Abstract
It is well known that tobacco consumption is a leading cause of preventable deaths worldwide and has been linked to major diseases ranging from cancer to chronic obstructive pulmonary disease, atherosclerosis, stroke and a host of neurological/neurodegenerative disorders. In the past decade a number of alternative vaping products have hit the market, rapidly gaining consumers especially among the younger population. Electronic nicotine delivery systems or e-cigarettes have become the sought-after product due to the belief that they are much safer than traditional cigarettes. However, inadequate research and lack of regulatory guidelines for both the manufacturing process and the content of the vaping solution of the e-cigarette has become a major concern. Highly debated and unresolved questions such as whether e-cigarettes may help smokers quit and whether e-cigarettes will promote the use of nicotine among non-smokers add to the confusion of the safety of e-cigarettes. In this review article, we summarize the current understanding (and lack thereof) of the potential health impacts of e-cigarettes. We will also highlight the most recent studies (in vivo/in vitro) which seem to conflict with the broad safety claims put forward by the manufacturers. Finally, we provide potential solutions to overcome the research gap of the short and long-term health impact of e-cigarettes.
Keywords: Electronic cigarettes, e-liquids, Inflammation, Brain, Toxicology, Research, Alternatives, teen smoking, teen vaping
1. Introduction
Electronic cigarettes (e-cigarettes), also known as electronic nicotine delivery systems (ENDS), are battery powered devices designed to vaporize (by heat) a solution of nicotine (e-liquid) and other additives (including propylene glycol, vegetable glycerin and ad hoc flavoring agents) into an aerosol which is then inhaled by the user (called vaping). These products are often made to look like cigarettes, cigars, pens or pipes in order to closely resemble traditional tobacco-based combustion products (TC) (Naik and Cucullo, 2015). Hon Lik, a Chinese pharmacist, invented the modern version of the e-cigarette in 2003, which was later patented internationally in 2007 (Electronic Atomization Cigarette: US 20070267031 A1) and was subsequently introduced into the global market including the U.S. (Rahman et al., 2014). The market size of this non-regulated product is booming because of the increase in popularity among young adults of the millennial generation (Grana et al., 2014). The accelerated adoption of e-cigarettes is supported by the common belief that they are indeed a safer alternative to traditional tobacco-based products {2016 14 /id} (Goniewicz et al., 2014b) (Regan et al., 2013) or that they can be used to facilitate smoking cessation despite some opposing evidence (Kalkhoran and Glantz, 2016). A recent study on e-cigarette awareness and harm perception has shown that ≈ 95% of interviewed subjects (US adult population) believe e-cigarettes to be; 1) cleaner and healthier than conventional products, 2) cheaper (93%), 3) can be used to circumvent smoke-free policies (76 – 88%) and 4) are trendy (≈73%) (Pearson et al., 2012).
A recent report (Zhu et al., 2014) states that an astonishing 7,764 unique flavors were available online in January 2014, with 242 new flavors being added per month, and sales occurring under 466 brands. A number of e-cigarette manufacturers minimized the health risk concerns on their products stating that the ingredients, including the flavoring agents, are not dangerous since they are all ‘food grade’ and ‘generally recognized as safe’ (GRAS). However, GRAS designation by the Flavor Extracts Manufacturers Association (FEMA) refers to use of these substances up to specific concentrations for specific purposes in specific foods and does not pertain to the use of the same compounds for inhalation. In some instance e-liquids contain very high levels of flavoring agents, possibly exceeding concentrations sufficient to elicit irritant effects and inflammation in the respiratory and cardiovascular systems. This concern was recently brought up by a study from Farsalinos et al. {Farsalinos, 2015 15 /id}in which the authors tested 159 sweet e-cigarette flavors including chocolate, toffee, and caramel. The results clearly showed that ≈ 74% of the samples contained diacetyl and/or acetyl propionyl which has been associated with bronchiolitis obliterans. Furthermore, certain flavored e-liquids demonstrated nicotine-independent in vitro cytotoxicity to various cells (Farsalinos et al., 2013a; Tierney et al., 2016). In addition to propylene glycol, glycerin and other flavoring agents, e-liquids contain a number of aldehydes (formaldehyde, benzaldehyde, acrolein, etc.) that form during the heating process. Small amounts of heavy metals and at least 20 known carcinogens and teratogenic agents have also been identified in the e-liquid as well as the vapors (Bahl et al., 2012; Grana et al., 2014; McAuley et al., 2012). Toxic aerosols released by e-cigarettes contain ultra-fine particles that can, in conjunction with air pollution, contribute to pulmonary and systemic inflammatory processes while decreasing macrophage and neutrophil antimicrobial activities (Hwang et al., 2016).Despite the proof that e-cigarettes are not as safe as popular belief, the long term health effects of e-cigarette vaping has only been marginally addressed (Orellana-Barrios et al., 2015). Hence, many unanswered questions remain about the overall toxicological effects, safety, efficacy of harm reduction, as well as the overall health impact of e-cigarettes.
2. The Device: Basic Design & Operation
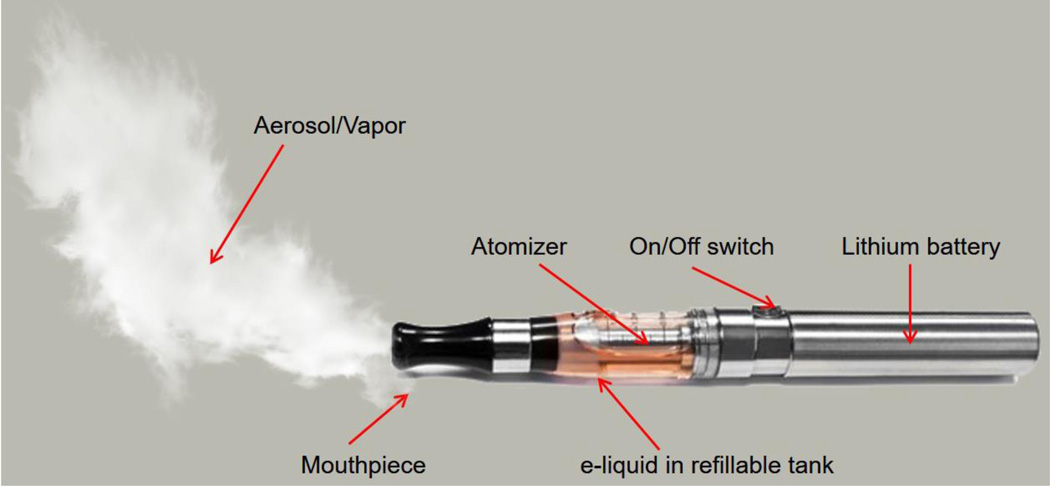
A variety of e-cigarette models have emerged over the last decade. Although the complexity in design and features has evolved, the key principle is the same. An e-cigarette has three basic parts: a rechargeable lithium battery, an atomizer and a reservoir (collectively known as a cartomizer; see Fig. 1) containing the e-liquid.. Activation of the heating coil within the atomizer generates the nicotine aerosol (or mist)..
Figure 1. e-Cigarette schematic illustration.
This device is primarily a battery operated product designed to turn nicotine and other chemicals (including solvents and flavoring agents) into a vapor; an atomizer heats the e-liquid producing the vapor inhaled by the user. A rechargeable battery (w/wo LED indicator) of different sizes and output powers to the atomizer. Note that the battery output and the resistance of the heating coils in the atomizer determine the vaping capacity of the e-cigarette.
Automatic models feature an airflow sensor which activates the heating coil when a user starts inhaling. Most models however, require the user to manually trigger the atomizer. By pressing and holding on an on/off switch a signal is sent to the battery to supply current to a heat coil which raises the temperature of the atomizer (up to 500 °F) vaporizing the e-liquid drawn from the cartridge/reservoir. This aerosol is then puffed by the users through a mouthpiece connected to the cartridge/ reservoir. E-cigarettes feature rechargeable batteriesavailable in different shapes and output power levels (expressed in milli-ampere-hours - mAH).. Many e-cigarettes are equipped with a flame-looking light-emitting diode (LED) at the end of the battery to replicate the appearance of a regular burning cigarette (Orellana-Barrios et al., 2015; Rahman et al., 2014).
A user’s vaping pattern and taste varies widely. Some consumers prefer to vape mild to moderate amounts of aerosol while others love to experience a smoke-like sensation or “hit” in their throat by generating huge amount of vapors. To meet the consumer taste, e-cigarette manufacturers have developed customizable products which enable the user to adjust the atomizer’s resistance (ohm, Ω) and voltage (V) through replaceable parts and adjustable dials. These parameters work following the basic principle of Ohms law. Replaceable coils can vary in size to alter the resistance of the atomizer (1.2 – 1.8 ohms), inversely affecting the flow of the current and heat generated by the coil. This ultimately allows for the production of a denser vapor with intense taste and enormous feeling of throat “hit”. Both variable voltage and variable wattage type manual devices are available in the marketplace. Variable voltage (usually 3.0–5.0 volts) type devices allow the user to adjust the amount of current flow and hence the amount of aerosol depends on the resistance of the atomizer. Later models termed “temperature controlling vaping” enable the user to program a fixed temperature into the microchip. Like a thermostat, the current is maintained through digital adjustment to the voltage. These devices ensure fixed current output regardless of the resistance of the atomizer.
3. Chemical Profile of e-Liquid and Nicotine Intake
E-cigarettes are either disposable (both the battery and the cartridge are to be discarded once the e-liquid has been consumed) or intended for multiple uses in which case, the reservoir (tank style) needs to be refilled with e-liquid or the prefilled cartridge needs to be replaced. E-liquid is the solution or “smoke juice” containing nicotine in an edible solvent approved by the FDA (usually propylene glycol or a mixture of propylene glycol and glycerin). Nicotine-free e-cigarettes are also available in the market. E-cigarette manufacturers usually categorize the e-liquid strength as zero (0 mg/ml), low (6, 12 mg/ml), medium (18 mg/ml) and high (24 mg/ml) based on the nicotine concentration. E-cigarette retail shops can purchase concentrated nicotine solutions (100 mg/ml) and dilute them according to the customer demand. E-liquids exceeding 24 mg/ml of nicotine (up to 36mg/ml) are also available in the market (Bhatnagar et al., 2014). Wide ranges of flavoring agents can be added to the nicotine solution including chocolate, caramel, mint, menthol, coffee, cherry, apple and many more.
Although the popularity of e-cigarettes is rapidly increasing, a comprehensive chemical characterization of the e-liquids (including sources and manufacturing processes, chemical composition of the solvents and flavoring agents, and the nicotine content of the aerosol) are seldom disclosed by the manufacturers. Chemical analyses of the e-liquids and popular e-cigarettes brands revealed high variability in the nicotine content (between refills) and nicotine delivery efficiency (between nominally identical e-cigarette models). Significant inconsistencies between the nominal (labeled) and the actual nicotine content of the e-liquids have been reported by a host of investigators (Etter et al., 2013; Famele et al., 2015; Goniewicz et al., 2015; Goniewicz et al., 2014a; Hahn et al., 2014; Lisko et al., 2015) These also include the presence of nicotine in “nicotine-free e-liquids” as well as variations (up to 12%) in nicotine concentration (Goniewicz et al., 2014a) and pH (Lisko et al., 2015) within the same batch of e-liquids. In addition to nicotine and flavoring agents the presence of other toxic compounds (especially carcinogens) have been recently reported. These include a cohort of toxic carbonyl compounds (acrolein, formaldehyde and acetaldehyde etc.), heavy metals (such as mercury -Hg, Cadmium - Cd, lead – Pb), volatile organic compounds (benzene, toluene), and nitrosamines (such as nitrosonornicotine, 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone – NNK; see Table-1). Since many e-cigarette manufacturers use tobacco-derived nicotine, toxic chemicals are likely to be found in the vape as well (although to levels several hundred folds lower than those observed in TCs). A very recent but highly controversial report suggests that the reaction between the e-liquid vehicle (propylene glycol and glycerol) and formaldehyde (a known degradation byproduct of propylene glycol generated by heat) during vaping e-cigarette at high voltage (5.0 V) can lead to the formation of a highly carcinogenic hemiacetal in significant concentrations (Jensen et al., 2015). Hemiacetals release formaldehyde and are commonly used as biocides. The presence of diacetyl, 2,3-pentanedione, and acetoin have also been reported in flavoring agents (Allen et al., 2015). Chronic inhalation of these chemicals may lead to respiratory complications including popcorn lung.
Table-1.
Toxic components present in e-Cigarette aerosol and e-liquid
| Components | Concentration | e-liquid/Aerosol | References |
|---|---|---|---|
| Carbonyl compounds | |||
| Formaldehyde | 2.0–6.3 µg | 150 puff | (Goniewicz et al., 2014) |
| 1.9–120 µg | 10 puff | (Uchiyama et al., 2016) | |
| 0.05–51 µg | 1 puff | (Gillman et al., 2016) | |
| 0.1– 9.0 ug | 1 g of e-liq | (Varlet et al., 2015) | |
| Acetaldehyde | 1.1–13.6 µg | 150 puff | (Goniewicz et al., 2014) |
| 1.4 −73 µg | 10 puff | (Uchiyama et al., 2016) | |
| 0.03–40.07 µg | 1 puff | (Gillman et al., 2016) | |
| 0.05–10..2 µg | 1 g of e-liq | (Varlet et al., 2015) | |
| Acrolein | 0.7– 41.9 µg | 150 puff | (Goniewicz et al., 2014) |
| 2.1–16 µg | 10 puff | (Uchiyama et al., 2016) | |
| <0.02–5.5 µg | 1 puff | (Gillman et al., 2016) | |
| Propionaldehyde | 110 ng | 1 puff | (Tayyarah and Long, 2014) |
| 40–1500 ng | 1 puff | (Bekki et al., 2014) | |
| Volatile Organic Compounds | |||
|
Toluene p,m-xylene |
0.2–6.3 µg 0.1–0.2 µg |
150 puff 150 puff |
(Goniewicz et al., 2014) |
| Tobacco-Specific Nitrosamines | |||
| N-nitrosonornicotine (NNN) | 0.8–4.3 ng | 150 puff | |
| 0.5–16.7 ng | 1 ml of e-liq | (Goniewicz et al., 2014) | |
|
4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone (NNK) |
1.1– 28.3 ng | 150 puff | (Farsalinos et al., 2015) |
| 3.2 −10.8 ng | 1 ml of e-liq | ||
| Metals | |||
| Ni | 0.11–0.29 µg | 150 puff | |
| Cd | 0.01–0.22 µg | 150 puff | (Goniewicz et al., 2014) |
| Pb | 0.03–0.57 µg | 150 puff | |
Reference List
Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N (Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11:11192–11200.2014).
Farsalinos KE, Gillman IG, Melvin MS, Paolantonio AR, Gardow WJ, Humphries KE, Brown SE, Poulas K, Voudris V (Nicotine levels and presence of selected tobacco-derived toxins in tobacco flavoured electronic cigarette refill liquids. Int J Environ Res Public Health 12:3439–3452.2015).
Gillman IG, Kistler KA, Stewart EW, Paolantonio AR (Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 75:58–65.2016).
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, III, Benowitz N (Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23:133–139.2014).
Tayyarah R, Long GA (Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 70:704–710.2014).
Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N (Determination of Chemical Compounds Generated from Second-generation E-cigarettes Using a Sorbent Cartridge Followed by a Two-step Elution Method. Anal Sci 32:549–555.2016).
Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF (Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health 12:4796–4815.2015).
A drawback of these studies however, is that the quantitative and qualitative analytical methods were (in most cases) not properly validated. The use of dissimilar experimental conditions and tools with diverse detection limits, resolution or linearity ranges impacts data reproducibility and comparison, thus impairing their translational relevance (Goniewicz et al., 2014b). One such challenge is the pattern and strength of vape inhalation (vaping proficiency) and the vacuum needed to produce nicotine. Unfortunately, (for research purposes) the vaping proficiency of the users and the e-cigarette response to the user vaping (vacuum needed to activate the atomizer) vary widely. Usually smoke density remains unaltered throughout the entire burning of a conventional cigarette, while for e-cigarette aerosol density decreases with continued use (Norton et al., 2014; Trtchounian et al., 2010). Addressing this variance is of crucial importance for the preparation of experimental samples to be used for toxicological testing (Behar et al., 2015; Farsalinos et al., 2013b). Researchers have recently attempted to measure the amount and size of the particulate matter contained in the e-cigarette vapes vs TCs. Ruprecht et al. found lower levels of particulate matter in e-cigarettes (irrespective of the nicotine content) compared to TCs but these levels still exceed WHO guidelines. In other studies, the size of the particulate matter found in the vapor released by the e-cigarette was comparable to that of TCs’ sidestream smoke until the aerosol reached the saturation point (first few puffs) but increased once the saturation point was crossed (Hua et al., 2013; Pellegrino et al., 2012; Ruprecht et al., 2014). The diameters of the e-cigarette aerosol particles were comparable to that found in sidestream smoke from conventional cigarettes with an average size of 250–450 nm (Ingebrethsen et al., 2012). In respect to nicotine delivery, a conventional cigarette releases approximately 1 mg of nicotine into the blood (Bhatnagar et al., 2014). When compared to that of e-cigarettes one-fourth to one-third of the nicotine content of the e-liquid will actually reach the blood stream (Farsalinos et al., 2014). Thus the total amount of nicotine effectively delivered into the blood stream depends on the effective strength of the e-liquid and the amount of e-liquid vaped. Considering a nominal nicotine concentration of 24 mg/mL then the total amount of nicotine potentially delivered into the blood stream of the vaper could be in the range of 6 to 8 mg. Nicotine delivery efficiency of the newest generation of e-cigarettes is even higher.
4. Toxicological Studies and Public Health Concern
The most common solvents used in the preparation of e-liquids are either propylene glycol and/or vegetable glycerin. Glycol mist (the same additive used in e-cigarettes) is also used in the show business and aviation industries to create “fog machine smoke”. Varughese et al. studied the lung function of 101 employees at 19 theatres exposed to glycol fog during their work. The study revealed impaired lung function associated with dry throat and cough upon either acute or chronic exposure (Varughese et al., 2005). Wieslander et al. tested acute effects of glycol aerosol on personnel involved in aviation emergency training and demonstrated ocular and upper respiratory tract irritation in non-asthmatic people. In a few cases airway obstruction was also observed (Wieslander et al., 2001). Although a direct parallelism with e-cigarette exposure cannot be established, concerns remain regarding the use of these solvents in the preparation of e-liquids. Also troublesome is the fact that although glycerin is hygroscopic in nature and reduces the consistency of bronchial fluid it is still considered a safe additive (Callahan-Lyon, 2014).
Acute e-cigarette exposure may impair human lung function. As recently reported, e-cigarette exposure decreased the fraction of exhaled nitric oxide thus enhancing the total peripheral resistance (Vardavas et al., 2012). A 3% FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity; the volume of air that can be maximally forcefully exhaled) reduction was also reported in response to e-cigarette exposure (Flouris et al., 2013). In vitro studies revealed direct cytotoxicity of e-liquid on human and mouse stem cells as although no effect was observed on human pulmonary fibroblast (hPF) (Bahl et al., 2012). Lerner et al., further demonstrated that e-cigarette aerosols induce oxidative stress in human lung epithelial cells and trigger an inflammatory response. They also observed similar results pre-clinically when exposing mice to e-cigarette aerosols (Lerner et al., 2015) (see Table-2).
Table-2.
Toxicological studies of e-Cigarette
| Study | Adverse effects | Reference | |
|---|---|---|---|
| 1. | Human embryonic stem cells (hESC), mouse neural stem cells (mNSC), and human pulmonary fibroblasts (hPF) were exposed to e-Cigarette refill liquid. |
Cytotoxicity was observed excluding hPF cells. |
(Bahl et al., 2012) |
| 2. | Human airway epithelial cells (H292) and wild type C57BL/6J mice were exposed to e- Cigarette aerosol. |
Oxidative stress and Inflammatory response. |
(Lerner et al., 2015) |
| 3. | Thirty healthy smoker inhaled e-Cigarette for 5 minutes. |
Fraction of exhaled nitric oxide (FENO) was reduced while total peripheral airway resistance was enhanced. |
(Vardavas et al., 2012) |
| 4. | Fifteen smokers underwent acute e-Cigarette exposure. |
3% reduction in FEV1/FVC | (Flouris et al., 2013) |
| 5. | Long term (6 months) use of e-Cigarette by forty participants. |
Irritation of mouth and throat | (Polosa et al., 2014) |
Reference List
Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P (Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34:529–537.2012).
Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace HA, Tsatsakis AM, Koutedakis Y (Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 25:91–101.2013).
Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I (Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10:e0116732.2015).
Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, Amaradio M, Fisichella A (Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med 9:537–546.2014).
Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK (Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141:1400–1406.2012).
The principal component of the e-liquids delivered by the e-cigarettes is nicotine. Adverse effects associated with nicotine exposure include nausea, vomiting, dizziness and severe topical damage in case of accidental spill of highly concentrated nicotine solutions (Callahan-Lyon, 2014). Risk of accidental poisoning can arise from the use of undiluted nicotine concentrates if mistakenly used as “ready to vape” e-liquids. Few fatal cases from ingestion of e-liquid have been reported {Chen, 2015 16 /id} (2015; Ordonez et al., 2015; Thornton et al., 2014).and although the number of deaths is not highly significant this remain a concern to be addressed (ref-16) The physiological impact of e-cigarette exposure has been investigated mainly on heart, lung and the vasculature suggesting sympathomimetic effects {Nelluri, 2016 17 /id}. Transient irritation of mouth and throat during the initial phase of e-cigarette inhalation has also been reported (Polosa et al., 2014). No change in complete blood count (CBC) has been observed in response to acute e-cigarette exposure (Flouris et al., 2012).
4.1 Availability of e-Cigarette to Vulnerable Population
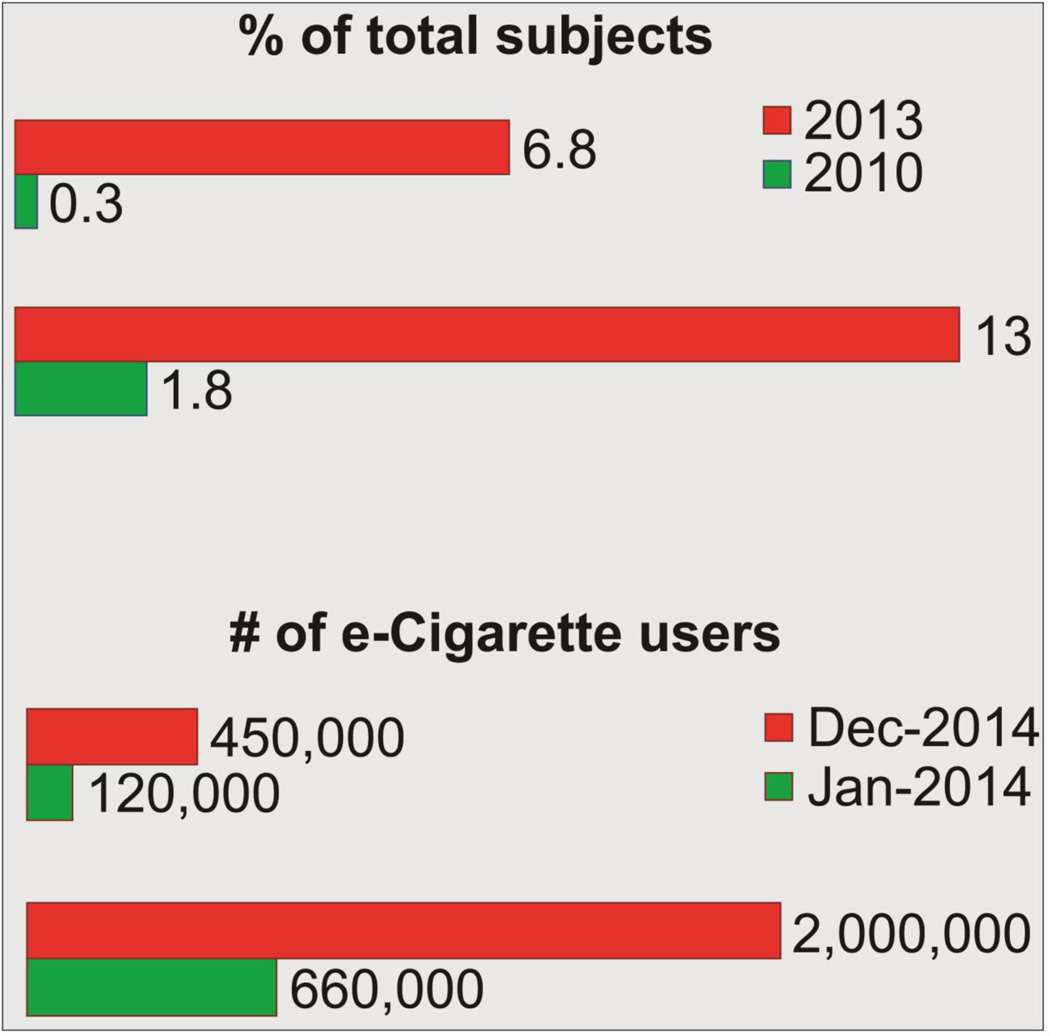
The most recent nationwide survey conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC) estimated 12.6% US adults vaped e-cigarettes at least once and 3.7% are current users (Schoenborn and Gindi, 2015). According to the CDC and the Center for Tobacco Products (CTP) of the Food & Drug Administration (FDA), the National Youth Tobacco Survey (2014) revealed an alarming rise of e-cigarette use among adolescents (see Fig. 2). It has been estimated that e-cigarette consumption has increased from 660,000 to 2 million and 120,000 to 450,000 among high and middle school students respectively (15 A.D.). The report also states that e-cigarette vaping topped all other tobacco products consumed by students in the last three years (2011–2014) and their use has increased by ≈ 800% among high school students (2016b). The number of recurrent users has also increased from 0.3% to 6.8% (McMillen et al., 2014). The report also revealed that the number of young vapers (millennials) currently outpaces that of adults (14.2% vs. 8.6%). A 2014 study showed that over 8% of 8th graders, 16% of 10th graders and approximately 17% of 12th graders in US have vaped an e-cigarette during the one month survey period (Schraufnagel, 2015).
Figure 2.
Rise in e-Cigarette use among U.S. adults (2010–2014); Sample size >3000 each year (15 A.D.; McMillen et al., 2014).
According to a recent public workshop by the FDA (Electronic Cigarettes and the Public Health; March 9–10 2015) (16 A.D.), e-cigarette manufacturers have developed specific marketing strategies (including social networking services) to draw the attention and interest of young consumers. In contrast to TCs, the e-cigarettes market is not (yet) regulated, thus allowing anyone to purchase these products without the proof of age. E-cigarette’s manufacturers have also replaced the term “cigarette” (which has a negative inference among the general population) with “vaping device” or “hookah pen” to distance their products from TCs. In addition to traditional market advertising young consumers are further incentivized to purchase e-cigarettes with coupons and promotional (free) products (Schraufnagel, 2015). A recent study on more than 15000 American adolescents (from 6th through 12th grade) of different races and ethnic groups was conducted demonstrating lesser prevalence of e-cigarette smoking among female and young African-Americans. By contrast Hispanics, Caucasian, Asian, males and (in general) adolescents that already experienced smoking traditional cigarettes and/or have smoker friends are more prone to adopt e-cigarettes. The desire to quit smoking was not the driving force initiating e-cigarette vaping (Lippert, 2014).
5. The Success of e-Cigarette in Smoking Cessation Program
The “Center for Drug Evaluation and Research” of the FDA assesses the potential therapeutic benefit over the risks of smoking cessation products and regulates their production, advertisement and sale. Accordingly, nicotine replacement therapies (NRTs) - e.g. nicotine patch/gum, bupropion SR, and varenicline are approved by the FDA for smoking cessation. This however, is not the case for e-cigarettes (despite the popular belief) since the rather scarce and quite contradictory scientific evidences do not presently justify their use as smoking cessation tools (Orr and Asal, 2014).
Bullen et al. conducted a randomized clinical trial on 657 people to observe the effect of e-cigarettes on reducing cravings and the relapse rate in smokers. Subjects were grouped into three different cohorts including nicotine patches, e-cigarettes and e-cigarette placebos. The study was inconclusive since the low success rate in quitting smoking among the participants did not yield sufficient statistical power to draw any conclusion on the effectiveness of e-cigarettes as smoking cessation tools when compared to nicotine patches. The investigators concluded that more extensive studies were needed (Bullen et al., 2013). Caponnetto et al. carried a recent randomized clinical study on 300 smokers with no desire to quit smoking. The difference in number of cigarettes smoked after vaping e-cigarettes was considered the main parameter to assess smoking cessation. Only 8.7% of smokers completely restrained themselves from smoking after 52 weeks. However, the investigators noticed a marked reduction of withdrawal syndrome and concluded that irrespective of the nicotine content e-cigarette vaping reduced the number of cigarettes smoked (Caponnetto et al., 2013). One of the most recent studies by Brose et al. suggests that the daily vaping of e-cigarettes bolsters the user effort to stop smoking or helps reduc the number of cigarettes smoked. By contrast, occasional vaping did not have any of these aforementioned effects (Brose et al., 2015).
The rate of smoking is considerably higher in people with mental disorders despite being more susceptible to tobacco related damage and injury. Studies aimed at evaluating the use of e-cigarettes as smoking cessation tools in healthy vs. mentally ill patients did not show statistically different results although the relapse rate in the latter category was higher (O'Brien et al., 2015). Further, no statistically significant differences were found when comparing the effectiveness of e-cigarettes vs. other nicotine delivery systems (e.g., nicotine patches, etc.) as a smoking cessation tool although the acceptability and consumer compliance was higher with e-cigarettes. The use of e-cigarettes as a replacement/alternative to TCs in cancer patients was also evaluated but results were not conclusive (Fillon, 2015).
6. E-Cigarette: Regulatory Issues
There is a rising demand by the regulatory authorities to bring the marketing, production and sale of e-cigarettes under legislative control and to limit their accessibility to vulnerable populations. Few countries have already developed regulatory guidelines for e-cigarettes such as Brazil, Uruguay, Singapore, Canada etc. In the US e-cigarettes will be brought under the FDA authority by August 8 2016 although the regulatory guidelines and the approaches to implement them are still a working in process far from being completed due to the scarce scientific data available to the regulators. Further, regulatory issues concerning the premises where e-cigarettes can or cannot be used is beyond the FDA’s mandate; state and local governments have the authority to impose any restriction on e-cigarettes use in public places or any non-smoking zones (Orellana-Barrios et al., 2015).
From a regulatory perspective, if any product contains synthetic compounds and claims any therapeutic effect it should be treated as a drug and must be approved by the appropriate controlling agency. The initial approach of the FDA was to treat e-cigarettes as drug delivery devices. This argument was met with significant resistance by the manufacturers which counter-argued that no therapeutic claims were linked to e-cigarettes. In the US, products containing synthetic nicotine are regulated and need to meet specific standards; if nicotine is obtained from a plant source it is treated as a tobacco product. Since the e-liquids manufacturers seldom disclose the constituents and the nicotine source(s) used in the preparation of their products, these issues remain unresolved (Rahman et al., 2014). Grana et al. in a comprehensive review on e-cigarette use advocated for the following guidelines to be included in the corresponding legislative policy: the use of e-cigarettes needs to be put under levels of restriction similar to conventional cigarettes; these include e-cigarette use in public places (smoke free zone), age requirement to purchase the product, premise for sale and commercialization methods (television, social media, etc.). Further, the use of flavoring agents should also be strongly regulated requiring detailed characterization (toxicological studies) for all the ingredients used in the preparation of e-liquids. Any unproven (deceiving) marketing claim should be forbidden until verified by the scientific community (e.g., e-cigarette use as smoking cessations tools). Finally, any marketing policy promoting the dual use of both e-cigarettes and TCs should be prohibited or at least strongly discouraged by the legislators (Orellana-Barrios et al., 2015).
7. Secondary Exposure and Non-User Risk of e-Cigarette
E-cigarettes have been reported to cause secondary exposure to nicotine but to a much lesser extent compared to TCs. The exhaled e-cigarette aerosol is also free from combustible toxic ingredients present in traditional cigarettes (Czogala et al., 2014). Schober et al. conducted a study on indoor air quality after six vaping sessions of two-hour duration by nine e-cigarette smokers. The results demonstrated an elevated number of suspended particles containing nicotine, glycerin and 1,2-propanediol (Schober et al., 2014). After evaporation of the solvents from the exhaled e-cigarette aerosols the residual nicotine deposited on solid surfaces was shown to react with atmospheric nitrous acid yielding tobacco specific nitrosamines which are carcinogenic in nature (Kuschner et al., 2011; Riker et al., 2012). Flavoring agents used in the preparation of e-liquids could also be appealing to children. The intrinsic risk is that children could be tempted into ingesting the e-liquid which also contains a high concentration of nicotine. The effect could be potentially fatal (Callahan-Lyon, 2014). As for the use of e-cigarettes during pregnancy (also as a “safer” alternative to TCs), it is important to note that there are no data currently available to assess the potential risks to the fetus.
8. Promoting e-Cigarette Research
To establish a rationale and sustainable policy to regulate e-cigarette manufacturing, marketing and usage, the long term biological and physiological impact on these products need to be dissected out and evaluated accordingly. The physical characteristics and mode of operation of e-cigarettes are rapidly evolving and their modulatory effects on aerosol generation (time & force required while inhaling), composition (presence of any unwanted degradation products), physical characteristics (diameter of aerosol particles) and user exposure (total amount and density) need to be investigated.
Traditional cigarettes are usually classified as full flavor, light and ultra-light in order of decreasing nicotine and tobacco residue (TAR) concentrations whereas consumers might not be fully aware of the exact nicotine content truly delivered by e-cigarettes as discussed above. Although nominal nicotine concentrations (0–24+ mg/ml) are clearly labeled on every e-liquid refill or cartridge, the effective nicotine yield delivered in the vapor is highly variable, thus extensive pharmacokinetic studies are needed to assess the bioavailability and relevant physiological parameters (absorption, distribution, metabolism and excretion) of nicotine from various e-liquid refills (nicotine strengths) and e-cigarette designs (Etter et al., 2013). Establishing e-cigarettes as a controlled and dose dependent nicotine delivery system might open a new avenue for the efficient delivery of chemicals other than nicotine. Chemical profiling of both inhaled and exhaled aerosols of e-cigarettes needs to be done with no discrepancy in sample collection and experimental conditions. About half of the chemical profiling studies that have been performed so far are on products sold outside US. Thorough analysis of products available in US market is, as of now, scarce. The discrepancies (Jensen et al., 2015) regarding heat-generated formaldehyde during vaping and the presence of di-acetal in e-liquid vehicles should be further investigated and resolved. Further, stringent regulatory parameters relative to the design, and technical characteristics of e-cigarettes (e.g., max battery output and temperature of the heat coil) should be developed and enforced.
8.1 Health Impact of e-Cigarette: The Little We Know
One of the major obstacles in regulating e-cigarettes is that the possible long term adverse effects are still unknown. Although a few studies are available with mixed results, the data are insufficient to draw any conclusion. The effects of e-cigarettes in altered medical conditions such as asthma, chronic obstructive pulmonary disease (COPD) or even pregnancy have not been investigated. Both COPD and asthma can be triggered/ aggravated by chronic exposure to traditional combustible cigarettes (Moerloose et al., 2006). Once these patients are diagnosed the best possible way to prevent worsening of these conditions is to quit smoking. However, the craving for nicotine and/or the act of smoking itself becomes a strong deterrent to quit and a catalyst for switching to e-cigarettes. According to the American Lung Association, approximately 80% of COPD deaths are caused by smoking (2014) and a most recent CDC survey revealed that about 21% of asthmatic patients in the US currently smoke (2016a). Further, recent in vivo studies have shown that e-cigarette exposure can induce proteostasis/autophagy impairment leading to oxidative stress, apoptosis, and senescence, thus suggesting a potential role in COPD-emphysema pathogenesis (Shivalingappa et al., 2015).
Several preclinical studies have been conducted on the effects of nicotine on the brain and neurological disorders such as stroke. Adverse effects (Bradford et al., 2011) have been reported where nicotine has been administered subcutaneously via osmotic pump or direct injection but did not expose the animals to nicotine aerosols to simulate chronic and frequent use of e-cigarettes. This is a serious concern with respect to adolescents because of the negative impact of nicotine on cognitive function and brain development (Yuan et al., 2015). As smoking has been reported to cause neurobiological dysfunction with altered blood flow and morphology in different parts of the brain (Durazzo et al., 2015; Toda and Toda, 2010) similar effects arising from the chronic use of e-cigarettes are likely. Vaporized e-cigarette liquids have been shown to induce increased DNA strand breaks and cell death independently from their nicotine content (Holliday et al., 2016; Yu et al., 2016). However, nicotine enhances the leakiness of the blood-brain barrier (BBB) by modulating expression and translocation of tight junction protein (Hawkins et al., 2004) that may originate or aggravate neurodegenerative disease conditions.
A recent pilot study has clearly shown that e-cigarette vapors can trigger oxidative stress and inflammation of lung endothelial cells with concurrent loss of lung endothelial barrier functions (Schweitzer et al., 2015). Hence, the lack of studies focusing on the effect of chronic e-cigarette exposure on BBB permeability is quite concerning and should be addressed. Alternatively, nicotine has been shown to have beneficial effects on Parkinson’s (Jurado-Coronel et al., 2016; Li et al., 2015; Liu et al., 2015; Lombardo and Maskos, 2015; Quik et al., 2015) and Alzheimer’s disease (Gao et al., 2014; Kem, 2000; Kihara et al., 1997; Xue et al., 2015). Further, nicotine’s major non-addictive metabolite cotinine has been proven to reduce the burden of post-traumatic stress disorder (PTSD) and schizophrenia (SCHZ) (Barreto et al., 2015; Echeverria et al., 2016; Echeverria and Zeitlin, 2012; Echeverria et al., 2011). Perhaps aside from the recreational purpose, e-cigarettes might be reconfigured as efficient nicotine delivery systems (devoid of all the other chemicals and flavoring agents) to treat these disease conditions but extensive preclinical and clinical studies need to be performed to prove substantial therapeutic efficacy.
9. Conclusion and future directions
The common belief that “e-cigarettes release merely water-based vapors” is incorrect. In addition to nicotine, e-cigarette vapors contain potentially toxic substances which are solvent byproducts (generated by heat) released in the vapor and/or trace constituents of the flavoring additives. Many of the toxic substances present in TC’s sidestream and mainstream smoke are absent or negligible although e-cigarette vapors have been shown to contain traces of heavy metals as well as carcinogenic and teratogenic agents derived from flavoring additives.
The rapid adoption of e-cigarettes among consumers of all ages and especially millennials is a major concern. The primary reasons are; 1) the lack of studies addressing the long term health impact of these products (Cheng, 2014), 2) the lack of standardized manufacturing processes and 3) the lack of regulatory guidelines concerning the use of e-cigarettes. Claims concerning the possible use of e-cigarettes not only as recreational devices but also as smoking cessation tools are unverified and need to be scientifically proven since the scant data available today are rather contradictory or lack sufficient power analysis to be relevant. If these claims are not confirmed, then e-cigarettes should be treated on par with TC products and regulated likewise.
As more studies addressing the potential biological and pathogenic impact associated with e-cigarette vaping becomes available there is the significant possibility that many of the safety claims put forward to the public by the manufacturers will fade. To further these investigations, organ specific long term functional and toxicological studies will be necessary. These should include side by side comparative studies against TC products, thus providing a sound scientific basis to help the FDA regulate the manufacturing processes of e-cigarettes (including functional vaping parameters and technical standards of the hardware components) and the allowable contents (qualitative and quantitative parameters) of the e-liquids (Ribisl et al., 2016).
Acknowledgments
Manuscript preparation was supported in part by NIH/NIDA R01-DA029121-01A1 and Alternative Research Development Foundation grants received by Luca Cucullo.
Abbreviations
- TC
tobacco-based combustion products
- GRAS
generally recognized as safe
- FEMA
Flavor Extracts Manufacturers Association
- FHA
first hand aerosol
- mAH
milli-ampere-hours
- NNK
nitrosonornicotine, 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone
- FEV1/FVC
forced expiratory volume in 1 second/forced vital capacity
- hPF
human pulmonary fibroblast
- CDC
Centers for Disease Control and Prevention
- CTP
Center for Tobacco Products
- NRTs
nicotine replacement therapies
- TAR
tobacco residue
- ADME
absorption, distribution, metabolism and excretion
- COPD
Chronic obstructive pulmonary disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
Author’s contribution
Mohammad A. Kaisar drafted the review; Shikha Prasad and Taylor Liles also contributed to the drafting of the manuscript and proof reading. Luca Cucullo supervised the work and provided guidance during manuscript preparation and revisions. All authors have read and approved the final version of the manuscript.
Reference List
E-cigarette use triples among middle and high school students in just one year. 15 A.D. CDC.
Ref Type: Report
Electronic Cigarettes and the Public Health:
Second Public Workshop. ELECTRONIC CIGARETTES AND THE PUBLIC HEALTH: A PUBLIC WORKSHOP. 16 A.D.
Ref Type: Conference Proceeding
The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. U.S. Department of Health and Human Services.
Ref Type: Report
(Electronic cigarettes: poisoning in children. Prescrire Int 24:21.2015).
Percentage of People with Asthma who Smoke. 2016a. Centers for Disease Control and Prevention.
Ref Type: Report
Youth Tobacco Use: Results from the 2014 National Youth Tobacco Survey. 2016b. FDA.
Ref Type: Report
- Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2015 doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Yarkov A, Avila-Rodriguez M, Aliev G, Echeverria V. Nicotine-Derived Compounds as Therapeutic Tools Against Post-Traumatic Stress Disorder. Curr Pharm Des. 2015;21:3589–3595. doi: 10.2174/1381612821666150710145250. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10:e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol. 2011;300:H1518–H1529. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption?A survey with a 1-year follow-up. Addiction. 2015 doi: 10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(Suppl 2):ii36–ii40. doi: 10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):i11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16:655–662. doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Murray DE. Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. Int J Environ Res Public Health. 2015;12:8198–8213. doi: 10.3390/ijerph120708198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Alex GJ, Barreto GE. Neuroinflammation: A Therapeutic Target of Cotinine for the Treatment of Psychiatric Disorders? Curr Pharm Des. 2016;22:1324–1333. doi: 10.2174/138161282210160304112511. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R. Cotinine: a potential new therapeutic agent against Alzheimer's disease. CNS Neurosci Ther. 2012;18:517–523. doi: 10.1111/j.1755-5949.2012.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-beta aggregation and improves memory in Alzheimer's disease mice. J Alzheimers Dis. 2011;24:817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- Famele M, Ferranti C, Abenavoli C, Palleschi L, Mancinelli R, Draisci R. The chemical components of electronic cigarette cartridges and refill fluids: review of analytical methods. Nicotine Tob Res. 2015;17:271–279. doi: 10.1093/ntr/ntu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013a;10:5146–5162. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013b;10:2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillon M. Electronic cigarettes might not help cancer patients quit smoking. J Natl Cancer Inst. 2015;107:496. doi: 10.1093/jnci/dju496. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace HA, Tsatsakis AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM, Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50:3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Gao J, Adam BL, Terry AV., Jr Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer's disease. Bioorg Med Chem Lett. 2014;24:1472–1478. doi: 10.1016/j.bmcl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, Kosmider L, Sobczak A. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the US, Korea, and Poland. Int J Drug Policy. 2015 doi: 10.1016/j.drugpo.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014a;109:500–507. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, III, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014b;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana RA, Ling PM. "Smoking revolution": a content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46:395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, Kuballa T, Lachenmeier DW. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12:23. doi: 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Holliday R, Kist R, Bauld L. E-cigarette vapour is not inert and exposure can lead to cell damage. Evid Based Dent. 2016;17:2–3. doi: 10.1038/sj.ebd.6401143. [DOI] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems. ENDS from YouTube videos. Tob Control. 2013;22:103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. Mol Med. Berl. 2016 doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372:392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- Jurado-Coronel JC, Avila-Rodriguez M, Capani F, Gonzalez J, Moran VE, Barreto GE. Targeting the Nicotinic Acetylcholine Receptors (nAChRs) in Astrocytes as a Potential Therapeutic Target in Parkinson's Disease. Curr Pharm Des. 2016;22:1305–1311. doi: 10.2174/138161282210160304112133. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer's disease: studies with DMXBA (GTS-21) Behav Brain Res. 2000;113:169–181. doi: 10.1016/s0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, Maeda T, Akaike A. Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann Neurol. 1997;42:159–163. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- Kougias M, Vardavas CI, Anagnostopoulos N, Matsunaga Y, Tzwrtzi A, Lymberi M, Connolly GN, Behrakis PK. The acute effect of cigarette smoking on the respiratory function and FENO production among young smokers. Exp Lung Res. 2013;39:359–364. doi: 10.3109/01902148.2013.830654. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette Use Among High School and Middle School Adolescents in Connecticut. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner WG, Reddy S, Mehrotra N, Paintal HS. Electronic cigarettes and thirdhand tobacco smoke: two emerging health care challenges for the primary care provider. Int J Gen Med. 2011;4:115–120. doi: 10.2147/IJGM.S16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson's disease: A meta-analysis. Arch Gerontol Geriatr. 2015;61:510–516. doi: 10.1016/j.archger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Lippert AM. Do Adolescent Smokers Use E-Cigarettes to Help Them Quit? The Sociodemographic Correlates and Cessation Motivations of U.S. Adolescent E-Cigarette Use. Am J Health Promot. 2014 doi: 10.4278/ajhp.131120-QUAN-595. [DOI] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, Ji J, Fan W, Huang Z, Hu J. Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson's disease. Neuropharmacology. 2015;91:87–96. doi: 10.1016/j.neuropharm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Lombardo S, Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. Neuropharmacology. 2015;96:255–262. doi: 10.1016/j.neuropharm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol Appl Pharmacol. 2014;278:9–15. doi: 10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24:850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- Michael L. Trehy, Wei Ye, Michael E. Hadwiger, Terry W. Moore, James F. Allgire, Jeffrey T. Woodruff, Shafiq S. Ahadi, John C. Black, Benjamin J. Westenberger. ANALYSIS OF ELECTRONIC CIGARETTE CARTRIDGES, REFILL SOLUTIONS, AND SMOKE FOR NICOTINE AND NICOTINE RELATED IMPURITIES. Journal of Liquid Chromatography & Related Technologies. 2011;34:1442–1458. [Google Scholar]
- Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res. 2006;7:49. doi: 10.1186/1465-9921-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P, Cucullo L. Pathobiology of tobacco smoking and neurovascular disorders: untied strings and alternative products. Fluids Barriers CNS. 2015;12:25. doi: 10.1186/s12987-015-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KJ, June KM, O'Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12:17. doi: 10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis. 2015;13:5. doi: 10.1186/s12971-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez JE, Kleinschmidt KC, Forrester MB. Electronic cigarette exposures reported to Texas poison centers. Nicotine Tob Res. 2015;17:209–211. doi: 10.1093/ntr/ntu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic Cigarettes-A Narrative Review for Clinicians. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Orr KK, Asal NJ. Efficacy of electronic cigarettes for smoking cessation. Ann Pharmacother. 2014;48:1502–1506. doi: 10.1177/1060028014547076. [DOI] [PubMed] [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino RM, Tinghino B, Mangiaracina G, Marani A, Vitali M, Protano C, Osborn JF, Cattaruzza MS. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter. PM. Ann Ig. 2012;24:279–288. [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, Amaradio M, Fisichella A. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2014;9:537–546. doi: 10.1007/s11739-013-0977-z. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, Zhang D, Perez XA. Nicotine and Nicotinic Receptor Drugs: Potential for Parkinson's Disease and Drug-Induced Movement Disorders. Int Rev Neurobiol. 2015;124:247–271. doi: 10.1016/bs.irn.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Hann N, Wilson A, Worrall-Carter L. Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues. Tob Induc Dis. 2014;12:21. doi: 10.1186/1617-9625-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the 'e-cigarette' in the USA. Tob Control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- Ribisl KM, Seidenberg AB, Orlan EN. COMPREHENSIVE E-CIGARETTE REGULATION AS A STEP TOWARD HARM REDUCTION. LDI Issue Brief. 2016;35:492–495. doi: 10.1002/pam.21899. [DOI] [PubMed] [Google Scholar]
- Riker CA, Lee K, Darville A, Hahn EJ. E-cigarettes: promise or peril? Nurs Clin North Am. 2012;47:159–171. doi: 10.1016/j.cnur.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Ruprecht AA, De MC, Pozzi P, Munarini E, Mazza R, Angellotti G, Turla F, Boffi R. Comparison between particulate matter and ultrafine particle emission by electronic and normal cigarettes in real-life conditions. Tumori. 2014;100:e24–e27. doi: 10.1700/1430.15833. [DOI] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jorres RA, Fromme H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628–637. doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States, 2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- Schraufnagel DE. Electronic Cigarettes: Vulnerability of Youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2–6. doi: 10.1089/ped.2015.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer KS, Chen SX, Law S, Van DM, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309:L175–L187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivalingappa PC, Hole R, Westphal CV, Vij N. Airway Exposure to E-Cigarette Vapors Impairs Autophagy and Induces Aggresome Formation. Antioxid Redox Signal. 2015 doi: 10.1089/ars.2015.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton SL, Oller L, Sawyer T. Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol. 2014;10:202–204. doi: 10.1007/s13181-014-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25:e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Toda H. Nitric oxide-mediated blood flow regulation as affected by smoking and nicotine. Eur J Pharmacol. 2010;649:1–13. doi: 10.1016/j.ejphar.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes. e-cigarettes have different smoking characteristics. Nicotine Tob Res. 2010;12:905–912. doi: 10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- Varughese S, Teschke K, Brauer M, Chow Y, van NC, Kennedy SM. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med. 2005;47:411–418. doi: 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med. 2001;58:649–655. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Zhu L, Zhang J, Qiu J, Du G, Qiao Z, Jin G, Gao F, Zhang Q. Low dose nicotine attenuates Abeta neurotoxicity through activation early growth response gene 1 pathway. PLoS One. 2015;10:e0120267. doi: 10.1371/journal.pone.0120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, Ongkeko WM. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593:3397–3412. doi: 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]