Abstract
Stress granules are assemblies of untranslating mRNPs that form from mRNAs stalled in translation initiation. Stress granules form through interactions between mRNA binding proteins that link together populations of mRNPs. Interactions promoting stress granule formation include conventional protein-protein interactions, as well as interactions involving intrinsically disordered regions of proteins. Assembly and disassembly of stress granules are modulated by a variety of post-translational modifications as well as a number of ATP dependent RNP or protein remodeling complexes, illustrating that stress granules represent an active liquid wherein energy input maintains their dynamic state. Stress granule formation modulates the stress response, viral infection, and signaling pathways. Persistent or aberrant stress granule formation contributes to neurodegenerative disease and some cancers.
Keywords: Stress Granule, RNP Granule, Phase Separation, intrinsically disorder protein
RNP Granules
A variety of non-membrane bound cellular compartments are termed RNP granules due to their high concentrations of protein and RNA. These include nuclear granules such as Cajal bodies, paraspeckles, and the nucleolus, as well as cytoplasmic granules such as stress granules and processing bodies [1]. Other examples of RNP granules include neuronal granules and germ cell granules, which function in synaptic remodeling and maternal mRNA storage in early development [2,3]. RNP granules are generally dynamic and dependent on RNA for their assembly. Therefore, the formation of dynamic RNP granules to concentrate specific cellular components is a conserved strategy across multiple organisms and in different cellular compartments.
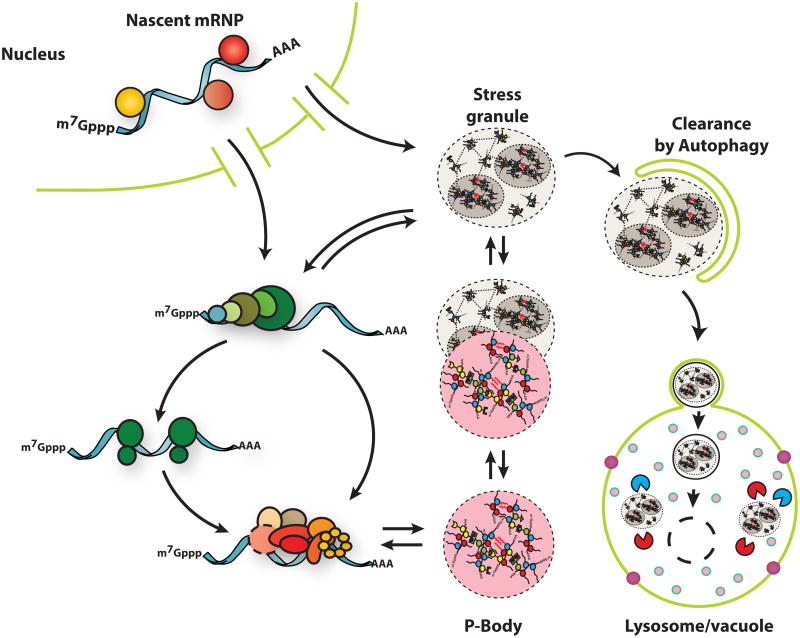
Stress granules and P-bodies are two conserved cytoplasmic mRNP granules that form from pools of untranslating mRNA [4–6]. Stress granules form from mRNAs stalled in translation initiation and contain various translation initiation factors, a variety of RNA binding proteins, and many non-RNA binding proteins [7]. P-bodies contain mRNAs associated with translational repressors, and the mRNA decay machinery. mRNAs within P-bodies can be targeted for decapping and degradation but mRNAs can also be degraded outside of P-bodies [8]. P-bodies and stress granules can dock and/or overlap in both yeast and mammalian cells suggesting a dynamic mRNA cycle wherein mRNPs can be remodeled within these assemblies and exchange between stress granules and P-bodies ([9,10]; Figure 1). During RNP granule disassembly mRNPs within P-bodies and stress granules can return to translation or, in some cases, can be targeted for autophagy (Figure 1), which provides a second system for stress granule clearance [11–13].
Figure 1. Stress Granules are dynamic and have multiple fates.
Stress granules form from untranslating mRNPs. They can interact with P-bodies, exchange components with the cytoplasm, and undergo autophagy.
Stress granules are of interest for four reasons. First, stress granule formation and dynamics can affect mRNA localization, translation and degradation, as well as signaling pathways and antiviral responses. Second, stress granules share many components with maternal mRNP and neuronal granules suggesting they reveal a conserved mechanism of mRNP compartmentalization (e.g [14]). Third, mutations that increase stress granule formation and/or limit stress granule clearance are causative in some neurodegenerative diseases [15,16]. Finally, as representative of non-membrane bound organelles, an understanding of their assembly and function illustrates an exciting new area of cell biology.
What are stress granules?
Three observations suggest stress granules represent assemblies of mRNPs stalled in translation initiation. First, stress granules form when translation initiation is inhibited either by drugs or by stress responses [4]. Similarly, stress granule-like RNP granules exist in neurons and embryos where there are significant pools of untranslating mRNPs [17]. Second, stress granules fail to form when mRNAs are trapped in polysomes, suggesting that mRNAs associated with ribosomes are unable to enter stress granules [5]. Third, stress granules are observed to contain translation initiation factors, and specific mRNAs that are stalled in steps of translation initiation such as TOP mRNAs [18].
Stress granules contain a diverse proteome. Based on proteomic analysis of stable substructures within stress granules referred to as “cores”, ∼50% of stress granule components are a subset of RNA binding proteins [7]. Stress granule components that do not bind RNA are presumably recruited to stress granules through protein-protein interactions. Such non-RNA binding proteins include post-translation modification enzymes, metabolic enzymes, and protein or RNA remodeling complexes, which can affect stress granule assembly and disassembly. Stress granules also contain key components of signaling pathways, [7,19] which highlight how the formation of stress granules can alter signaling. An overlapping group of proteins form aggregates during extreme heat stress in yeast and some of those aggregating proteins are shown to be components of stress granules [20,21]. Collectively, these experiments show that the composition of stress granules can vary under different conditions revealing that they are both complex and variable assemblies [5].
Stress granules are not uniform structures and contain internal sub-structures as judged by either electron dense regions in EM micrographs,[22] or as regions identified by super-resolution fluorescence microscopy with higher concentrations of proteins and mRNAs [7]. These structures are referred to as “cores”, and can be biochemically purified [7], suggesting that stress granules have two distinct layers (Figure 1), a core structure that is surrounded by a less concentrated, and potentially more dynamic shell. These two regions of stress granules may have different components, functions and dynamics.
Beyond stress granules, a common substructure may be a general principle of all RNP granules. First, the nucleolus contains dense fibrillarin cores as a substructure [23]. Second, C. elegans P granules show spatial orientation when bound to the nuclear pore, forming a consistent “tripartite sandwich” [24]. Third, lattice-light sheet microscopy on P-granules in live C. elegans reveals substructures [25]. Finally, FISH on Drosophila germline granules reveals foci of specific mRNAs implying a sub-granular organizing principle [26].
Stress granules are dynamic structures. In mammalian cells, stress granules undergo fusion, fission and flow in the cytosol [10]. Moreover, by FRAP, most components of stress granules exchange rapidly with half-times for recovery of less than 30 seconds (reviewed in [5]). Interestingly, these FRAP experiments have also revealed an “immobile pool” of protein that does not exchange on a similar timescale suggesting that a subset of the molecules within stress granule components exchange slowly. One intriguing possibility is that components in the shell structure can exchange rapidly, while stress granule components in the core layer may be less dynamic [7].
Interactions influencing stress granule assembly
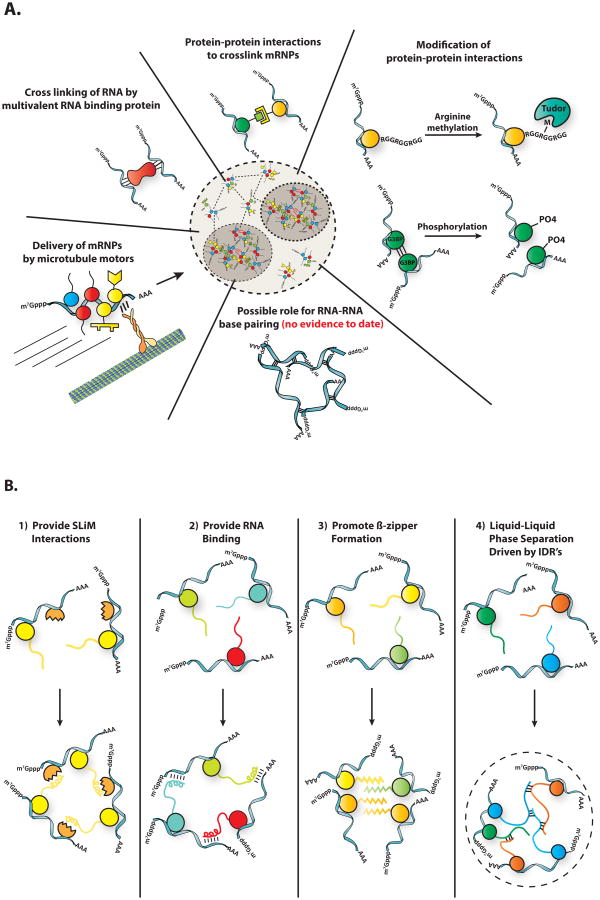
Stress granules assemble when untranslating mRNPs interact through protein-protein interactions between mRNA binding proteins (Figure 2a). Analyses of the proteomes of yeast and mammalian stress granule cores identified a dense network of protein-protein interactions between stress granule components that could contribute in redundant manners to stress granule formation [7]. For example, in both mammals and yeast, Atx2/Pbp1, or TIA1/Pub1 proteins promote, but are not absolutely required for, stress granule assembly [9,27]. The redundancy of interactions suggests that stress granule formation under different conditions can occur by different interactions. For example, the paralogs G3BP1 and G3BP2 play important roles in stress granule formation in mammalian cells in oxidative stress, both by self-interaction,[28] and by interaction with the caprin RNA binding protein [29]. However, during osmotic stress G3BP1/2 and caprin are not required for stress granule formation [30]. Similarly, in yeast Gtr1, Rps1b, and Hgh1 promote stress granule formation during glucose starvation, but suppress stress granule formation during heat shock [31]. Therefore, granule assembly is highly redundant, and the mechanism of assembly can be context specific. This suggests that granules can assemble differently in response to specific cellular conditions, and that stress granules may have different functions for different stresses.
Figure 2. Intermolecular interactions that drive stress granule assembly.
A) A diverse set of intermolecular interactions are important for granule assembly. B) Various mechanisms by which intrinsically disordered regions could contribute to granule assembly.
Protein methylation, phosphorylation and glycosylation influence stress granule assembly, presumably by altering specific protein-protein interactions. For instance, phosphorylation of G3BP impairs its ability to multimerize, which impairs granule assembly [28]. Similarly, granule disassembly during recovery is promoted by the phosphorylation of the granule protein Grb7 [32], and the DYRK3 kinase [33]. Many stress granule proteins contain RGG motifs that are sites of arginine methylation [34]. This methylation can impact stress granules through the recruitment of Tudor domains. For example, the Tudor domain of TDRD3 is both sufficient and necessary for the recruitment of that protein to stress granules, and point mutants that impair TDRD3 binding to methylated arginine impair its localization to stress granules [35]. O-Glc-NAc glycosylation of proteins also enhances stress granule formation [36]. Based on over-expression and inhibitor studies, acetylation and parylation have also been suggested to play a role in stress granule assembly in mammalian cells [37,38].
Stress Granule Assembly: Possible roles for Intrinsically disordered domains
Given the dynamic behavior of RNP granules in cells, and the behavior of RNP granule components in vitro, a current model is that many RNP granules are liquid-liquid phase separations (LLPS) driven by dynamic and promiscuous interactions between IDRs [34,39–42]. A LLPS occurs when a molecule, or mixture of molecules, forms a network of multivalent weak interactions, which allows those molecules to concentrate into a separate phase. When applied to stress granules, this model for assembly consists of three aspects worthy of discussion.
One issue is whether stress granules represent a phase separation wherein multivalent interactions between their components lead to the formation of a higher order structure. By definition, any assembly larger than a dimer requires multivalent interactions for its formation. Moreover, stress granules appear to form through the cross linking of untranslated mRNAs that can provide a scaffold for multiple mRNA binding proteins, thereby allowing each mRNP multivalent interactions and stress granule formation. Thus, stress granules can certainly be thought of as forming by phase separation, or as a multivalent assembly. Because of this nature, stress granules will have two interesting properties. First, because of the diversity of interactions promoting their formation, stress granules will not be specifically defined, and the interactions between components can vary, and even be rearranging. In addition, macromolecules below a certain size should enter, diffuse within, and exit stress granules. This principle is illustrated wherein 40 kDa, but not 155 kDa, dextrans can diffuse into the related P-granules in nematodes [43]. It should be noted that the liquid-like nature of an assembly is not due to its formation by phase separation. Indeed, protein crystals are also formed by phase separation. The material properties of the assembly are derived from the on-off rates of the interactions driving the transition. Macromolecules interacting with slow off-rates will phase separate into a solid, while interactions with fast off-rates will lead to liquid assemblies. With core structures surrounded by more dilute shell, stress granules could be thought of as a combination of liquid and solid phases.
A second issue is how prevalent the role is for intrinsically disordered regions of proteins in stress granule formation, which is based on the following observations. First, many RNA binding proteins found in RNP granules contain intrinsically disordered regions (IDRs), also referred to as low complexity sequences (LCS), and containing the more narrowly defined prion-like domains (PrLD), which are identified by amino acid composition similar to fungal prion proteins predicted to have high cross-beta zipper forming abilities [44–46]. Second, the IDR/prion-like-domain of the human TIA-1 protein promotes stress granule formation and can be substituted with the prion-like domain of the yeast Sup35 protein [27]. Although other evidence that IDRs affecting stress granule formation is limited, IDRs do affect other RNP granules. Specifically, assembly of P-granules in C. elegans requires an FG repeat region on the PGL proteins,[47] the IDR of RBM14 is required for paraspeckle assembly,[48] and P-body assembly in yeast is promoted by PrLDs on Lsm4 and several other P-body components [44,45]. However, since components of stress granules show a dense network of defined protein-protein interactions[7], a reasonable hypothesis is that stress granules form by a number of different protein-protein interactions, some of which involve IDRs.
The final issue to consider is whether stress granules are held together by weak promiscuous interactions of IDRs. In principle, IDRs could play four possible roles in stress granule assembly (Figure 2b). First, IDRs could provide access to Short Linear Motifs (SLiMs), short protein sequences that typically fit into binding sites on other well-folded domains. Precedent for IDRs functioning as important sites of SLiMs comes from the analysis of P-bodies, where many SLiMs in IDRs contribute to P-body assembly [49]. Second, IDRs can contain regions that bind RNA [39,40] and therefore might provide additional interactions between mRNPs in granules. Third, since IDRs can often form amyloid-like fibers in vitro, including both hetero- and homotypic interactions, perhaps IDRs function to stabilize granules by the formation of cross-strand beta zippers [45,46,39,50]. A fourth manner by which IDRs might affect RNP granule assembly is through weak dynamic interactions between IDRs, which then promote a liquid-liquid phase separation (LLPS). This model has gathered support from the observation that many IDRs from RNP granule components undergo LLPS in vitro, including the RNP granule components hnRNP A1,[39,40] Ddx4,[34] LAF- 1,[42] FUS [51], and Whi3 [52]. Interestingly, LLPS triggered by IDRs in vitro are initially dynamic and liquid-like, consistent with their formation through numerous weak interactions, but over time the high concentration of IDRs within the LLPS can promote the formation of stronger interactions, including amyloid-like structures [39,40,51,53,52].
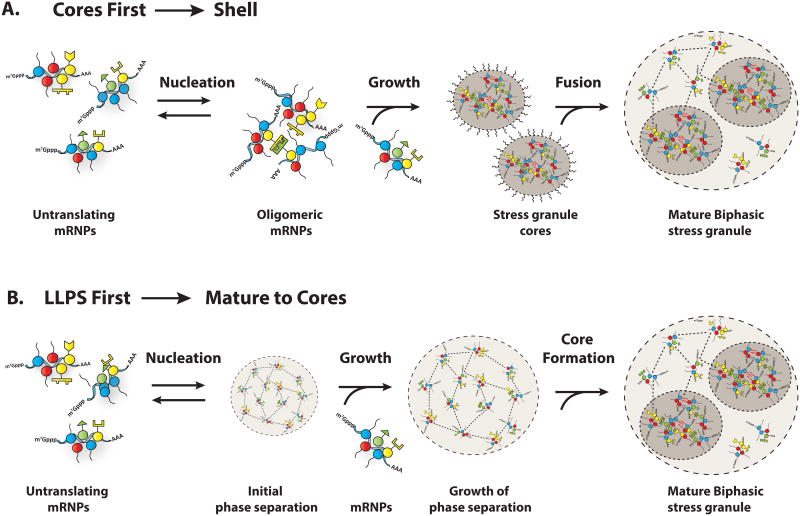
One complication with considering stress granules as a simple LLPS is that they contain stable core substructures, which implies more stable interactions are also present. [7]. Given this, there are two models for how LLPS could contribute to stress granule formation (Figure 3A&B) in the context of stable substructure. In one model, stress granules form first by a LLPS through weak dynamic interactions,[39,40,51] and the high concentration of components in such a LLPS promotes the formation of the core structures, analogous to the formation of amyloid fibers in LLPS in vitro [39,40,51].
Figure 3. Two models for discrete phases of stress granule assembly.
Stress granules can by hypothesized to undergo three phases of assembly.
A) In the “Cores First” model, cores precede assembly of large stress granules. The first phase of assembly is the nucleation of translationally repressed RNPs into oligomers. The second phase of assembly is growth of these oligomers into larger assemblies by addition of more translationally repressed RNPs. The third phase of assembly is fusion of these core assemblies and recruitment of the dynamic shell to form the large, microscopically visible granules typically observed in cells. Some of the stability of cores may be due to amyloid interactions, as indicated by squiggly red lines.
B) In the “LLPS First” model, the formation of large stress granules precedes core assembly. The first phase of this model is the nucleation of translationally repressed RNPs into initial phase separated droplets, held together by weak dynamics interactions. The second phase is growth of initial droplets by the addition of translationally repressed RNPs. The third phase of assembly is core formation within phase separated granules due to the high local concentration of proteins within the droplets. In this model, the formation of cores may be driven in part by amyloid interactions, as indicated by squiggly red lines.
Alternatively, since IDRs can be promiscuous in their interactions, we favor a model wherein mRNPs first condense into stable core structures through strong, specific interactions. Then the high local concentration of IDRs on stress granule components would trigger a LLPS, which may explain the dynamic shell structure surrounding the cores [7].
Multiple phases of Stress granule assembly
One can identify multiple steps in stress granule assembly (Figure 3). In the most parsimonious model, nucleation occurs wherein we hypothesize the formation of oligomeric assemblies of untranslating mRNPs, whose assembly can be controlled by post-translational modifications and/or RNP remodelers. For example, defects in the CCT chaperonin complex give more stress granules in yeast, which is consistent with the CCT complex limiting nucleation, either by remodeling interactions between mRNPs, or by limiting misfolded proteins, which in some contexts can overlap and potentially seed stress granule formation [7,20,21,41,54]. Second, the nucleated states then grow by the joining of additional mRNPs to form small stress granules of ∼200 nm in both yeast and mammals [7]. Under some conditions, the oligomeric seeds of stress granules may form by transitions in mRNP composition that occur at P-bodies. This is suggested by the observation that stress granules tending to form after and on P-bodies in yeast during glucose deprivation, and the observation that some stress granules in mammalian cells appear to grow out of P-bodies [9]. In mammals, a third step occurs wherein, in a microtubule transport-dependent manner, [55–57] smaller stress granules merge and form higher order assemblies with stable core structures surrounded by a more dynamic and less concentrated “shell” structure.
Dynamics, disassembly and clearance of stress granules
Stress granules are dynamic structures and exhibit liquid-like behavior, rapid exchange rates of components, disassembly into translating mRNPs, and clearance by autophagy. Several lines of evidence now suggest a model where the dynamics of stress granules arises, at least in part, by ATP dependent remodeling complexes. For example, acute pharmacological impairment of ATP production eliminates stress granule movement, fusion and fission [7]. Moreover, ATP depletion increases the pools of G3BP that fails to recover after photobleaching,[7] suggesting that at least some of the protein exchange is dependent on ATP. Interesting, some G3BP protein does still recover, which could either be due to residual ATP in the cell, or could suggest that some G3BP is recruited to stress granules by interactions with intrinsically high off rates.
The ATP dependence of granule dynamics supports a general model of dynamic RNP assemblies as “active liquids”, where the energy of ATP driven remodeling events keeps the assembly in a dynamic state [23]. For example, nucleoli require ATP for their own liquidlike behavior, and are 10 –fold less dynamic when ATP is depleted [23]. Similarly, germline RNP processing bodies in C. elegans form solid, crystalline-like aggregates when the helicase CGH-1 is non-functional (DDX6 in humans) [66].
In this view, stress granule proteins and mRNA can form stable interactions that are disrupted by ATPases. During stress, when concentrations of granule components like non-translating RNA are high, ATPases transiently disrupt such interactions and thereby contribute to fast exchange rates. During recovery this disruption leads to disassembly. Potentially, residual material is then subject to autophagy. The ATPases involved in stress granule dynamics are likely to include complexes that directly affect interactions within stress granules such as protein chaperones and RNA helicases (Figure 4), as well as those affecting cellular transport, such as microtubule dependent motors [55–57].
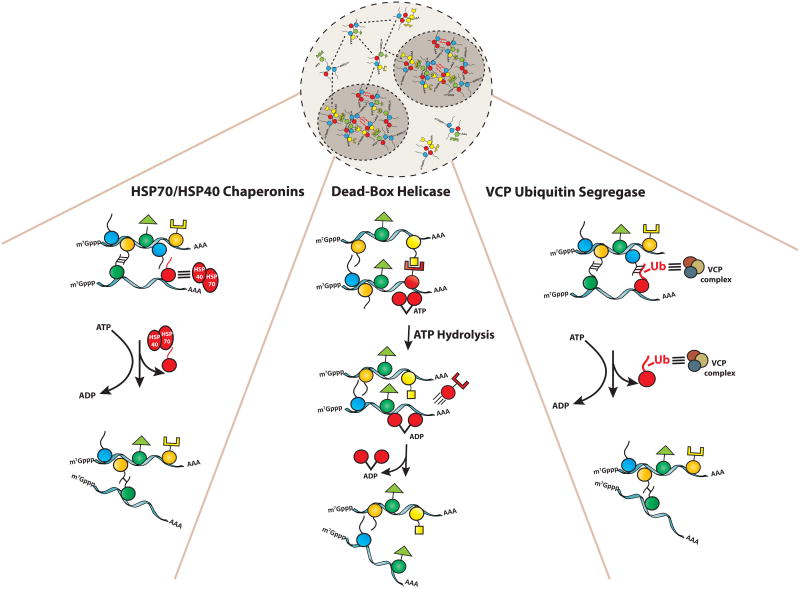
Figure 4. Various ATPases impact granule assembly.
Heat shock proteins, helicases, and VCP all impact stress granule assembly by remodeling specific interactions utilizing the energy of ATP hydrolysis.
Several observations argue RNA/DNA helicases, which utilize the energy of ATP hydrolysis to either unwind DNA/RNA or to displace proteins bound to nucleic acids, also play roles in controlling stress granule assembly and disassembly (Figure 4). Stress granules contain a variety of different helicases including many members of the common DEAD-box helicase family[7]. Moreover, the DEAD box helicases Ded1 (mammalian orthologue: DDX3) is a conserved component of stress granules and promotes stress granule assembly [58]. However, mutations in Ded1 that block its ATPase activity trap mRNAs in stress granules and lead to the inhibition of translation, indicating an important role for ATP hydrolysis in the release of mRNAs back into the cytosol. Similarly, the exchange rate of the RHAU DEAD box from mammalian stress granules is slowed dramatically by cis acting mutations in the ATPase active site [59].
The MCM and Rvb helicase complexes are conserved components of stress granules, and affect the rate of clearance of stress granules from cells. The key observation is that loss of function of either the MCM or Rvb complexes leads to an increased rate of stress granule disassembly during stress recovery in both yeast and mammalian cells [7]. Since the MCM complex is known to act as a DNA helicase and function in DNA replication [60] a role in stress granules would be a novel function for this complex. The Rvb complex also primarily acts on DNA but has been seen to affect snoRNA biogenesis and even function in translation control of HIV transcripts, which may be related to how it affects stress granules [61]. Since helicases would generally be expected to disassemble RNA-protein assemblies, it remains to be determined how these helicases function to increase the stability of stress granules.
The AAA-ATPase VCP/Cdc48 appears to remodel stress granules in a manner that promotes their targeting to autophagy, and may also affect their disassembly. The key observation is that inhibition of VCP/Cdc48 function in yeast or mammals leads to the accumulation of stress granules in the cytosol, as well as a reduction in stress granules that can be trapped in intravacuolar vesicles [13]. VCP/Cdc48 is an ubiquitin segregase and uses the energy of ATP hydrolysis to extract ubiquinated proteins from complexes [62]. Thus one hypothesis is that VCP/Cdc48 may potentially extract some ubiquinated protein from stress granules and that process can, at a minimum, allow stress granules to be targeted for autophagy (Figure 4).
A number of protein chaperones affects stress granule assembly or disassembly. For example, inhibition of Hsp70 function in yeast or mammals leads to either increased stress granule formation and/or delayed disassembly of stress granules [54,63,64]. Moreover, both Hsp70 and Hsp40 proteins can localize to stress granules in yeast and mammalian cells [41,54,63,64]. Interestingly, in yeast different Hsp40 proteins, which often provide substrate specificity to Hsp70s, affect stress granules in different manners. Ydj1 plays a role in disassembling stress granules to promote new translation, while Sis1 functions to trigger stress granules entering autophagy [64]. This implies that different remodeling complexes can lead to different fates of stress granule components.
Under extreme heat shock conditions, the yeast Hsp104 chaperone is also required for stress granule disassembly and timely resumption of translation [41,54]. Interestingly, under extreme heat shock in yeast and Drosophila cells, stress granules appear to overlap with unfolded proteins, and this interaction is limited by Hsp104 [54]. One interpretation of this observation is that under extreme heat shock IDRs within stress granules are prone to misfolded and thereby interact with other misfolded proteins. Interactions between misfolded proteins and stress granules could suggest that in some contexts mutations causing misfolded proteins might enhance stress granule formation, and/or that stress granule formation might stimulate proteins misfolding, which might contribute to some pathologies [65].
Functions of Stress Granules
Stress granule formation is expected to affect biological reactions in two manners. First, due to the high local concentration of components, equilibriums of interacting molecules will shift towards associated states. For example, during viral infections stress granules recruit numerous antiviral proteins including RIG-1, PKR, OAS, and RNaseL, stimulating their activation, and thereby enhance induction of the innate immune response and viral resistance [67–69]. Given this function, many viruses employ mechanisms to block stress granule induction including proteolytic cleavage of G3BP [70]. Stress granule formation might also promote the interaction of mRNAs with translation factors, and thereby enhance the formation of translation initiation complexes [9].
A second manner by which stress granules may affect biological reactions is by limiting the interactions of sequestered components with the bulk cytosol. In this manner, stress granules have been proposed to modulate signaling pathways by sequestering components of TOR, RACK1, or TRAF2 signaling pathways [71–74]. Because stress granules also sequester numerous proteins involved in RNA physiology and/or metabolism, the formation of stress granules is likely to have broad effects on the physiology of cells. However, it should be noted that how stress granule assembly fully affects either the regulation of mRNA function, and/or other aspects of cell physiology remains to be established.
Stress granules in disease
Mutations that affect stress granule formation, or persistence, contribute to the formation of several degenerative diseases including ALS, FTLD, and some myopathies.[15,16] Strikingly, in many cases the mutations are in RNA binding proteins (e.g. hnRNPA1, FUS, TDP-43, Atx2, TIA1) increase their self-assembly properties in vitro, and in cells lead to the formation of stress granule-like assemblies in the absence of stress. How related these pathogenic assemblies are to stress granules remains to be seen [16,75]. Moreover, mutations in VCP, an AAA-ATPase ubiquitin segregase, both inhibit the clearance of stress granules [13] and trigger the same family of diseases as hyper-assembly mutations in RNA binding proteins [76,77]. Consistent with autophagy being important in clearing stress granules, mutations in other proteins that can affect autophagy (optineurin, ubiquilin-2, DNAJb6, and p62) also lead to neuro- or muscular degenerative diseases [64,78,79]. Moreover, because patient biopsies from these diseases often contain aggregates including various stress granule components and RNA [15,16], an emerging model is that persistent stress granules trigger a series of events that leads to cell death.
One appealing model is that the persistence of stress granules in these diseases increases the probability of prion-like domains on stress granule components forming very stable beta-amyloid structures, which might be largely irreversible in cells [15,16]. Consistent with that possibility, several groups have recently shown that when stress granule components undergo a LLPS in vitro, which generates a high local concentration, they show an increased tendency to form amyloid-like fibers [39,40,51]. The accumulation of such hyper-stable stress granule-like assemblies might then trigger cell death by altering regulation of RNA biogenesis and function, misregulation of signaling pathways, and/or triggering defects in axonal or nuclear-cytoplasmic transport of mRNPs [16,80–82].
Stress granules also seem to be involved in both tumor progression and treatment (reviewed in [83]). For example, many chemotherapeutic agents promote stress granule formation [84,85]. Moreover, mutations in DDX3 promoting the WNT subclass of pediatric medulloblastoma inhibit the ATPase activity of DDX3, which would be expected to trap mRNPs in stress granules based on analogous mutations in the yeast ortholog Ded1 [58]. Similarly, YB-1 overexpression upregulates G3BP levels in human sarcomas, correlates with poor survival and in mouse models G3BP promotes metastasis [86]. Given the diversity of mechanisms by which stress granules can affect cell signaling and survival under stress conditions, one anticipates that stress granule formation will have multiple roles in both tumor progression and the outcome of chemotherapeutic treatments.
Concluding Remarks
Stress granules are dynamic assemblies with yet to be fully appreciated roles in cell function (see Outstanding Questions). The interactions that drive their assembly appear complex and may include both traditional protein-protein interactions as well as yet to be defined roles for IDRs. Stress granule dynamics, assembly, and disassembly are modulated by numerous post-translational modifications, RNP and protein remodeling complexes, and movement of components on microtubules giving a complex set of interactions that modulate these structures. Finally, stress granules have a clear impact on a growing number of diseases and understanding this connection may lead to therapeutic interventions targeting stress granule assembly or disassembly.
Outstanding Questions.
What is the actual structure and organizing principles of stress granules?
What are the mechanisms by which ATP-dependent machines make RNP granules dynamic?
What is the full effect of stress granule formation on both post-transcriptional control o mRNAs, and on other aspects of cell physiology?
How does stress granule organization and dynamics impact their function? Is there an important role for regions of substructure?
Why do disruptions in stress granule dynamics and homeostasis promote neurodegenerative disease?
Trends Box.
Stress granules are conserved RNP granules formed from pools of untranslating mRNPs.
Stress granules are dynamic and show liquid-like behaviors but also contain stable substructures.
Numerous interactions promote stress granule assembly including those involving intrinsically disordered protein domains.
Stress granules assembly, disassembly and dynamics are affected by protein chaperons, RNA helicases, and many post-translational modifications.
Mutations that alter stress granule formation contribute to some neurodegenerative diseases and some cancers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spector DL. SnapShot: Cellular Bodies. Cell. 2006 Dec;127(5):1071.e1–1071.e2. doi: 10.1016/j.cell.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Barbarese E, Ifrim MF, Hsieh L, Guo C, Tatavarty V, Maggipinto MJ, et al. Conditional Knockout of Tumor Overexpressed Gene in Mouse Neurons Affects RNA Granule Assembly, Granule Translation, LTP and Short Term Habituation. In: Baudry M, editor. PLoS One. 8. Vol. 8. 2013. Aug 6, p. e69989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009 Jun 26;324(5935):1729–32. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10(6):430–6. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- 5.Buchan JR, Parker R. Eukaryotic Stress Granules: The Ins and Outs of Translation. Mol Cell. 2009 Dec 1;36(6):932–41. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker R, Sheth U. P Bodies and the Control of mRNA Translation and Degradation. Mol Cell. 2007 Mar;25(5):635–46. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell [Internet] 2016 Jan; doi: 10.1016/j.cell.2015.12.038. [cited 2016 Jan 25]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S009286741501702X. [DOI] [PMC free article] [PubMed]
- 8.Aizer A, Kalo A, Kafri P, Shraga A, Ben-Yishay R, Jacob A, et al. Quantifying mRNA targeting to P-bodies in living human cells reveals their dual role in mRNA decay and storage. J Cell Sci. 2014 Oct 15;127(20):4443–56. doi: 10.1242/jcs.152975. [DOI] [PubMed] [Google Scholar]
- 9.Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008 Nov 3;183(3):441–55. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005 Jun 20;169(6):871–84. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-Mediated Translational Repression in Human Cells Subjected to Stress. Cell. 2006 Jun;125(6):1111–24. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310(5747):486–9. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan JR, Kolaitis RM, Taylor JP, Parker R. Eukaryotic Stress Granules Are Cleared by Autophagy and Cdc48/VCP Function. Cell. 2013 Jun;153(7):1461–74. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, et al. Staufen-and FMRP-Containing Neuronal RNPs Are Structurally and Functionally Related to Somatic P Bodies. Neuron. 2006 Dec;52(6):997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013 Apr 29;201(3):361–72. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramaswami M, Taylor JP, Parker R. Altered Ribostasis: RNA-Protein Granules in Degenerative Disorders. Cell. 2013 Aug;154(4):727–36. doi: 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin KC, Ephrussi A. mRNA Localization: Gene Expression in the Spatial Dimension. Cell. 2009 Feb;136(4):719–30. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damgaard CK, Lykke-Andersen J. Translational coregulation of 5′TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011 Oct 1;25(19):2057–68. doi: 10.1101/gad.17355911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan JR. mRNP granules: Assembly, function, and connections with disease. RNA Biol. 2014 Aug 3;11(8):1019–30. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherkasov V, Grousl T, Theer P, Vainshtein Y, Gläßer C, Mongis C, et al. Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett. 2015 Nov 30;589(23):3654–64. doi: 10.1016/j.febslet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Wallace EWJ, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, et al. Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell. 2015 Sep;162(6):1286–98. doi: 10.1016/j.cell.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souquere S, Mollet S, Kress M, Dautry F, Pierron G, Weil D. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J Cell Sci. 2009 Oct 15;122(20):3619–26. doi: 10.1242/jcs.054437. [DOI] [PubMed] [Google Scholar]
- 23.Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci. 2011 Mar 15;108(11):4334–9. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheth U, Pitt J, Dennis S, Priess JR. Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development. 2010 Apr 15;137(8):1305–14. doi: 10.1242/dev.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JT, Smith J, Chen BC, Schmidt H, Rasoloson D, Paix A, et al. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife. 2015;3:e04591. doi: 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little SC, Sinsimer KS, Lee JJ, Wieschaus EF, Gavis ER. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat Cell Biol. 2015 Apr 6;17(5):558–68. doi: 10.1038/ncb3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004 Dec 1;15(12):5383–98. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tourriere H. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003 Mar 17;160(6):823–31. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, et al. Distinct Structural Features of Caprin-1 Mediate Its Interaction with G3BP-1 and Its Induction of Phosphorylation of Eukaryotic Translation Initiation Factor 2, Entry to Cytoplasmic Stress Granules, and Selective Interaction with a Subset of mRNAs. Mol Cell Biol. 2007 Mar 15;27(6):2324–42. doi: 10.1128/MCB.02300-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, et al. G3BP–Caprin1–USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol. 2016 Mar 28;212(7):845–60. doi: 10.1083/jcb.201508028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Shen Y, Garre E, Hao X, Krumlinde D, Cvijović M, et al. Stress Granule-Defective Mutants Deregulate Stress Responsive Transcripts. In: Anderson P, editor. PLoS Genet. 11. Vol. 10. 2014. Nov 6, p. e1004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai NP, Ho PC, Wei LN. Regulation of stress granule dynamics by Grb7 and FAK signalling pathway. EMBO J. 2008 Jan 1;27(5):715–26. doi: 10.1038/emboj.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L. Dual Specificity Kinase DYRK3 Couples Stress Granule Condensation/Dissolution to mTORC1 Signaling. Cell. 2013 Feb;152(4):791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol Cell. 2015 Mar;57(5):936–47. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulet I, Boisvenue S, Mokas S, Mazroui R, Cote J. TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules. Hum Mol Genet. 2008 Jul 9;17(19):3055–74. doi: 10.1093/hmg/ddn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohn T, Kedersha N, Hickman T, Tisdale S. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell … [Internet] 2008 Jan 1; doi: 10.1038/ncb1783. Available from: http://www.nature.com/ncb/journal/v10/n10/abs/ncb1783.html. [DOI] [PMC free article] [PubMed]
- 37.Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007 Dec 15;21(24):3381–94. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung AKL, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-Ribose) Regulates Stress Responses and MicroRNA Activity in the Cytoplasm. Mol Cell. 2011 May 1;42(4):489–99. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y, Protter DSW, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015 Oct;60(2):208–19. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, et al. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell. 2015 Sep;163(1):123–33. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroschwald S, Maharana S, Mateju D, Malinovska L, Nüske E, Poser I, et al. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife. 2015;4:e06807. doi: 10.7554/eLife.06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CCH, Eckmann CR, Myong S, et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci. 2015 Jun 9;112(23):7189–94. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Updike DL, Hachey SJ, Kreher J, Strome S. P granules extend the nuclear pore complex environment in the C. elegans germ line. J Cell Biol. 2011 Mar 21;192(6):939–48. doi: 10.1083/jcb.201010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol. 2007 Nov 5;179(3):437–49. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reijns MAM, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. J Cell Sci. 2008 Aug 1;121(Pt 15):2463–72. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, et al. Cell-free Formation of RNA Granules: Low Complexity Sequence Domains Form Dynamic Fibers within Hydrogels. Cell. 2012 May 11;149(4):753–67. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanazawa M, Yonetani M, Sugimoto A. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J Cell Biol. 2011 Mar 21;192(6):929–37. doi: 10.1083/jcb.201010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hennig S, Kong G, Mannen T, Sadowska A, Kobelke S, Blythe A, et al. Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles. J Cell Biol. 2015 Aug 17;210(4):529–39. doi: 10.1083/jcb.201504117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonas S, Izaurralde E. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 2013 Dec 15;27(24):2628–41. doi: 10.1101/gad.227843.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011 Jun 12;18(7):822–30. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015 Aug;162(5):1066–77. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, et al. RNA Controls PolyQ Protein Phase Transitions. Mol Cell. 2015 Oct;60(2):220–30. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang S, Kato M, Wu LC, Lin Y, Ding M, Zhang Y, et al. The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei. Cell. 2015 Nov;163(4):829–39. doi: 10.1016/j.cell.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, et al. Coordination of Translational Control and Protein Homeostasis during Severe Heat Stress. Curr Biol. 2013 Dec;23(24):2452–62. doi: 10.1016/j.cub.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 55.Chernov KG, Barbet A, Hamon L, Ovchinnikov LP, Curmi PA, Pastre D. Role of Microtubules in Stress Granule Assembly: Microtubule Dynamical Instability Favors the Formation of Micrometric Stress Granules in Cells. J Biol Chem. 2009 Dec 25;284(52):36569–80. doi: 10.1074/jbc.M109.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loschi M, Leishman CC, Berardone N, Boccaccio GL. Dynein and kinesin regulate stress-granule and P-body dynamics. J Cell Sci. 2009 Nov 1;122(21):3973–82. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadezhdina ES, Lomakin AJ, Shpilman AA, Chudinova EM, Ivanov PA. Microtubules govern stress granule mobility and dynamics. Biochim Biophys Acta BBA - Mol Cell Res. 2010 Mar;1803(3):361–71. doi: 10.1016/j.bbamcr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-Box Protein Ded1 Modulates Translation by the Formation and Resolution of an eIF4F-mRNA Complex. Mol Cell. 2011 Sep;43(6):962–72. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalupnikova K, Lattmann S, Selak N, Iwamoto F, Fujiki Y, Nagamine Y. Recruitment of the RNA Helicase RHAU to Stress Granules via a Unique RNA-binding Domain. J Biol Chem. 2008 Dec 12;283(50):35186–98. doi: 10.1074/jbc.M804857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bell SD, Botchan MR. The Minichromosome Maintenance Replicative Helicase. Cold Spring Harb Perspect Biol. 2013 Nov 1;5(11):a012807–a012807. doi: 10.1101/cshperspect.a012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mu X, Fu Y, Zhu Y, Wang X, Xuan Y, Shang H, et al. HIV-1 Exploits the Host Factor RuvB-like 2 to Balance Viral Protein Expression. Cell Host Microbe. 2015 Aug;18(2):233–42. doi: 10.1016/j.chom.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Meyer H, Weihl CC. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014 Sep 15;127(18):3877–83. doi: 10.1242/jcs.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazroui R, Di Marco S, Kaufman RJ, Gallouzi IE. Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol Biol Cell. 2007 Jul 1;18(7):2603–18. doi: 10.1091/mbc.E06-12-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walters RW, Muhlrad D, Garcia J, Parker R. Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. RNA. 2015 Sep;21(9):1660–71. doi: 10.1261/rna.053116.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, et al. Contrasting Pathology of the Stress Granule Proteins TIA-1 and G3BP in Tauopathies. J Neurosci. 2012 Jun 13;32(24):8270–83. doi: 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hubstenberger A, Noble SL, Cameron C, Evans TC. Translation Repressors, an RNA Helicase, and Developmental Cues Control RNP Phase Transitions during Early Development. Dev Cell. 2013 Oct;27(2):161–73. doi: 10.1016/j.devcel.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, et al. Critical Role of an Antiviral Stress Granule Containing RIG-I and PKR in Viral Detection and Innate Immunity. In: Kanai A, editor. PLoS ONE. 8. Vol. 7. 2012. Aug 13, p. e43031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reineke LC, Lloyd RE. The Stress Granule Protein G3BP1 Recruits Protein Kinase R To Promote Multiple Innate Immune Antiviral Responses. In: Sandri-Goldin RM, editor. J Virol. 5. Vol. 89. 2015. Mar 1, pp. 2575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reineke LC, Kedersha N, Langereis MA, van Kuppeveld FJM, Lloyd RE. Stress Granules Regulate Double-Stranded RNA-Dependent Protein Kinase Activation through a Complex Containing G3BP1 and Caprin1. mBio. 2015 May 1;6(2):e02486–14. doi: 10.1128/mBio.02486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reineke LC, Lloyd RE. Diversion of stress granules and P-bodies during viral infection. Virology. 2013 Feb;436(2):255–67. doi: 10.1016/j.virol.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008 Nov;10(11):1324–32. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 72.Kim WJ, Back SH, Kim V, Ryu I, Jang SK. Sequestration of TRAF2 into Stress Granules Interrupts Tumor Necrosis Factor Signaling under Stress Conditions. Mol Cell Biol. 2005 Mar 15;25(6):2450–62. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahara T, Maeda T. Transient Sequestration of TORC1 into Stress Granules during Heat Stress. Mol Cell. 2012 Jul;47(2):242–52. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Kläsener K, Ruf S, et al. Inhibition of mTORC1 by Astrin and Stress Granules Prevents Apoptosis in Cancer Cells. Cell. 2013 Aug;154(4):859–74. doi: 10.1016/j.cell.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 75.Klar J, Sobol M, Melberg A, Mäbert K, Ameur A, Johansson ACV, et al. Welander Distal Myopathy Caused by an Ancient Founder Mutation in TIA1 Associated with Perturbed Splicing. Hum Mutat. 2013 Jan;:n/a–n/a. doi: 10.1002/humu.22282. [DOI] [PubMed] [Google Scholar]
- 76.Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, et al. Exome Sequencing Reveals VCP Mutations as a Cause of Familial ALS. Neuron. 2010 Dec;68(5):857–64. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimonis VE, Mehta SG, Fulchiero EC, Thomasova D, Pasquali M, Boycott K, et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A. 2008 Mar 15;146A(6):745–57. doi: 10.1002/ajmg.a.31862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cipolat Mis MS, Brajkovic S, Frattini E, Di Fonzo A, Corti S. Autophagy in motor neuron disease: Key pathogenetic mechanisms and therapeutic targets. Mol Cell Neurosci. 2016 Apr;72:84–90. doi: 10.1016/j.mcn.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Li S, Zhang P, Freibaum BD, Kim NC, Kolaitis RM, Molliex A, et al. Genetic interaction of hnRNPA2B1 and DNAJB6 in a Drosophila model of multisystem proteinopathy. Hum Mol Genet. 2016 Mar 1;25(5):936–50. doi: 10.1093/hmg/ddv627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015 Aug 26;525(7567):129–33. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ling SC, Polymenidou M, Cleveland DW. Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis. Neuron. 2013 Aug;79(3):416–38. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015 Aug 26;525(7567):56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson P, Kedersha N, Ivanov P. Stress granules, P-bodies and cancer. Biochim Biophys Acta BBA - Gene Regul Mech. 2015 Jul;1849(7):861–70. doi: 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adjibade P, St-Sauveur Valérie Grenier, Quevillion Huberdeau Miguel, Furnier Marie-Josée, Savard Andreanne, Coudert Laetitia, et al. Sorafenib, a multikinase inhibitor, induces formation of stress granules in hepatocarcinoma cells. Oncotarget. 2015 Aug 20;6(41):43927–43. doi: 10.18632/oncotarget.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaehler C, Isensee J, Hucho T, Lehrach H, Krobitsch S. 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 2014 Apr 11; doi: 10.1093/nar/gku264. gku264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Somasekharan SP, El-Naggar A, Leprivier G, Cheng H, Hajee S, Grunewald TGP, et al. YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J Cell Biol. 2015 Mar 30;208(7):913–29. doi: 10.1083/jcb.201411047. [DOI] [PMC free article] [PubMed] [Google Scholar]