Abstract
The phylum Nematoda comprises a diverse group of roundworms that includes parasites of vertebrates, invertebrates, and plants. Human-parasitic nematodes infect approximately one billion people worldwide and cause some of the most common neglected tropical diseases, particularly in low-resource countries [1]. Parasitic nematodes of livestock and crops result in billions of dollars in losses each year [1]. Many nematode infections are treatable with low-cost anthelmintic drugs, but repeated infections are common in endemic areas and drug resistance is a growing concern with increasing therapeutic and agricultural administration [1]. Many parasitic nematodes have an environmental infective larval stage that engages in host seeking, a process whereby the infective larvae use sensory cues to search for hosts. Host seeking is a complex behavior that involves multiple sensory modalities, including olfaction, gustation, thermosensation, and humidity sensation. As the initial step of the parasite-host interaction, host seeking could be a powerful target for preventative intervention. However, host-seeking behavior remains poorly understood. Here we review what is currently known about the host-seeking behaviors of different parasitic nematodes, including insect-parasitic nematodes, mammalian-parasitic nematodes, and plant-parasitic nematodes. We also discuss the neural bases of these behaviors.
Keywords: parasitic nematodes, parasitic helminths, host-seeking behavior, olfactory behavior, skin-penetrating nematodes, entomopathogenic nematodes
Graphical Abstract
Host seeking by entomopathogenic nematodes
Entomopathogenic nematodes (EPNs) in the genera Heterorhabditis and Steinernema are parasites that infect and kill insects. EPNs are known as “beneficial nematodes” because they infect a wide variety of insect pests and disease vectors, and are used commercially throughout the world for biocontrol. EPNs are of interest not only as biocontrol agents, but also as models for understanding human-parasitic nematodes. EPNs are broadly distributed geographically, having been found on every continent except Antarctica [2]. Most EPNs, including many species commonly used for biocontrol such as Steinernema carpocapsae and Heterorhabditis bacteriophora, are generalists that are capable of infecting and killing many different insect species (Figure 1A). However, some EPNs are specialists that primarily target a single type of insect [2]. For example, Steinernema scapterisci targets mole crickets [3] and Steinernema diaprepesi targets the larval stages of the root weevil Diaprepes abbreviates, a citrus pest [4].
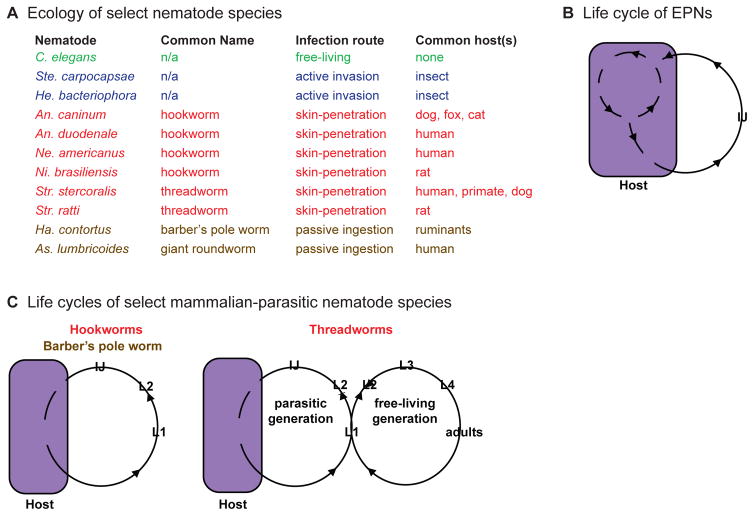
Figure 1. Ecology and life cycles of parasitic nematodes.
A. Ecology of select nematode species, with common hosts indicated [28, 29, 31, 96–98]. Green = free-living, blue = insect-parasitic, red = skin-penetrating, brown = passively ingested. B. The life cycle of EPNs. EPN infective juveniles (IJs) infect by invasion through orifices or by penetration of the cuticle. The IJs and their associated bacteria rapidly kill the host. The nematodes develop and reproduce within the host cadaver for a number of generations, where they feed on bacteria in the insect body and the cadaver tissue. Once resources in the cadaver are exhausted, IJs emerge and search for a new host to infect [96]. C. Life cycles of mammalian-parasitic nematodes. Hookworms infect by skin penetration or orally. Inside the host, hookworms develop into adults that reproduce in the small intestine. Eggs are excreted with the host’s feces and develop into IJs. IJs leave the feces in search of new hosts. Hookworms must infect a new host every generation [99]. Threadworms of the Strongyloides genus have a similar life cycle but can develop through a limited number of free-living generations outside the host. Some of the eggs excreted with the host’s feces develop into IJs, while others develop into free-living adults that mate outside the host [28]. The life cycle outside the host of the barber’s pole worm Ha. contortus is similar to that of hookworms, except that the IJs enter the host only by passive ingestion [97]. For B–C, L1–L4 are the first through fourth larval stages. For some parasitic nematode species IJs are alternatively described as infective third-stage larvae (L3i). Figures are adapted from Castelletto et al., 2014 [26].
Life cycle of EPNs
EPNs are infective during a particular life stage called the infective juvenile (IJ), or alternatively the infective third-stage larva (L3i). IJs invade insect hosts by entering through an orifice such as the mouth or spiracles, or by penetrating through the cuticle [5]. The IJs contain a bacterial endosymbiont in their gut, and upon host entry they deposit their symbiotic bacteria into the insect. The worms and bacteria rapidly kill the insect, typically within 48 hours of host entry. The worms grow and reproduce inside the insect cadaver, feeding off bacteria and cadaver tissue until food sources are depleted. New IJs then form and disperse into the environment to search for new hosts (Figure 1B) [5].
Host-seeking strategies of EPNs
The host-seeking strategies of EPN species are typically described as varying along a continuum ranging from ambushing, in which the IJs remain relatively stationary and latch on to passing hosts, to cruising, in which the IJs disperse in search of hosts [6]. Ambushers often nictate, where the IJ stands on its tail and waves to facilitate attachment to passing hosts. Some ambushing Steinernema species also jump, where the IJ stands on its tail, curls, and propels itself into the air [6]. In general, ambushers are most effective at targeting motile hosts, while cruisers are most effective at targeting non-motile hosts [7]. However, recent studies suggest that many species are capable of engaging in either ambushing or cruising depending on the environmental context. For example, although Ste. carpocapsae is generally considered a classical ambusher, it moves more in peat than sandy soil, suggesting that it can ambush or cruise depending on its environment [8]. The extent to which Ste. carpocapsae moves in the soil also depends on which insect hosts are present [7, 9]. In addition, all EPN species examined so far exhibit robust chemotaxis in the presence of insect-derived odorants [10, 11]. Thus, most EPNs appear to be capable of cruising toward host-emitted sensory cues under at least some conditions.
Responses of EPNs to olfactory cues
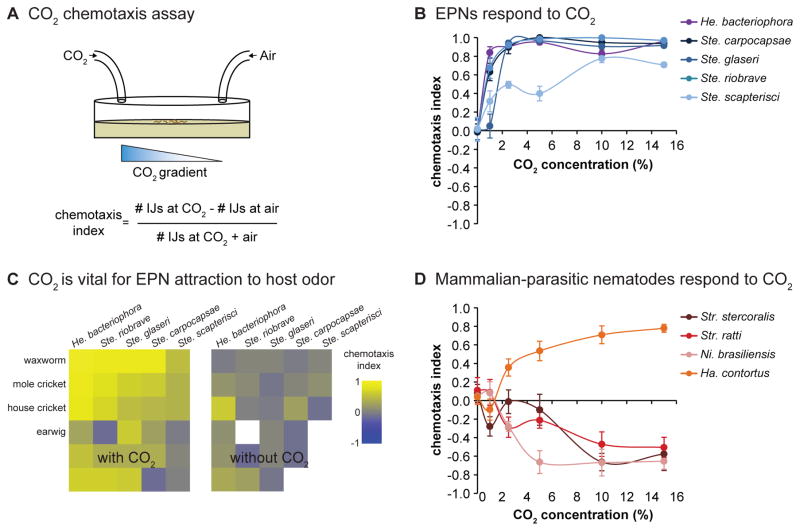
A number of studies have demonstrated that EPNs use olfactory cues to locate hosts to infect. IJs are attracted to the odor blends emitted by live insects and to a diverse array of insect-emitted odorants, including carbon dioxide (CO2) (Figure 2A–B) [10–15]. CO2 is an essential host cue for EPNs: attraction to insect odor blends is greatly reduced or eliminated when CO2 is removed (Figure 2C) [11, 12]. Jumping in Steinernema is stimulated by insect odor blends, CO2, and host-specific odorants [10, 11, 16]. EPNs are also attracted to volatile components of insect feces [17]. A large-scale comparative analysis of olfactory behaviors across species revealed that different EPN species respond differently to odorants [10, 11]; thus, EPNs appear to have specialized olfactory systems that contribute to host selection.
Figure 2. Responses of different parasitic nematode species to CO2.
A. A CO2 chemotaxis assay. CO2 is pumped into one side of a plate, and an air control is pumped into the other side. IJs are placed in the center of the plate and allowed to migrate in the CO2 gradient for 1 hour. The number of IJs underneath the CO2 inlet and the number underneath the air inlet are then counted, and a chemotaxis index (CI) is calculated according to the formula indicated. The chemotaxis index ranges from +1 to −1, with positive values indicating attraction to CO2 and negative values indicating repulsion from CO2. B. Responses of EPNs to CO2 in a chemotaxis assay. All EPN species tested are attracted to CO2 across concentrations. Data are from Dillman et al., 2012 [11]. C. CO2 is required for normal attraction of EPNs to insect odor blends. Left, EPN responses to host odor blends in a chemotaxis assay. Right, EPN responses to host odor blends with CO2 chemically removed. Attraction of EPNs to insect odor is reduced or eliminated in the absence of CO2. Responses are shown as a heatmap; yellow indicates attraction and blue indicates repulsion. White boxes in the heatmap indicate EPN-host combinations that were not tested with CO2 removed because they were not attractive with CO2 present. Reproduced from Dillman et al., 2012 [11]. D. Responses of mammalian-parasitic nematodes to CO2 in a chemotaxis assay. The skin-penetrating nematodes Str. stercoralis, Str. ratti, and Ni. brasiliensis are repelled by CO2, while the passively ingested nematode Ha. contortus is attracted to CO2. Data are from Castelletto et al., 2014 [26].
EPNs also respond to odorants emitted by insect-damaged plants [18]. For example, the odorant (E)-β-caryophyllene is released by maize roots in response to insect feeding and attracts the EPN Heterorhabditis megidis [19]. Similarly, Ste. diaprepesi is attracted to volatiles released by plant roots that have been damaged by its host D. abbreviates [4]. CO2 acts synergistically with root volatiles to attract EPNs [18]. Thus, EPNs appear to use CO2, insect odorants, and plant odorants to find insects to infect.
Responses of EPNs to other sensory cues
In addition to responding to olfactory cues, EPNs respond to a number of other sensory cues that may contribute to host seeking. For example, EPNs have been shown to aggregate at temperatures that approximate insect body temperature, which is slightly (<1°C) above ambient temperature due to insect metabolic processes [20]. EPNs also respond to salt gradients. Ste. carpocapsae IJs can navigate in gradients of Na+, Mg2+, Ca2+, CO32−, and Cl− and accumulate at different preferred concentrations for each ion [21]. EPNs also respond to electric fields, magnetic fields, vibration, and mechanical stimulation [22–26]. These other sensory responses are presumed to facilitate environmental navigation and/or host finding.
Host seeking by mammalian-parasitic nematodes
Nematode parasites of humans are widespread and pose dangerous health risks. Approximately one billion people worldwide harbor at least one nematode infection, mostly in low-income tropical and sub-tropical regions of the world [27]. Many parasitic nematode species are co-endemic and mixed infections are frequently observed. Parasitic nematode infections can cause chronic gastrointestinal distress, anorexia, anemia, and stunted physical and cognitive development in children. Select nematode species can cause severe symptoms such as permanent disfigurement and blindness, and some can even be fatal for infants and the immunocompromised [27]. In addition, nematode parasites of non-human animals are widespread, and preventing or controlling nematode infections in commercial livestock and household pets costs billions of dollars annually [1].
Many mammalian-parasitic nematodes infect only one or a limited number of host species. For example, Strongyloides fulleborni kellyi is a human parasite, while Strongyloides stercoralis has a limited host range that includes humans, primates, and dogs (Figure 1A) [28]. Mammalian-parasitic nematodes can infect hosts by skin penetration, passive ingestion, or direct transmission via intermediate vectors [1]. Vector-borne parasitic nematodes such as Wuchereria bancrofti and Onchocerca volvulus, the causative agents of lymphatic filariasis and onchocerciasis, respectively, rely on the host-seeking capabilities of their intermediate insect vectors to infect their definitive hosts [27]. By contrast, skin-penetrating nematodes and passively ingested nematodes use environmental and host-emitted stimuli to seek out nearby hosts or position themselves in advantageous locations for host ingestion. We focus here on skin-penetrating and passively ingested nematodes.
Life cycles of mammalian-parasitic nematodes
Skin-penetrating nematodes such as the human hookworms Ancylostoma duodenale and Necator americanus, and the human threadworm Str. stercoralis, have similar life cycles inside the host (Figure 1A, C). Parasitic adults colonize the mucosa of the host intestine and shed eggs that are passed with feces. The nematodes develop on the host feces to the IJ stage, and the IJs then find and infect new hosts. The soil-dwelling IJs infect by skin penetration, commonly through the feet [28, 29]. The IJs typically migrate through the circulatory system to the lungs, where they penetrate the alveoli and cause irritation and a dry cough. The IJs are coughed up and swallowed, and then pass through the stomach into the intestine, where they resume development into parasitic adults. Hookworms can also infect orally [30]. Strongyloides species can undergo one or a limited number of free-living generations outside of the host, and Str. stercoralis can cycle through multiple generations inside the same host (Figure 1C) [28, 29].
Passively ingested nematodes vary in their life cycles. For example, the human-parasitic giant roundworm Ascaris lumbricoides colonizes the host intestine and eggs are passed in the feces. As. lumbricoides larvae developmentally arrest while still inside the egg, and development resumes when the host ingests infective eggs [31]. In contrast, the ruminant parasite Haemonchus contortus is passively ingested as IJs and has a life cycle outside the host that is similar to that of skin-penetrating hookworms (Figure 1A, C) [32]. However, unlike hookworms, the in-host life cycle of Ha. contortus is constrained to the gut. Ha. contortus IJs exsheath in the rumen and travel to the abomasum, where they develop into parasitic adults [33].
Host-seeking strategies of mammalian-parasitic nematodes
Like EPNs, mammalian-parasitic nematodes vary in their host-seeking strategies. In the absence of stimulation, the dog hookworm Ancylostoma caninum and the human hookworms An. duodenale and Ne. americanus have been described as ambushers that remain relatively motionless [34, 35]. In the presence of host-emitted cues, the IJs crawl or nictate [34, 35]. By contrast, the Strongyloides species appear to be cruisers that spend more time crawling than nictating in the absence of stimulation [26, 36]. Human-parasitic Str. stercoralis IJs crawl faster than rat-parasitic skin-penetrating IJs and EPN IJs in the absence of sensory stimulation, suggesting that Str. stercoralis may have evolved longer-distance dispersal mechanisms to accommodate for more motile hosts [26]. In contrast, passively ingested Ha. contortus is less motile than the skin-penetrating species and appears to be an ambusher [26]. As with EPNs, some mammalian-parasitic nematodes have foraging behaviors that are intermediate between traditionally classified cruising and ambushing behaviors. For example, the rat hookworm Nippostrongylus brasiliensis is capable of crawling at a speed comparable to that of the skin-penetrating rat parasite Strongyloides ratti, yet unlike Str. ratti, Ni. brasiliensis prefers to nictate on certain surfaces [26]. Thus, like EPNs, mammalian-parasitic nematodes appear to modulate their foraging strategy depending on environmental conditions.
Responses of mammalian-parasitic nematodes to CO2
There is now substantial evidence that mammalian-parasitic nematodes use host-emitted chemosensory cues to identify potential hosts. One important host cue for some mammalian-parasitic nematodes is CO2, which is exhaled by mammals at concentrations of 4–5% during respiration (compared to 0.04% in air) [37]. CO2 induces nictation behavior in An. caninum [34]. It also increases random crawling in Str. stercoralis and An. caninum, but decreases random crawling in Ha. contortus [36].
In the presence of a CO2 gradient, the skin-penetrating nematodes Str. stercoralis, Str. ratti, and Ni. brasiliensis are repelled by high CO2 but neutral to low CO2 (Figure 2D) [26]. Avoidance of high CO2 by skin-penetrating nematodes is consistent with the fact that they typically infect around the feet and lower extremities, where CO2 concentrations are less than 1% [38]. However, whether CO2 is attractive in combination with other host cues has not yet been investigated. In contrast to the skin-penetrating nematodes, passively ingested Ha. contortus is attracted to CO2 concentrations at or above 2.5% (Figure 2D) [26]. Ha. contortus infects grazing animals and has been shown to migrate vertically between herbage and soil in response to changes in environmental factors such as temperature and humidity [39, 40]. Attraction to host-emitted CO2 may stimulate Ha. contortus to migrate up the herbage toward the mouths of grazing ruminants and thereby increase the chances of a successful infection.
Responses of mammalian-parasitic nematodes to soluble host cues
Mammalian-parasitic nematodes respond to a number of chemicals present in mammalian skin, sweat, and serum. For example, An. caninum is attracted to hydrophilic components extracted from dog skin [34]. In addition, An. duodenale and Ne. americanus show increased random crawling speeds and skin-penetration behaviors in the presence of human skin extract [35]. More quantitative studies are needed to determine if human hookworms are attracted to human skin extracts. In the case of threadworms, Str. stercoralis is attracted to dog skin extracts, human serum, and human sweat, while Str. ratti is attracted to mammalian serum components [41–43]. Furthermore, both Str. stercoralis and Str. ratti are capable of navigating in sodium chloride gradients and accumulate at concentrations comparable to the concentrations found in sweat [44, 45].
Responses of mammalian-parasitic nematodes to volatile host cues
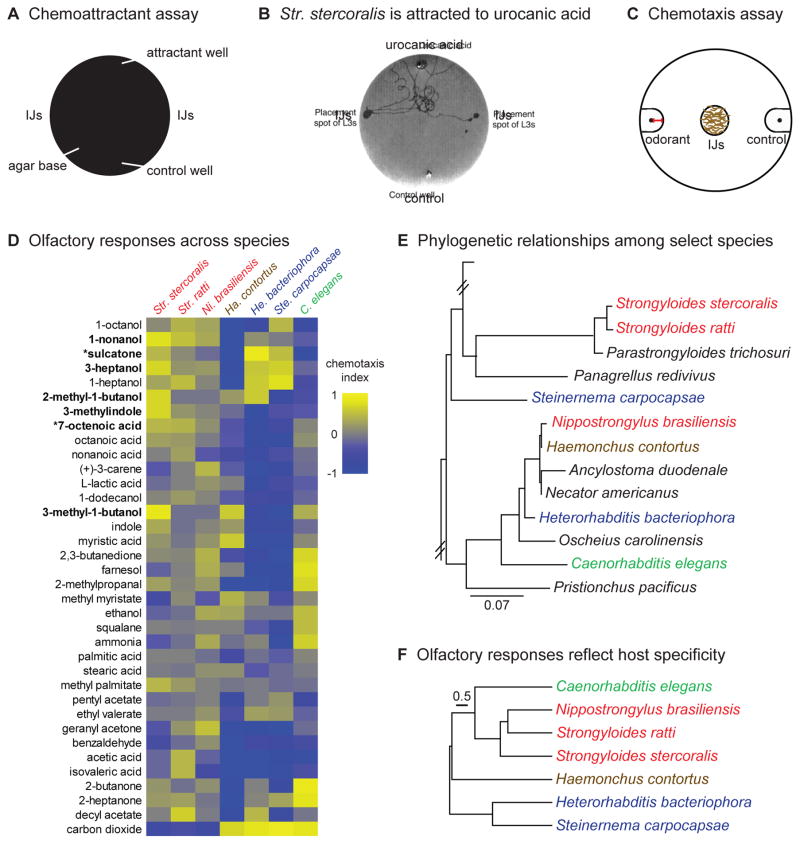
Humans and other mammals emit hundreds of odorants from skin, sweat, skin microbiota, and breath [46, 47]. Since host-emitted odor blends are species-specific, olfaction is likely to contribute to the species-specificity of many parasites, including parasitic nematodes [48]. One of the first skin odorants identified as an attractant for mammalian-parasitic nematodes was urocanic acid. Str. stercoralis IJs are robustly attracted to urocanic acid even at low concentrations (Figure 3A–B) [42]. Urocanic acid is found on the skin of many mammals, but in humans it is especially abundant on the feet, where Str. stercoralis infections commonly occur [42]. Thus, urocanic acid is likely to be an important host-seeking cue for Str. stercoralis.
Figure 3. Responses of mammalian-parasitic nematodes to volatile host cues.
A. A chemoattractant assay for Str. stercoralis IJs. IJs are placed on each side of the plate and allowed to migrate in the chemical gradient for 28 min. B. Str. stercoralis IJs are attracted to urocanic acid. For A–B, data are reproduced from Safer et al., 2007 [42]. C. A chemotaxis assay for IJs. Odorant is placed on one side of the plate and control is placed on the other (black dots). IJs are placed in the center of the plate and allowed to migrate in the odorant gradient for 3 hours. The number of IJs in each scoring region (extended circles around the black dots) is then counted and a chemotaxis index (CI) is calculated as: CI = (# IJs at odorant − # IJs at control)/(# IJs at odorant + control). The chemotaxis index ranges from +1 to −1, with positive values indicating attraction to the odorant and negative values indicating repulsion from the odorant. Red scale bar = 1 cm. D. Olfactory responses across species. CI values are color-coded as shown to the right of the heatmap. For nematode species included, red = skin-penetrating; brown = passively ingested; blue = insect-parasitic; green = free-living. Odorants in bold are known attractants for anthropophilic mosquito species. Odorants denoted by an asterisk are abundant in humans relative to other mammals. E. Phylogenetic relationships among select nematode species, based on Castelletto et al., 2014 [26] and Dillman et al., 2012 [11]. Species tested for olfactory behavior are color-coded. F. Behavioral dendrogram constructed from odorant responses in D. Olfactory responses reflect preferred host rather than genetic relatedness. For C–F, data are from Castelletto et al., 2014 [26].
More recently, Str. stercoralis, Str. ratti, Ni. brasiliensis, and Ha. contortus IJs were tested in chemotaxis assays to assess their responses to a large panel of human-emitted odorants (Figure 3C–D) [26]. Each of the species tested showed a unique odor response profile, demonstrating that like EPNs, mammalian-parasitic nematodes have species-specific olfactory preferences (Figure 3D) [26]. In the case of Str. stercoralis, nearly all of the strongest attractants identified are also known attractants for anthropophilic mosquitoes, suggesting that mosquitoes and nematodes may utilize similar olfactory cues to locate human hosts (Figure 3D). Two of the attractive odorants identified, 7-octanoic acid and 6-methyl-5-hepten-2-one (sulcatone), are thought to be highly enriched in human body odor relative to the body odor of other mammals [49, 50]. In addition, sulcatone response in certain Aedes aegypti mosquito populations was recently associated with preference for human hosts [50]. The finding that Str. stercoralis is attracted to sulcatone raises the possibility that it also uses sulcatone to preferentially target humans [26]. We note that sulcatone is also an insect pheromone [51, 52], perhaps explaining why it is an attractant for EPNs as well as Str. stercoralis. In contrast to the skin-penetrating nematodes, passively ingested Ha. contortus was repelled by most skin and sweat odorants tested. However, Ha. contortus was attracted to fresh grass extracts, as well as methyl myristate and myristic acid, known components of cow and goat milk (Figure 3D) [26]. These responses may allow Ha. contortus IJs to position themselves in grazing areas frequented by ruminants.
A quantitative comparison of olfactory behavior in skin-penetrating IJs, passively ingested IJs, EPN IJs, and dauer larvae of the free-living nematode Caenorhabditis elegans revealed that species with similar host ranges responded more similarly to odorants, even when phylogenetically distant (Figure 3E–F) [26]. For example, Str. ratti and Ni. brasiliensis are distantly related but share a rodent host, and they respond more similarly to odorants than Str. ratti and Str. stercoralis. Thus, the olfactory preferences of mammalian-parasitic nematodes reflect their host specificity rather than their phylogenetic relationships. These results suggest that mammalian-parasitic nematodes have specialized olfactory systems that support host finding and host selection.
Thermosensory behaviors of mammalian-parasitic nematodes
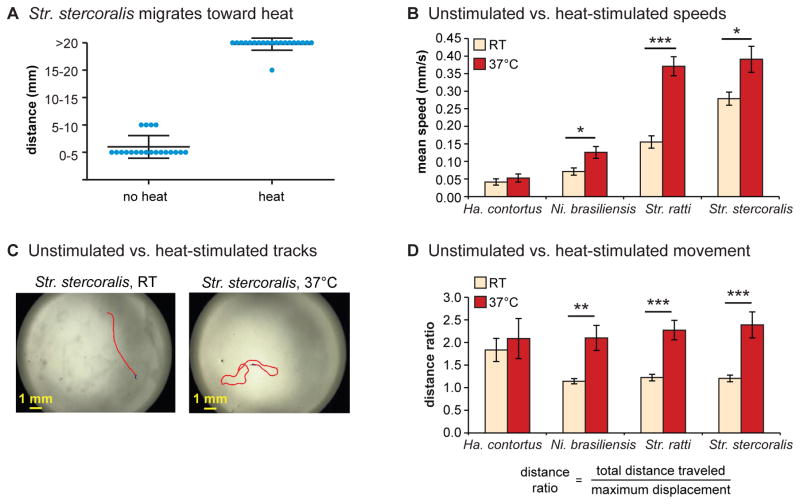
Skin-penetrating nematodes respond robustly to thermal stimulation. Both hookworms and Strongyloides species can navigate through thermal gradients and accumulate at temperatures approximating mammalian body temperature (Figure 4A) [34, 35, 53, 54]. In addition, Str. ratti and Str. stercoralis display relatively straight crawling trajectories at room temperature but increased crawling speeds and highly curved trajectories at 37°C (Figure 4B–D) [26]. This observation suggests that heat stimulates local search and thereby increases the likelihood of host attachment. In contrast, Ha. contortus does not migrate to a heat source and does not increase its crawling speed in response to heat (Figure 4B) [26, 55]. Ha. contortus instead migrates to its cultivation temperature, a behavioral strategy resembling that of C. elegans [55]. Thus, passively ingested IJs may not use heat as a host-seeking cue. However, more quantitative studies will be necessary to better understand how mammalian-parasitic nematodes move within temperature gradients.
Figure 4. Skin-penetrating nematodes respond to thermosensory cues.
A. Str. stercoralis IJs migrate toward heat. In the absence of a thermal gradient, Str. stercoralis IJs do not migrate far from their placement point (“no heat” condition). When the IJs are placed at 26°C in a thermal gradient ranging from 22°C to 43°C, they migrate toward the heated end (“heat” condition). Blue dots = migration distances of individual IJs from their initial placement point during a 1 minute assay; center bars = mean migration distances; upper and lower bars = standard deviations. Dot-plot data are reproduced from Lopez et al., 2000 [54] with permission. B. Unstimulated vs. heat-stimulated speeds of mammalian-parasitic IJs. IJs of skin-penetrating nematode species exposed to an acute 37°C stimulus increase their crawling speeds. C. Representative tracks of Str. stercoralis from 20 s recordings at room temperature versus 37°C. D. Movement patterns at room temperature versus 37°C. Distance ratios were calculated according to the formula shown; a greater distance ratio indicates a more curved trajectory. Skin-penetrating nematodes exposed to an acute 37°C stimulus show curved crawling trajectories, indicative of local-search behavior. For B–D, data are from Castelletto et al., 2014 [26].
Responses of mammalian-parasitic nematodes to other sensory cues
Mammalian-parasitic nematodes respond to a number of additional sensory cues that may contribute to host seeking or promote host contact in the environment. An. caninum, An. duodenale, and Ne. americanus IJs respond to vibration and humidity changes by actively crawling [30, 34, 35]. An. duodenale and Ne. americanus IJs are also activated by light, but only Ne. americanus migrates toward light [30, 35]. Mechanical stimulation increases crawling speed in Str. ratti IJs [26]. How these responses to vibration, humidity, light, and mechanical stimulation contribute to host-seeking behaviors for skin-penetrating nematodes remains to be elucidated. Finally, Ha. contortus IJs exhibit migration toward light (phototaxis) and moisture (hygrotaxis) [39, 40]. Phototaxis may allow Ha. contortus IJs to move vertically on herbage during the day when grazing animals are more active. Hygrotaxis may serve as a protection mechanism, allowing IJs to migrate down herbage and into the relatively damp soil when surface conditions are unfavorable.
Host seeking by plant-parasitic nematodes
Some plant-parasitic nematodes (PPNs) also engage in host seeking, although their host-seeking behaviors remain poorly understood. For example, PPNs such as the potato cyst nematode Globodera pallida and root-knot nematodes in the genus Meloidogyne are attracted to plant roots [56–58]. Ethylene signaling in the plant modulates root attractiveness to PPNs, although whether ethylene signaling directly regulates the production of specific PPN attractants has not yet been determined [59]. Meloidogyne species are attracted to low pH, consistent with the fact that growing roots create a low pH environment [58]. CO2 attracts a number of PPN species, including Meloidogyne incognita and Rotylenchulus reniformis [13, 60]. However, at least in the case of Meloidogyne species, the observed attraction to CO2 may be primarily a response to low pH rather than molecular CO2 [58]. PPNs are also attracted to some of the same root volatiles emitted by insect-damaged plants that attract EPNs [61]. Thus, the emission of specific volatiles by plants in response to insect damage comes at a potential ecological cost.
The neural basis of host-seeking behavior
The neural basis of host-seeking behavior in parasitic nematodes is poorly understood, due to the technical difficulty of working with these organisms and a disconnect between the fields of parasitology and neurobiology. However, the neural basis of sensory behavior is well-studied in the model free-living nematode C. elegans, and neural anatomy and function are often conserved across free-living and parasitic nematode species [48, 62, 63]. In addition, C. elegans has an developmentally arrested, long-lived alternative life stage called the dauer larva, which is developmentally analogous to the IJ stage of parasitic nematodes [64]. C. elegans dauers form when environmental conditions are unfavorable and engage in phoresy, using insects and other invertebrates for transport to more favorable environmental niches [65, 66]. A number of dauer behaviors, including nictation, are shared with parasitic IJs [66]. Thus, knowledge of the neural basis of behavior in C. elegans, particularly dauer behavior, can be leveraged to better understand the neural basis of host seeking in parasitic nematodes.
The neural basis of sensory behaviors in C. elegans
C. elegans responds robustly to sensory stimuli, including a wide variety of volatile compounds [67, 68]; water-soluble compounds such as cations, anions, nucleotides, and amino acids [68]; pheromones [69]; the gases O2 and CO2 [70]; and temperature [71]. The responses to most chemicals and temperature are mediated primarily by head sensory neurons that extend processes toward the tip of the anterior of the worm [68]. Odorants and pheromones are detected by large families of seven-transmembrane domain G protein-coupled receptors (GPCRs) [68, 72], while the gustatory response requires receptor guanylate cyclases (rGCs) [73]. CO2 detection is mediated in part by the rGC GCY-9 [74–76]; however, GCY-9-independent mechanisms of CO2 detection appear to operate in some sensory neurons but have not yet been characterized [77, 78]. O2 detection is mediated by soluble guanylate cyclases and globins [79–81]. Thermosensation involves rGCs and TRP channels [82].
Dauer-specific nictation behavior in C. elegans is mediated by a set of six cholinergic sensory neurons in the head called IL2 neurons [66]. The processes of the IL2 neurons undergo extensive remodeling during dauer development, and the furan homolog kpc-1 is required for both dauer-specific IL2 remodeling and nictation behavior [83]. Whether IL2 neurons and/or a kpc-1 homolog are required for host seeking by parasitic nematodes has not yet been investigated.
The neural basis of chemosensation in parasitic nematodes
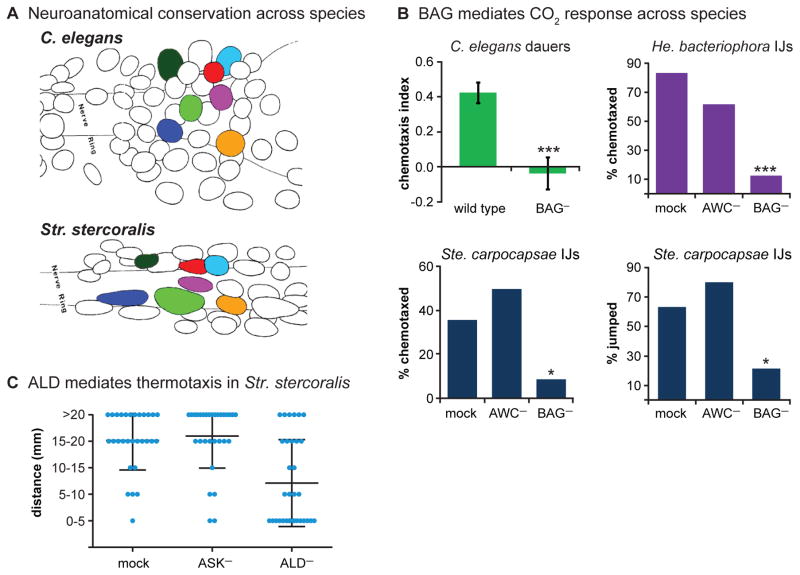
A number of sensory neurons that contribute to host seeking have been identified in parasitic nematodes based on analogy with C. elegans neurons (Figure 5A). For example, CO2 chemotaxis in C. elegans was shown to require a pair of head sensory neurons called the BAG neurons, which directly sense molecular CO2 [75, 76, 84, 85]. Subsequently, BAG neurons were shown to mediate CO2 chemotaxis in the EPNs Ste. carpocapsae and He. bacteriophora, and CO2-evoked jumping in Ste. carpocapsae (Figure 5B) [10]. Thus, the neural basis of CO2 responsiveness is at least partly conserved across free-living and parasitic nematode species. Similarly, the ASE and ASH neurons mediate responses to gustatory cues in both C. elegans and the skin-penetrating human parasite Str. stercoralis [44]. The olfactory sensory neurons that mediate responses to host-specific odorants have not yet been identified in parasitic nematodes.
Figure 5. Sensory neuron function is often conserved across free-living and parasitic nematodes.
A. Schematics of neurons in the head regions of C. elegans and Str. stercoralis. Color-coding indicates a few of the analogous sensory neurons. Schematics are reproduced from Ashton et al., 1995 [86] with permission. B. BAG neurons mediate CO2 response across species. Wild-type C. elegans dauer larvae are attracted to CO2, but animals containing a genetic ablation of the BAG neurons (BAG−) do not respond to CO2. Reproduced from Hallem et al., 2011 [10]. Wild-type He. bacteriophora and Ste. carpocapsae IJs, and IJs in which the AWC chemosensory neurons have been laser-ablated (AWC−), are attracted to CO2. However, IJs in which the BAG neurons have been laser-ablated (BAG−) no longer respond to CO2. BAG ablation also eliminates CO2-evoked jumping by Ste. carpocapsae IJs (lower right graph). Reproduced from Hallem et al., 2011 [10]. C. ALD neurons mediate thermotaxis in Str. stercoralis. Wild-type Str. stercoralis IJs, and IJs in which the ASK chemosensory neurons have been laser-ablated (ASK−), migrate toward heat when placed at 26°C in a thermal gradient ranging from 22°C to 43°C. However, IJs in which the ALD neurons have been laser-ablated (ALD−) do not migrate toward heat. Blue dots = migration distances of individual IJs from their initial placement point during a 1 minute assay; center bars = mean migration distances; upper and lower bars = standard deviations. Dot-plot data are reproduced from Lopez et al., 2000 [54] with permission.
The neural basis of thermosensation in parasitic nematodes
In C. elegans, the primary thermosensory neurons are the AFD neurons, although the AWC and ASI chemosensory neurons are also thermosensory and contribute to thermotaxis [82]. The AFD neurons have a finger-like dendritic structure that results in an increased surface area in the amphid chemosensory organs, which is thought to be important for temperature sensing [82]. The positional analogs of the AFD neurons in the dog hookworm Ancylostoma caninum and the passively ingested ruminant parasite Ha. contortus also have a finger-like dendritic structure and are also required for thermotaxis [53, 55]. Moreover, the RIA interneurons, which function downstream of AFD to mediate thermosensation in C. elegans [82], were also found to be required for thermosensation in Ha. contortus [55]. Thus, as is the case for chemosensation, the neural basis of thermosensation is at least partly conserved across free-living and parasitic species.
Temperature sensing in Str. stercoralis may be somewhat different because Str. stercoralis does not have a pair of neurons with a finger-like dendritic structure [86]. Instead, Str. stercoralis has a pair of neurons, called the ALD neurons, with a lamellar dendritic structure that also results in a large surface area in the amphids [86]. Ablation of the ALD neurons in Str. stercoralis disrupted thermotaxis, demonstrating that the ALD neurons are required for thermosensory behavior (Figure 5C) [54]. The anatomical position of the ALD neurons appears to most closely resemble that of the C. elegans AWC neurons [54]. However, whether ALD neurons are functionally more analogous to the C. elegans AWC or AFD neurons remains unclear.
Unanswered questions regarding neural circuit function in parasitic nematodes
The studies described above demonstrate that sensory neuron function is often conserved across free-living and parasitic species. However, the extent to which functional conservation across species exists at the interneuron level remains unknown. With the exception of the RIA interneurons mentioned above, interneuron function has not yet been explored in parasitic nematodes. The fact that sensory neuron function is often conserved across species, yet sensory microcircuits support species-specific behaviors, suggests that significant differences in interneuron function exist across species. While the connections between neurons have been almost completely mapped for C. elegans [87, 88], connectome data is not yet available for parasitic nematodes. Thus, whether positionally analogous interneurons participate in the same microcircuits across species but have different functional properties, or whether positionally analogous interneurons participate in different microcircuits across species, is not yet clear.
The molecular basis of host seeking by parasitic nematodes also remains to be investigated. Some insights into possible molecular mechanisms of host seeking have come from studies of the necromenic nematode Pristionchus pacificus, which uses beetles for transport to new environmental niches and feeds off beetle cadavers [89]. Closely related Pristionchus species show species-specific responses to insect pheromones and plant volatiles [89]. Natural variation in the response to insect pheromone across P. pacificus strains is associated with the cGMP-dependent protein kinase gene egl-4, suggesting a role for cGMP signaling in the host-seeking behavior of P. pacificus [90]. Future studies will be necessary to determine whether cGMP signaling also regulates host seeking in parasitic nematodes.
Conclusions and future directions
In summary, parasitic nematodes use multiple sensory modalities to find and infect hosts, including olfaction, gustation, thermosensation, and hygrosensation. Moreover, all parasitic nematode infective larvae that have so far been examined respond robustly to sensory cues, suggesting that parasitic nematodes rely on these to maximize their chances of a successful infection regardless of their host range, host-seeking strategy, or infection route. Although we are still at the early stages of understanding the neural basis of host seeking, a more detailed understanding of the molecular and cellular basis of this crucial behavior is likely to emerge over the next few years. Mechanistic studies of neural circuit function in parasitic nematodes are now feasible due to the large-scale sequencing of parasitic nematode genomes [91] and the development of new methods for genetic transformation of parasitic worms [92]. In addition, targeted gene disruption has recently been achieved in multiple free-living nematodes using the CRISPR-Cas9 system [93–95], and this system is likely to be applicable to parasitic nematodes. These exciting developments pave the way for in-depth molecular, cellular, and circuit-level analyses of the host-seeking behaviors of parasitic nematodes. A better understanding of neural circuit function in parasitic nematodes will provide important insights into how parasites target their hosts, and more generally, how the nervous systems of parasites evolve to mediate parasitic behaviors such as host seeking and host invasion. In addition, a more mechanistic understanding of host seeking may enable the development of new strategies for preventing harmful nematode infections of animals and plants, and for enhancing the efficacy of beneficial nematodes as biocontrol agents.
Summary.
Parasitic nematodes use a diverse array of host-emitted sensory cues to find and infect their hosts.
Highlights.
Parasitic worms use sensory cues to find and infect hosts
Host seeking is a complex behavior that involves multiple sensory modalities
Parasitic worms have specialized olfactory systems that support host finding
Sensory neural function is often conserved across free-living and parasitic worms
Mechanisms of host seeking are being elucidated based on knowledge of C. elegans
Acknowledgments
We thank Taylor Brown, Michelle Castelletto, and Joon Ha Lee for insightful comments on the manuscript. This work was funded by the Whitcome Predoctoral Training Program, UCLA Molecular Biology Institute; Ruth L. Kirschstein National Research Service Award AI007323; and a National Science Foundation East Asia and Pacific Summer Institute Fellowship (Award #1414655) to S.S.G.; and a MacArthur Fellowship, McKnight Scholar Award, Searle Scholar Award, Rita Allen Foundation Fellowship, and NIH New Innovator Award (1DP2DC014596-01) to E.A.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Spencer S. Gang, Email: sgang@ucla.edu.
Elissa A. Hallem, Email: ehallem@microbio.ucla.edu.
References
- 1.Jasmer DP, Goverse A, Smant G. Parasitic nematode interactions with mammals and plants. Annu Rev Phytopathol. 2003;41:245–270. doi: 10.1146/annurev.phyto.41.052102.104023. [DOI] [PubMed] [Google Scholar]
- 2.Hominick WM. Biogeography. In: Gaugler R, editor. Entomopathogenic Nematology. CABI Publishing; New York: 2002. pp. 115–143. [Google Scholar]
- 3.Nguyen KB, Hunt DJ, Mracek Z. Steinernematidae: species descriptions. In: Nguyen KB, Hunt DJ, editors. Entomopathogenic Nematodes: Systematics, Phylogeny, and Bacterial Symbionts, Nematology Monographs and Perspectives. Brill; Leiden: 2007. pp. 121–609. [Google Scholar]
- 4.Ali JG, Alborn HT, Stelinski LL. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol. 2010;36:361–368. doi: 10.1007/s10886-010-9773-7. [DOI] [PubMed] [Google Scholar]
- 5.Dowds BCA, Peters A. Virulence mechanisms. In: Gaugler R, editor. Entomopathogenic Nematology. CAB International; New York: 2002. pp. 79–98. [Google Scholar]
- 6.Lewis EE. Behavioral Ecology. In: Gauger R, editor. Entomopathogenic Nematology. CAB International; New York: 2002. pp. 205–223. [Google Scholar]
- 7.Bal HK, Grewal PS. Lateral dispersal and foraging behavior of entomopathogenic nematodes in the absence and presence of mobile and non-mobile hosts. PLoS ONE. 2015;10(6):e0129887. doi: 10.1371/journal.pone.0129887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MJ, Ehlers RU, Glazer I. Entomopathogenic nematode foraging strategies -- is Steinernema carpocapsae really an ambush forager? Nematol. 2012;14:389–394. [Google Scholar]
- 9.Santhi VS, Salame L, Nakache Y, Koltai H, Soroker V, Glazer I. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus) Biol Control. 2015;83:75–81. [Google Scholar]
- 10.Hallem EA, Dillman AR, Hong AV, Zhang Y, Yano JM, DeMarco SF, Sternberg PW. A sensory code for host seeking in parasitic nematodes. Curr Biol. 2011;21(5):377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillman AR, Guillermin ML, Lee JH, Kim B, Sternberg PW, Hallem EA. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc Natl Acad Sci USA. 2012;109(35):E2324–2333. doi: 10.1073/pnas.1211436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaugler R, Campbell JF, Gupta P. Characterization and basis of enhanced host-finding in a genetically improved strain of Steinernema carpocapsae. J Invert Pathol. 1991;57:234–241. [Google Scholar]
- 13.Robinson AF. Optimal release rates for attracting Meloidogyne incognita, Rotylenchulus reniformis, and other nematodes to carbon dioxide in sand. J Nematol. 1995;27:42–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Koppenhofer AM, Fuzy EM. Attraction of four entomopathogenic nematodes to four white grub species. J Invert Path. 2008;99:227–234. doi: 10.1016/j.jip.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.O’Halloran DM, Burnell AM. An investigation of chemotaxis in the insect parasitic nematode Heterorhabditis bacteriophora. Parasitol. 2003;127(Pt 4):375–385. doi: 10.1017/s0031182003003688. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JF, Kaya HK. Influence of insect-associated cues on the jumping behavior of entomopathogenic nematodes (Steinernema spp.) Behavior. 2000;137:591–609. [Google Scholar]
- 17.Schmidt J, All JN. Attraction of Neoaplectana carpocapsae (Nematoda: Steinernematidae) to common excretory products of insects. Environ Entomol. 1979;8(1):55–61. [Google Scholar]
- 18.Turlings TC, Hiltpold I, Rasmann S. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil. 2012;358:51–60. [Google Scholar]
- 19.Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434(7034):732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 20.Byers JA, Poinar JO. Location of insect hosts by the nematode, Neoaplectana carpocapsae, in response to temperature. Behavior. 1982;79:1–10. [Google Scholar]
- 21.Pye AE, Burman M. Neoaplectana carpocapsae: nematode accumulations on chemical and bacterial gradients. Exp Parasitol. 1981;51:13–20. doi: 10.1016/0014-4894(81)90037-0. [DOI] [PubMed] [Google Scholar]
- 22.Ilan T, Kim-Shapiro DB, Bock CH, Shapiro-Ilan DI. Magnetic and electric fields induce directional responses in Steinernema carpocapsae. Int J Parasitol. 2013;43(10):781–784. doi: 10.1016/j.ijpara.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro-Ilan DI, Campbell JF, Lewis EE, Elkon JM, Kim-Shapiro DB. Directional movement of steinernematid nematodes in response to electrical current. J Invertebr Pathol. 2009;100(2):134–137. doi: 10.1016/j.jip.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro-Ilan DI, Lewis EE, Campbell JF, Kim-Shapiro DB. Directional movement of entomopathogenic nematodes in response to electrical field: effects of species, magnitude of voltage, and infective juvenile age. J Invert Path. 2012;109:34–40. doi: 10.1016/j.jip.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Torr P, Heritage S, Wilson MJ. Vibrations as a novel signal for host location by parasitic nematodes. Int J Parasitol. 2004;34(9):997–999. doi: 10.1016/j.ijpara.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Castelletto ML, Gang SS, Okubo RP, Tselikova AA, Nolan TJ, Platzer EG, Lok JB, Hallem EA. Diverse host-seeking behaviors of skin-penetrating nematodes. PLoS Pathog. 2014;10(8):e1004305. doi: 10.1371/journal.ppat.1004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basanez MG. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6(4):e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viney ME, Lok JB. The biology of Strongyloides spp. WormBook. 2015 doi: 10.1895/wormbook.1.141.2. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 29.Periago MV, Bethony JM. Hookworm virulence factors: making the most of the host. Microbes Infect. 2012;14(15):1451–1464. doi: 10.1016/j.micinf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Haas W, Haberl B, Idris SI, Kersten S. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95(1):25–29. doi: 10.1007/s00436-004-1256-8. [DOI] [PubMed] [Google Scholar]
- 31.Dold C, Holland CV. Ascaris and ascariasis. Microbes Infect. 2011;13(7):632–637. doi: 10.1016/j.micinf.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor LJ, Walkden-Brown SW, Kahn LP. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet Parasitol. 2006;142(1–2):1–15. doi: 10.1016/j.vetpar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Laing R, Kikuchi T, Martinelli A, Tsai IJ, Beech RN, Redman E, Holroyd N, Bartley DJ, Beasley H, Britton C, Curran D, Devaney E, Gilabert A, Hunt M, Jackson F, Johnston SL, Kryukov I, Li K, Morrison AA, Reid AJ, Sargison N, Saunders GI, Wasmuth JD, Wolstenholme A, Berriman M, Gilleard JS, Cotton JA. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013;14(8):R88. doi: 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granzer M, Hass W. Host-finding and host recognition of infective Ancylostoma caninum larvae. Int J Parasitol. 1991;21:429–440. doi: 10.1016/0020-7519(91)90100-l. [DOI] [PubMed] [Google Scholar]
- 35.Haas W, Haberl B, Idris SI, Kallert D, Kersten S, Stiegeler P. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol Res. 2005;95(1):30–39. doi: 10.1007/s00436-004-1257-7. [DOI] [PubMed] [Google Scholar]
- 36.Sciacca J, Forbes WM, Ashton FT, Lombardini E, Gamble HR, Schad GA. Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol Int. 2002;51(1):53–62. doi: 10.1016/s1383-5769(01)00105-2. [DOI] [PubMed] [Google Scholar]
- 37.Pleil JD, Lindstrom AB. Measurement of volatile organic compounds in exhaled breath as collected in evacuated electropolished canisters. J Chromatogr B Biomed Appl. 1995;665(2):271–279. doi: 10.1016/0378-4347(94)00545-g. [DOI] [PubMed] [Google Scholar]
- 38.Alkalay I, Suetsugu S, Constantine H, Stein M. Carbon dioxide elimination across human skin. Am J Physiol. 1971;220(5):1434–1436. doi: 10.1152/ajplegacy.1971.220.5.1434. [DOI] [PubMed] [Google Scholar]
- 39.Rees G. Observations on the vertical migrations of the third-stage larva of Haemonchus contortus (Rud.) on experimental plots of Lolium perenne S24, in relation to meteorological and micrometeorological factors. Parasitol. 1950;40(1–2):127–143. doi: 10.1017/s0031182000017959. [DOI] [PubMed] [Google Scholar]
- 40.Callinan AP, Westcott JM. Vertical distribution of trichostrongylid larvae on herbage and in soil. Int J Parasitol. 1986;16(3):241–244. doi: 10.1016/0020-7519(86)90050-0. [DOI] [PubMed] [Google Scholar]
- 41.Koga M, Nuamtanong S, Dekumyoy P, Yoonuan T, Maipanich W, Rojekittikhun W, Waikagul J. Host-finding behavior of Strongyloides stercoralis infective larvae to sodium cation, human serum, and sweat. Southeast Asian J Trop Med Public Health. 2005;36:93–98. [PubMed] [Google Scholar]
- 42.Safer D, Brenes M, Dunipace S, Schad G. Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc Natl Acad Sci USA. 2007;104(5):1627–1630. doi: 10.1073/pnas.0610193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koga M, Tada I. Strongyloides ratti: chemotactic responses of third-stage larvae to selected serum proteins and albumins. J Helminthol. 2000;74(3):247–252. [PubMed] [Google Scholar]
- 44.Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120(3):189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Tobata-Kudo H, Higo H, Koga M, Tada I. Chemokinetic behavior of the infective third-stage larvae of Strongyloides ratti on a sodium chloride gradient. Parasitol Int. 2000;49(3):183–188. doi: 10.1016/s1383-5769(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 46.Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, Preti G. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159(4):780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cork A, Park KC. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10(3):269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 48.Chaisson KE, Hallem EA. Chemosensory behaviors of parasites. Trends Parasitol. 2012;28(10):427–436. doi: 10.1016/j.pt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu YT, Smallegange RC, van Loon JJ, Takken W. Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol. 2011;25(3):247–255. doi: 10.1111/j.1365-2915.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 50.McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, Ignell R, Vosshall LB. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515(7526):222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Funes H, Zerba E, Gonzalez-Audino P. Effect of release rate and enantiomeric composition on response to pheromones of Megaplatypus mutatus (Chapuis) in poplar plantations of Argentina and Italy. Bull Entomol Res. 2013;103(5):564–569. doi: 10.1017/S0007485313000175. [DOI] [PubMed] [Google Scholar]
- 52.Siljander E, Gries R, Khaskin G, Gries G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. J Chem Ecol. 2008;34(6):708–718. doi: 10.1007/s10886-008-9446-y. [DOI] [PubMed] [Google Scholar]
- 53.Bhopale VM, Kupprion EK, Ashton FT, Boston R, Schad GA. Ancylostoma caninum: the finger cell neurons mediate thermotactic behavior by infective larvae of the dog hookworm. Exp Parasitol. 2001;97(2):70–76. doi: 10.1006/expr.2000.4575. [DOI] [PubMed] [Google Scholar]
- 54.Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int J Parasitol. 2000;30(10):1115–1121. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Zhu X, Boston R, Ashton FT, Gamble HR, Schad GA. Thermotaxis and thermosensory neurons in infective larvae of Haemonchus contortus, a passively ingested nematode parasite. J Comp Neurol. 2000;424(1):58–73. doi: 10.1002/1096-9861(20000814)424:1<58::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.Rasmann S, Ali JG, Helder J, van der Putten WH. Ecology and evolution of soil nematode chemotaxis. J Chem Ecol. 2012;38(6):615–628. doi: 10.1007/s10886-012-0118-6. [DOI] [PubMed] [Google Scholar]
- 57.Farnier K, Bengtsson M, Becher PG, Witzell J, Witzgall P, Manduric S. Novel bioassay demonstrates attraction fo the white potato cyst nematode Globodera pallida (Stone) to non-volatile and volatile host plant cues. J Chem Ecol. 2012;38:795–801. doi: 10.1007/s10886-012-0105-y. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Bruening G, Williamson VM. Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. J Chem Ecol. 2009;35(10):1242–1251. doi: 10.1007/s10886-009-9703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fudali SL, Wang C, Williamson VM. Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol Plant Microbe Interact. 2013;26(1):75–86. doi: 10.1094/MPMI-05-12-0107-R. [DOI] [PubMed] [Google Scholar]
- 60.Pline M, Dusenbery DB. Responses of plant-parasitic nematode Meloidogyne incognita to carbon dioxide determined by video camera computer tracking. J Chem Ecol. 1987;13:873–888. doi: 10.1007/BF01020167. [DOI] [PubMed] [Google Scholar]
- 61.Ali JG, Alborn HT, Stelinski LL. Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol. 2011;99:26–35. [Google Scholar]
- 62.Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol. 1999;84(3–4):297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 63.Ashton FT, Schad GA. Amphids in Strongyloides stercoralis and other parasitic nematodes. Parasitol Today. 1996;12:187–194. doi: 10.1016/0169-4758(96)10012-0. [DOI] [PubMed] [Google Scholar]
- 64.Crook M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int J Parasitol. 2014;44:1–8. doi: 10.1016/j.ijpara.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frezal L, Felix MA. C. elegans outside the Petri dish. Elife. 2015;4:e05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee H, Choi MK, Lee D, Kim HS, Hwang H, Kim H, Park S, Paik YK, Lee J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci. 2012;15(1):107–112. doi: 10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- 67.Hart AC, Chao MY. From odors to behaviors in Caenorhabditis elegans. In: Menini A, editor. The Neurobiology of Olfaction. CRC Press; Boca Raton, FL: 2010. [PubMed] [Google Scholar]
- 68.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 69.Ludewig AH, Schroeder FC. Ascaroside signaling in C. elegans. WormBook. 2013 doi: 10.1895/wormbook.1.155.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 70.Carrillo MA, Hallem EA. Gas sensing in nematodes. Mol Neurobiol. 2015;51:919–931. doi: 10.1007/s12035-014-8748-z. [DOI] [PubMed] [Google Scholar]
- 71.Goodman MB, Klein M, Lasse S, Luo L, Mori I, Samuel A, Sengupta P, Wang D. Thermotaxis navigation behavior. WormBook. 2014 doi: 10.1895/wormbook.1.168.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 72.Braendle C. Pheromones: evolving language of chemical communication in nematodes. Curr Biol. 2012;22(9):R294–296. doi: 10.1016/j.cub.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 73.Ortiz CO, Faumont S, Takayama J, Ahmed HK, Goldsmith AD, Pocock R, McCormick KE, Kunimoto H, Iino Y, Lockery S, Hobert O. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19(12):996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, Pocock R, Ringstad N. A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS ONE. 2012;7(3):e34014. doi: 10.1371/journal.pone.0034014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Ratsch G, Miller DM, Horvitz HR, Sternberg PW, Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108(1):254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith ES, Martinez-Velazquez L, Ringstad N. A chemoreceptor that detects molecular carbon dioxide. J Biol Chem. 2013;288(52):37071–37081. doi: 10.1074/jbc.M113.517367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fenk LA, de Bono M. Environmental CO2 inhibits Caenorhabditis elegans egg-laying by modulating olfactory neurons and evokes widespread changes in neural activity. Proc Natl Acad Sci USA. 2015;112(27):E3525–3534. doi: 10.1073/pnas.1423808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, de Bono M. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69(6):1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430(6997):317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 80.Persson A, Gross E, Laurent P, Busch KE, Bretes H, de Bono M. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458(7241):1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- 81.McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, Kruglyak L, Bargmann CI. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61(5):692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aoki I, Mori I. Molecular biology of thermosensory transduction in C. elegans. Curr Opin Neurobiol. 2015;34:117–124. doi: 10.1016/j.conb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder NE, Androwski RJ, Rashid A, Lee H, Lee J, Barr MM. Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr Biol. 2013;23(16):1527–1535. doi: 10.1016/j.cub.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105(23):8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carrillo MA, Guillermin ML, Rengarajan S, Okubo R, Hallem EA. O2-sensing neurons control CO2 response in C. elegans. J Neurosci. 2013;33:9675–9683. doi: 10.1523/JNEUROSCI.4541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J Comp Neurol. 1995;357(2):281–295. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- 87.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans Royal Soc London B. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 88.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 2011;7(2):e1001066. doi: 10.1371/journal.pcbi.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong RL, Sommer RJ. Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr Biol. 2006;16(23):2359–2365. doi: 10.1016/j.cub.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 90.Hong RL, Witte H, Sommer RJ. Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL-4. Proc Natl Acad Sci USA. 2008;105(22):7779–7784. doi: 10.1073/pnas.0708406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dillman AR, Mortazavi A, Sternberg PW. Incorporating genomics into the toolkit of nematology. J Nematol. 2012;44:191–205. [PMC free article] [PubMed] [Google Scholar]
- 92.Lok J. piggyBac: a vehicle for integrative DNA transformation of parasitic nematodes. Mob Genet Elements. 2013;3(2):e24417. doi: 10.4161/mge.24417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witte H, Moreno E, CR, Kim J, Kim JS, Streit A, Sommer RJ. Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Dev Genes Evol. 2015;225:55–62. doi: 10.1007/s00427-014-0486-8. [DOI] [PubMed] [Google Scholar]
- 94.Lo TW, Pickle CS, Lin S, Ralston EJ, Gurling M, Schartner CM, Bian Q, Doudna JA, Meyer BJ. Precise and heritable genome editing in evolutionarily diverse nematodes using TALENs and CRISPR/Cas9 to engineer insertions and deletions. Genetics. 2013;195:331–348. doi: 10.1534/genetics.113.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods. 2013;10(8):741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dillman AR, Sternberg PW. Entomopathogenic nematodes. Curr Biol. 2012;22(11):R430–431. doi: 10.1016/j.cub.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilleard JS. Haemonchus contortus as a paradigm and model to study anthelmintic drug resistance. Parasitol. 2013;140:1506–1522. doi: 10.1017/S0031182013001145. [DOI] [PubMed] [Google Scholar]
- 98.Lok JB. Strongyloides stercoralis: a model for translational research on parasitic nematode biology. WormBook. 2007 doi: 10.1895/wormbook.1.134.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 99.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351(8):799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]