | β | p | β | p | |
| Model 1 | ||||||||
| Child SES | −.05 | .44 | −.08 | .25 | .02 | .80 | .04 | .54 |
| Psychological Resources | .10 | .15 | .09 | .15 | .10 | .15 | −.02 | .80 |
| Current SES | .13 | .07 | .12 | .10 | .02 | .81 | .10 | .17 |
| BMI (ln) | .13 | .05 | .14 | .04 | −.07 | .33 | −.12 | .10 |
| Race (1=White) | .16 | .02 | .17 | .01 | .06 | .35 | −.06 | .42 |
| Marital Status (1=married) | .05 | .43 | .06 | .33 | .01 | .84 | −.02 | .80 |
| Age (years) | −.04 | .54 | −.06 | .32 | −.14 | .04 | .10 | .13 |
| Current smoking (1= yes) | .01 | .93 | .00 | .99 | −.12 | .11 | −.01 | .88 |
| Task Demand | −.01 | .94 | −.14 | .03 | .07 | .32 | .01 | .83 |
| Model 2 | ||||||||
| Child SES X Psych Resources | .06 | .33 | .05 | .44 | −.01 | .90 | −.05 | .43 |
| Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | HR | HF-HRV (ln) | |||||
| β | p | β | p | β | p | β | p | |
| Model 1 | ||||||||
| Child SES | −.14 | .04 | .02 | .75 | −.14 | .04 | .10 | .14 |
| Psychological Resources | −.16 | .02 | −.01 | .88 | −.08 | .22 | −.07 | .31 |
| Current SES | .03 | .66 | −.02 | .74 | −.02 | .75 | −.16 | .04 |
| BMI (ln) | .06 | .42 | −.12 | .07 | .06 | .36 | −.11 | .11 |
| Race (1=White) | −.05 | .44 | −.12 | .08 | −.08 | .21 | −.05 | .44 |
| Marital Status (1=married) | .06 | .39 | −.03 | .65 | .04 | .54 | .00 | >.99 |
| Age (years) | .07 | .26 | .07 | .26 | −.15 | .03 | .13 | .05 |
| Current smoking (1= yes) | .14 | .06 | .07 | .33 | −.10 | .18 | −.08 | .29 |
| Task Demand | .03 | .64 | −.15 | .02 | −.01 | .83 | .03 | .60 |
| Model 2 | ||||||||
| Child SES X Psych Resources | .14 | .03 | .08 | .23 | .02 | .74 | −.08 | .25 |
Note. Model 2 included all terms from Model 1 and added an interaction term between childhood SES and psychological resources.
Race differences in the association between childhood SES and cardiovascular responses to stress were examined by testing interaction effects between childhood SES and race in the prediction of each cardiovascular parameter at baseline, reactivity, and recovery (see Supplemental Table 2). There was a significant interaction between childhood SES and race in the prediction of baseline DBP in fully adjusted models, β = .56, t(231) = 2.93, p = .004. In general, Blacks had higher DBP, but their childhood SES was not associated with baseline DBP, β = −.13, t(127) = 1.48, p = .14. Among Whites, higher childhood SES was associated with higher baseline DBP, β = .20, t(101) = 2.15, p = .03.
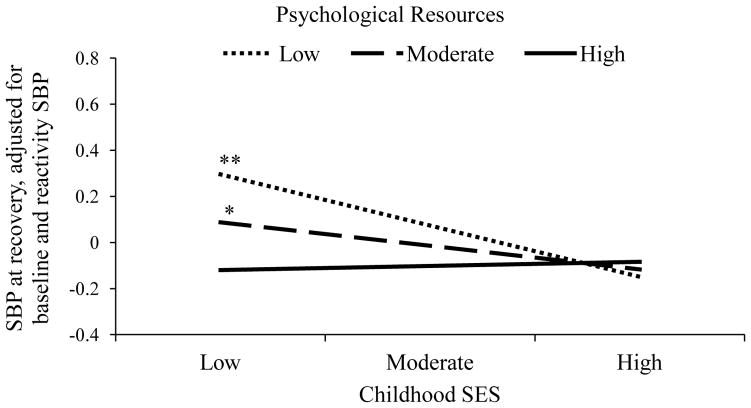
In order to examine whether psychological resources buffered the association between childhood SES and cardiovascular responses to stress, interactions between childhood SES overall and the psychological resource composite were tested. A significant interaction emerged between childhood SES and psychological resources in the prediction of SBP recovery (β = .14, t(231) = 2.21, p = .028). This interaction is depicted in Figure 1. A comparison of simple slopes at the mean and plus and minus one standard deviation from the mean on psychological resources revealed that there were significant, inverse associations between childhood SES and SBP recovery among those with low (β = −.29, t(232) = 3.03, p = .003) and moderate (β = −.14, t(232) = 2.07, p = .04) psychological resources, but this association was null for those with high psychological resources (β = .001, t(232) = 0.19, p = .85). No other interactions between childhood SES and psychological resources emerged as significant in the prediction of any other cardiovascular parameter (p’s > .15)1.
Figure 1.
The association between childhood SES and SBP at recovery at low, moderate, and high levels of psychological resources. High and moderate psychological resources bufferedthe association between childhood SES and SBP at recovery. **p < .01, *p < .05
Exploratory Analyses: Developmental Periods
Supplemental Table 1 presents associations among childhood SES in three separate developmental periods (middle childhood, late childhood, and adolescence), which did not differ appreciably from the overall average measure of SES. Although analyses of associations between SES and both SBP and HR recovery varied with respect to statistical significance, similar associations were revealed across developmental windows (SBP recovery: middle childhood: β = −.13, p = .055; late childhood: β = −.17, p = .01; adolescence: β = −.11, p = .10. HR recovery: middle childhood: β = −.14, p = .04; late childhood: β = −.17, p = .02; adolescence: β = −.10, p = .16). The interaction with psychological resources predicting SBP recovery was significant with SES in middle childhood (β = .14, p = .03) and late childhood (β = .14, p = .01), but not adolescence (p = .44).
The question arises whether we had sufficient change in SES across developmental periods to identify whether the impact of developmental periods would vary. To address this issue, change in SES was evaluated by categorizing participants into tertiles across developmental periods. Not surprisingly, approximately 60% of the sample remained in the same tertile of SES from middle childhood to adolescence. There were no significant differences in SBP or HR recovery among men who remained in the top (n = 51) or bottom (n = 50) tertile of SES or who increased (n = 29) or decreased (n = 26) in SES between middle childhood and adolescence (SBP recovery: F(3, 152) = 2.22, p = .09; HR recovery: F(3, 150) = 1.29, p = .28).
Discussion
The current study examined associations between childhood SES and cardiovascular responses to stress. Results demonstrated that low SES in childhood was associated with higher HR and SBP during the recovery window following acute stress in adulthood, independent of BMI, current smoking, current SES, age, race, marital status and perceptions of task demand. Childhood SES was not associated with cardiovascular parameters at baseline or in reactivity to stress. Greater psychological resources were also associated with lower SBP at recovery. However, the independent associations between SBP at recovery and childhood SES and psychological resources, respectively, were qualified by a significant moderation effect. That is, the inverse association between childhood SES and SBP at recovery was only significant among men with low and moderate psychological resources. There was no association between childhood SES and SBP recovery among those with high psychological resources.
Two unique strengths of this study were the prospective design and racially diverse sample, yielding the opportunity to examine developmental questions about the timing of SES exposure in childhood and its association with cardiovascular responses to stress in adulthood as well as whether these associations were different in Black and White men. Prospective data with multiple waves, like that in the current study, are necessary to discern if and when there are sensitive periods for when SES exposure has the most enduring effects on adult health (Cohen et al., 2010). The current data demonstrated rather consistent associations between cardiovascular responses to stress and childhood SES across developmental chunks. That is, sensitive periods for when SES exposure matters most for adult health were not identified, and lower SES was associated with higher SBP and HR during the recovery window throughout childhood, although findings were slightly weaker in adolescence (Pollitt, Rose, & Kaufman, 2005). Though the relation between recovery and adolescent SES is nominally, and non-significantly weaker, than the same relation in middle and late childhood, overall the continuity of the SES-recovery relationship is much more striking than any specificity to a developmental period. Change in SES across childhood and adolescence was also unrelated to SBP and HR during recovery. Childhood SES was relatively stable across childhood and adolescence in this sample (r’s ranged from .64 to .77 across developmental chunks), which may have affected the ability to identify differential effects across time. Further, little evidence for race differences was found in the associations between childhood SES and cardiovascular responses to stress, emphasizing that exposure to low SES in early life predicts poor SBP and HR recovery from stress ubiquitously in both Black and White men.
The finding that higher childhood SES was associated with higher baseline DBP among White men only was unexpected and inconsistent with prior literature demonstrating inverse associations between SES and hypertension, including in national samples (e.g., Colhoun, Hemingway, & Poulter, 1998; Janicki-Deverts, Cohen, Matthews, & Jacobs, 2012; Vargas, 2000). However, hypertension is much more common after age 50 (Mozaffarian et al., 2014), and the current sample may have been too young to discern notable SES disparities. Independent of childhood SES, higher adult SES was associated with lower HR at baseline and lower HF-HRV at recovery. The inverse association between adult SES and resting HR is consistent with the Whitehall II Study (Hemingway et al., 2005), and high HR has been linked to ischemic heart disease, sudden cardiac death, and mortality (Benetos, Rudnichi, Thomas, Safar, & Guize, 1999; Shaper, Wannamethee, Macfarlane, & Walker, 1993). The association with adult SES and HF-HRV at recovery was unexpected and may reflect less parasympathetic tone during recovery among higher SES men. This finding is in contrast to the findings from the Whitehall Psychobiology Study, where higher SES was associated with more adaptive recovery across several cardiovascular parameters, including HRV (Steptoe et al., 2002). Future research is needed to confirm and understand this incongruent finding.
Dysregulation in the autonomic nervous system in response to stress is often cited as a primary biological mechanism underlying SES disparities in health (reviewed in Cohen et al., 2010; Everson-Rose & Lewis, 2005). The current results support the autonomic nervous system as a physiological pathway by which low SES “gets under the skin,” as demonstrated by poor SBP and HR recovery following acute stressors. A recent meta-analysis found that poor recovery from laboratory challenges (mostly physical) was associated with increased risk for adverse cardiovascular events and all-cause mortality (Panaite et al., 2015), and that effects were comparable to meta-analytic findings of increased disease risk associated with heightened cardiovascular reactivity to acute stress in the laboratory (Chida & Steptoe, 2010). Reactivity did not differ as a function of childhood family SES in this sample, consistent with some prior research in African American male adolescents (Hackman et al., 2012). Other research supports associations between lower early life SES and either greater (Evans et al., 2013; Gump et al., 1999; Quas et al., 2014; Williams et al., 2008; Wilson et al., 2000), or lesser cardiovascular reactivity to acute psychological stressors in childhood and adolescence (Lovallo et al., 2012; Lovallo, 2013; McLaughlin et al., 2015). Our results raise the possibility that recovery may be a more stable effect of lower early life SES. Poor recovery, in particular, is thought to reflect the failure to regulate physiological systems over time following sustained or repeated challenges (McEwen, 1998), and as such, is considered especially important regarding vulnerability for downstream health effects (Dickerson & Kemeny, 2004). Few studies have had the opportunity to test prospective associations between SES in childhood and reactivity and recovery from laboratory stressors in adulthood. The current evidence suggests that the recovery window may be critical to understanding long-term cardiovascular consequences of exposure to low SES in childhood.
The second aim of the study was to examine psychological resources as a predictor of cardiovascular responses to stress and also as a moderator of the link between childhood SES and cardiovascular responses to stress. In line with predictions, psychological resources, a composite of positive affect, purpose in life, optimism, self-mastery, and self-esteem, predicted lower SBP at recovery and also moderated the association between childhood SES and SBP recovery. Among men with moderate and low psychological resources, lower childhood SES was associated with higher SBP at recovery, consistent with prior literature linking lower SES in adulthood to poorer cardiovascular recovery from stress (Merritt et al., 2004; Steptoe & Marmot, 2006; Steptoe, Willemsen, Kunz-Ebrecht, & Owen, 2003). However, there was no association between SES and SBP recovery among men with high psychological resources. Prior studies have found enhanced cardiovascular recovery from stress following positive mood inductions (Fredrickson & Levenson, 1998; Fredrickson et al., 2000) and also found associations between state and trait psychological resources and cardiovascular recovery (Brummett et al., 2009; Ong & Allaire, 2005; Papousek et al., 2010; Steptoe et al., 2007). The same moderation pattern for psychological resources was not apparent in the prediction of HR. Prior evidence shows inconsistent associations between SBP and HR recovery from stress (Steptoe & Marmot, 2006), and Gianaros and Wager (2015) demonstrate differential neural activation associated with HR and BP reactivity to stress, suggesting that the beneficial associations of psychological resources are via pathways specific to SBP.
The question remains, how might psychological resources contribute to recovery from stressful events? Being happier and optimistic, having higher self-esteem and self-mastery, and a greater purpose in life may reflect enhanced emotion regulation abilities and provide motivation to reappraise stressful events in an adaptive way, thus avoiding rumination, which is associated with poor cardiovascular recovery (e.g., Gerin, Davidson, Christenfeld, Goyal, & Schwartz, 2006; Radstaak, Geurts, Brosschot, Cillessen, & Kompier, 2011; Woody, Burkhouse, Birk, & Gibb, 2015). Psychological resources may represent the intrapersonal piece of reserve capacity, and together with other tangible and interpersonal resources, help low SES individuals cope with stressors (Gallo & Matthews, 2003). Regarding potential top-down mechanisms, psychological well-being, including purpose in life, has been linked to healthier brain functioning, such as greater left (than right) prefrontal activation profiles (Urry et al., 2004), faster automatic recovery from negative emotional stimuli (Schaefer et al., 2013), and sustained striatal and dorsolateral prefrontal cortex activity following positive stimuli (Heller et al., 2013). Together, the data support salubrious effects of psychological resources, and the moderation findings add to a growing body of literature that such resources are especially important for health among individuals with low, but not high, SES (Lachman & Weaver, 1998; Morozink et al., 2010; Turiano et al., 2014). Given the consistency of findings across the five resource measures, these psychological factors may represent several targets for intervention, and such efforts to increase psychological resources may have important physical health benefits as well, especially among those already at risk for poor health due to low SES.
Results from the current study should be interpreted in light of several limitations. Importantly, only men were included in the study, and thus it is unknown if the results would generalize to women. A subsample of adults from the original study (n=42) were incarcerated and thus were unable to participate in the current study. It is unclear whether their inclusion would have affected results, although we note that the current sample did not differ from the original parent sample on childhood SES. Also, the sample was restricted geographically and only included men who grew up and lived in proximity to Pittsburgh, PA. In addition, several analyses were conducted, yielding concerns about type I error. Our analytic strategy reflected the focus on childhood SES and psychological resources and associations with four distinct cardiovascular measures at three time points. Though replication is needed, results suggest that not all cardiovascular parameters or time points in response to stress have an equivalent, detrimental effect. Finally, we were unable to formally test reactivity and recovery from stress as mediators of the association between childhood SES and morbidity given limited data on overt cardiovascular disease due to the relatively young age of the sample. Notwithstanding these limitations, this study is the first to demonstrate differences in SBP and HR recovery from stress as a function of prospectively assessed childhood SES, and also the first to demonstrate buffering effects of psychological resources, finding that men with low childhood SES who nonetheless possess high psychological resources looked comparable to their higher SES counterparts in terms of SBP recovery from challenge.
Supplementary Material
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01HL111802, 5T32HL007560-32, R01HL101959).
Footnotes
All resource measures independently and significantly moderated the association between childhood SES and SBP at recovery, except self-mastery (interaction effect p = .067). When the other resource scales were included as covariates, moderation findings for any given resource scale were largely attenuated to marginal significance.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All authors declare no conflicts of interest.
References
- Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychological Reports. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33(1):44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychological Bulletin. 2012;138:655–91. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- Boylan JM, Ryff CD. Psychological well-being and metabolic syndrome: Findings from the Midlife in the United States National Sample. Psychosomatic Medicine. 2015;77:548–58. doi: 10.1097/PSY.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46:862–9. doi: 10.1111/j.1469-8986.2009.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Hemingway H, Poulter NR. Socio-economic status and blood pressure: an overview analysis. Journal of Human Hypertension. 1998;12:91–110. doi: 10.1038/sj.jhh.1000558. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Evans GW, Exner-Cortens D, Kim P, Bartholomew D. Childhood poverty and blood pressure reactivity to and recovery from an acute stressor in late adolescence: the mediating role of family conflict. Psychosomatic Medicine. 2013;75:691–700. doi: 10.1097/PSY.0b013e31829f9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson SA, Lynch JW, Kaplan GA, Lakka TA, Sivenius J, Salonen JT. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke. 2001;32:1263–70. doi: 10.1161/01.str.32.6.1263. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annual Review of Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62:387–90. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Gerin W, Davidson KW, Christenfeld NJS, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosomatic Medicine. 2006;68:64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, Matthews KA. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosomatic Medicine. 2005;67:553–60. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Science. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Malone S, Richardson EA, Cheadle JE, Williams DT. Perceived discrimination and markers of cardiovascular risk among low-income African American youth. American Journal of Human Biology. 2015;27:546–552. doi: 10.1002/ajhb.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Räikkönen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychology. 1999;18:140–50. doi: 10.1037//0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, van Reekum CM, Schaefer SM, Lapate RC, Radler BT, Ryff CD, Davidson RJ. Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science. 2013;24:2191–2200. doi: 10.1177/0956797613490744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111(23):3071–7. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Matthews KA, Jacobs DR. Sex differences in the association of childhood socioeconomic status with adult blood pressure change: the CARDIA study. Psychosomatic Medicine. 2012;74:728–35. doi: 10.1097/PSY.0b013e31825e32e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, Turpin G. Committee report. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18:226–31. doi: 10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Kamarck T, Jennings J, Manuck S. Psychometric applications in the assessment of cardiovascular reactivity. Homeostasis in Health and Disease. 1993;34:229–243. [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.PSY.0000030390.34416.3E. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Hoeber M, Loeber R, McTigue K. The Pittsburgh Girls Study: overview and initial findings. Journal of Clinical Child and Adolescent Psychology. 2010;39:506–521. doi: 10.1080/15374416.2010.486320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Park N, Sun JK, Smith J, Peterson C. Life satisfaction and frequency of doctor visits. Psychosomatic Medicine. 2014;76:86–93. doi: 10.1097/PSY.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Strecher VJ, Ryff CD. Purpose in life and use of preventive health care services. Proceedings of the National Academy of Sciences. 2014;111:16331–6. doi: 10.1073/pnas.1414826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Sun JK, Park N, Kubzansky LD, Peterson C. Purpose in life and reduced risk of myocardial infarction among older U.S. adults with coronary heart disease: a two-year follow-up. Journal of Behavioral Medicine. 2012;36:124–33. doi: 10.1007/s10865-012-9406-4. [DOI] [PubMed] [Google Scholar]
- Kim ES, Sun JK, Park N, Peterson C. Purpose in life and reduced stroke in older adults: The Health and Retirement Study. Journal of Psychosomatic Research. 2013;74:427–32. doi: 10.1016/j.jpsychores.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology. 1998;74:763–73. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. International Journal of Psychophysiology. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry. 2012;71:344–9. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Salomon K, Brady SS, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosomatic Medicine. 2003;65:410–5. doi: 10.1097/01.psy.0000057612.94797.5f. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences. 2015;112:201423363. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt MM, Bennett GG, Williams RB, Sollers John JI, Thayer JF. Low educational attainment, John Henryism, and cardiovascular reactivity to and recovery From personally relevant stress. Psychosomatic Medicine. 2004;66:49–55. doi: 10.1097/01.PSY.0000107909.74904.3D. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall MH, Richard Jennings J, Kamarck TW. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology. 2014;51:88–96. doi: 10.1111/psyp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychology. 2010;29:626–35. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Turner MB. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2014;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Musante L, Treiber FA, Kapuku G, Moore D, Davis H, Strong WB. The effects of life events on cardiovascular reactivity to behavioral stressors as a function of socioeconomic status, ethnicity, and sex. Psychosomatic Medicine. 2000;62:760–7. doi: 10.1097/00006842-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Ong AD, Allaire JC. Cardiovascular intraindividual variability in later life: the influence of social connectedness and positive emotions. Psychology and Aging. 2005;20:476–85. doi: 10.1037/0882-7974.20.3.476. [DOI] [PubMed] [Google Scholar]
- Panaite V, Salomon K, Jin A, Rottenberg J. Cardiovascular recovery from psychological and physiological challenge and risk for adverse cardiovascular outcomes and all-cause mortality. Psychosomatic Medicine. 2015;77:215–226. doi: 10.1097/PSY.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papousek I, Nauschnegg K, Paechter M, Lackner HK, Goswami N, Schulter G. Trait and state positive affect and cardiovascular recovery from experimental academic stress. Biological Psychology. 2010;83:108–15. doi: 10.1016/j.biopsycho.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. Journal of Health and Social Behavior. 1981;22:337–56. [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Quas JA, Yim IS, Oberlander TF, Nordstokke D, Essex MJ, Armstrong JM, … Boyce WT. The symphonic structure of childhood stress reactivity: patterns of sympathetic, parasympathetic, and adrenocortical responses to psychological challenge. Development and Psychopathology. 2014;26:963–82. doi: 10.1017/S0954579414000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radstaak M, Geurts SAE, Brosschot JF, Cillessen AHN, Kompier MAJ. The role of affect and rumination in cardiovascular recovery from stress. International Journal of Psychophysiology. 2011;81:237–44. doi: 10.1016/j.ijpsycho.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton: Princeton University Press; 1965. [Google Scholar]
- Schaefer SM, Boylan JM, van Reekum CM, Lapate RC, Norris CJ, Ryff CD, Davidson RJ. Purpose in life predicts better emotional recovery from negative stimuli. PLoS ONE. 2013;8:e80329. doi: 10.1371/journal.pone.0080329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Wrosch C, Baum A, Cohen S, Martire LM, Matthews KA, … Zdaniuk B. The Life Engagement Test: assessing purpose in life. Journal of Behavioral Medicine. 2006;29:291–8. doi: 10.1007/s10865-005-9044-1. [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Wasson LT, Davidson KW, Schwartz JE, Kirkland S, Shimbo D. Blood pressure reactivity to an anger provocation interview does not predict incident cardiovascular disease events: The Canadian Nova Scotia Health Survey prospective population study. International Journal of Hypertension. 2012;2012:658128. doi: 10.1155/2012/658128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, Macfarlane PW, Walker M. Heart rate, ischaemic heart disease, and sudden cardiac death in middle-aged British men. British Heart Journal. 1993;70(1):49–55. doi: 10.1136/hrt.70.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmund M, Puzzanchera C, editors. Juvenile offenders and victims: 2014 national report. Pittsburgh, PA: National Center for Juvenile Justice; 2014. [Google Scholar]
- Steptoe A, Feldman PJ, Kunz S, Owen N, Willemsen G, Marmot M. Stress responsivity and socioeconomic status. A mechanism for increased cardiovascular disease risk? European Heart Journal. 2002;23(22):1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine. 2003;65:461–470. doi: 10.1097/01.PSY.0000035717.78650.A1. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. Journal of Hypertension. 2005;23:529–36. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Psychosocial, hemostatic, and inflammatory correlates of delayed poststress blood pressure recovery. Psychosomatic Medicine. 2006;68:531–7. doi: 10.1097/01.psy.0000227751.82103.65. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Kunz-Ebrecht SR, Owen N. Socioeconomic status and hemodynamic recovery from mental stress. Psychophysiology. 2003;40:184–91. doi: 10.1111/1469-8986.00020. [DOI] [PubMed] [Google Scholar]
- Suchday S, Krantz DS, Gottdiener JS. Relationship of socioeconomic markers to daily life ischemia and blood pressure reactivity in coronary artery disease patients. Annals of Behavioral Medicine. 2005;30:74–84. doi: 10.1207/s15324796abm3001_9. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Chapman B, Agrigoroaei S, Infurna FJ, Lachman M. Perceived control reduces mortality risk at low, not high, education levels. Health Psychology. 2014;33:883–90. doi: 10.1037/hea0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, … Davidson RJ. Making a life worth living: Neural correlates of well-being. Psychological Science. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Vargas CM. Incidence of hypertension and educational attainment: The NHANES I Epidemiologic Followup Study. American Journal of Epidemiology. 2000;152:272–278. doi: 10.1093/aje/152.3.272. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Siegler IC, Barefoot JC, Helms MJ, Brummett BH, … Schanberg SM. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosomatic Medicine. 2008;70:32–9. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]
- Wilson DK, Kliewer W, Plybon L, Sica DA. Socioeconomic status and blood pressure reactivity in healthy Black adolescents. Hypertension. 2000;35:496–500. doi: 10.1161/01.hyp.35.1.496. [DOI] [PubMed] [Google Scholar]
- Woody ML, Burkhouse KL, Birk SL, Gibb BE. Brooding rumination and cardiovascular reactivity to a laboratory-based interpersonal stressor. Psychophysiology. 2015;52:722–5. doi: 10.1111/psyp.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.