Abstract
Chimeric antigen receptor (CAR) T cells have been developed to treat tumors and have shown great success against B cell malignancies. Exploiting modular designs and swappable domains, CARs can target an array of cell surface antigens and, upon receptor-ligand interactions, direct signaling cascades, thereby driving T cell effector functions. CARs have been designed using receptors, ligands, or scFv binding domains. Different regions of a CAR have each been found to play a role in determining the overall efficacy of CAR T cells. Therefore, this review provides an overview of CAR construction and common designs. Each CAR region is discussed in the context of its importance to a CAR’s function. Additionally, the review explores how various engineering strategies have been applied to CAR T cells in order to regulate CAR T cell function and activity.
Keywords: CAR, costimulation, immunotherapy, cancer, cell engineering
Introduction
Chimeric antigen receptors (CARs) are engineered molecules that are constructed by taking various domains from different proteins and combining them to create new receptors. These novel receptors will bind to a specific antigen and stimulate downstream cell signaling leading to an effector response. Thus, a large number of antigen-specific effectors cells can be created. These engineered receptors are an important step in immunotherapy because they have opened the potential to engineer effector cells with the power to regulate a wide variety of diseases. In this article, we review lessons learned from the use of different CARs on T cells and how the particular design of a CAR affects function.
Practical Application and Clinical Relevance
CAR technology in T cells has gained interest as an immunotherapeutic approach against cancer. Introduction of a CAR into a T cell allows that cell to bypass the requirements for MHC restriction and additional costimulation signals normally required for effective T cell activation and persistence (Dotti et al., 2014; Kershaw et al., 2013; Sadelain et al., 2013). Coupling this with the ability to specifically target tumor associated or tumor-specific antigens, CAR T cells can be activated to elicit an effective T cell mediated response against tumors, using both cytotoxicity and cytokine production (Barrett et al., 2014). Thus, CAR T cells provide a way to create a robust anti-tumor immune response, augment the ineffective host tumor-specific immune activity, and overcome the potent defense mechanisms of tumors and their microenvironments.
Clinical trials with CAR therapy have been successful in treating hematological cancers, and many patients have undergone complete remission of their cancers within weeks following adoptive transfer of CAR T cells (Cheadle et al., 2014; Grupp et al., 2013; Maude et al., 2014; Porter et al., 2015; Porter et al., 2011). Although efficacy has been observed in various hematological cancers, similar findings with solid tumors have been lackluster and remain a challenge for CAR T cell therapy to overcome in the clinic (Gilham et al., 2012; Kakarla and Gottschalk, 2014; Lipowska-Bhalla et al., 2012). Additionally, regulation of these CAR T cells once administered to a patient is a major issue, as uncontrolled secretion of pro-inflammatory cytokines or cell killing can cause cytokine release syndrome and autoimmune reactions which can result in severe inflammation and morbidity (Lipowska-Bhalla et al., 2012). Controlling cytotoxicity, cytokine secretion, proliferation, persistence, and memory induction of these CAR T cells is beneficial to maximize the therapeutic potential, while mitigating their potential for adverse side effects. Therefore, understanding how CAR design can influence the function, efficacy, and potential toxicity of CAR T cells is imperative for these therapies to be developed effectively.
CAR Regions and Generations
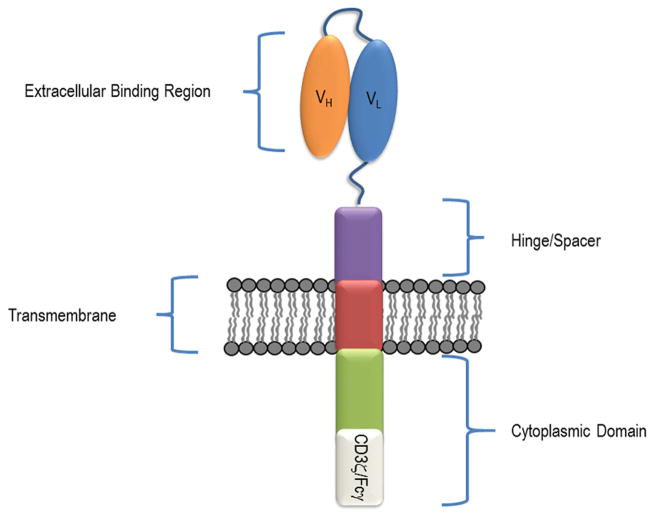
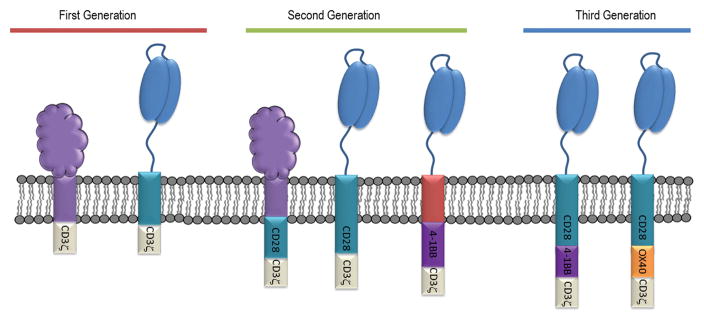
CARs are composed of four regions (Figure 1). These are (1) an extracellular binding domain(s), (2) a hinge or spacer region, (3) a transmembrane region, and (4) a cytoplasmic signaling region (Dotti et al., 2014; Sadelain et al., 2013). CARs are classified into three generations based on the number of costimulatory domains within the cytoplasmic region (Figure 2). First generation CARs contain one activation domain, second generation CARs contain an activation and one costimulatory domain, and third generation CARs contain an activation domain and multiple costimulatory domains. Each region contributes specific attributes to the new receptor, affecting receptor ligand interactions, expression, stability, and downstream signaling and activation.
Figure 1. Overview of chimeric antigen receptor structure.
CARs consist of an extracellular binding region, a hinge or spacer region, a transmembrane domain, and cytoplasmic domain. Most CARs use an scFv as their extracellular binding region, constructed by joining the variable (VH and VL) regions of an antibody. The cytoplasmic region is composed of an activation domain, such as CD3ζ and is often paired with a costimulatory domain (I.e. 4-1BB or CD28), which can influence CAR T cell effector function.
Figure 2. CAR Generations.
CAR generations are based upon how many co-stimulation domains have been incorporated into the cytoplasmic region of a CAR. First Generation CARs were constructed with only an activation domain. Second Generation CARs were constructed with an additional co-stimulatory domain to augment activation. Third Generation CARs were constructed with the intent of harnessing multiple co-stimulatory domains to further enhance CAR T cell function.
Extracellular Binding Domain
The extracellular binding regions of CARs have historically utilized the binding region of a receptor expressed on the cell surface, a receptor ligand, T cell receptor binding chains, or the antigen binding regions normally found within an antibody that has been formatted into single-chain variable fragment (scFv). Indeed, the first CARs that were created utilized binding portions of receptors that are naturally expressed on cells. Extracellular regions from CD4, CD8, or CD16 receptors were among the first to be fused to the CD3ζ or FcRγ cytoplasmic domains, which provided a primary stimulation signal to T cells (Irving and Weiss, 1991; Letourneur and Klausner, 1991; Romeo et al., 1992; Romeo and Seed, 1991). However, the purpose of creating these first CARs was to understand the signaling components and requirements needed to induce activation of a T cell, mediated by the signaling domains of either CD3ζ or FcRγ receptor. Initially, cross-linking antibodies that bound to the extracellular regions of these CARs were used to induce signaling (Irving and Weiss, 1991; Letourneur and Klausner, 1991; Romeo et al., 1992). However, some early studies (Romeo and Seed, 1991) used actual receptor-ligand mediated interactions, such as CD4 binding the HIV protein gp120, suggesting the potential to use CAR technology as a target-specific therapy. As applications for CAR therapy were explored, receptors for extracellular regions of CARs were carefully selected and developed to target specific ligands.
scFv-based Binding Domains
The largest group of extracellular binding regions used in construction of CARs are scFvs. These binding domains consist of a variable heavy and variable light chains fused together with a flexible linker. The variable domains are derived within an antibody, determining antigen specificity. One of the very first scFv based CARs was taken from a Sp6 anti-TNP mAb, in which the variable heavy region and the variable light regions of the antibody were joined by a linker (Eshhar et al., 1993). In this way, the CAR receptor will have the specificity and affinity of the original antibody yet also be expressed as one intact protein on the cell surface. Since then, CAR designs have used the scFv format to create CARs specific for all types of targets as well as targeting different epitopes on the same target. Many CARs were built to recognize tumor-specific antigens or tumor associated antigens. Prominent tumor targets include CD19, CD20, HER2, GD2, the ErbB receptor family, MUC1, and PSMA (Cheever et al., 2009; Dotti et al., 2014). However, reformatting variable chains from a full antibody to an scFv does not guarantee a functional CAR receptor. Potential reasons for this include ablated binding specificity, Fv-mediated protein aggregation, or ineffective expression on the cell surface. Furthermore, not all antibodies can be reformatted into an scFv and retain antigen binding. This must be taken into consideration when attempting to design scFvs with specific binding properties, such as when humanizing an scFv or attempting to affinity mature the scFv to improve antigen binding.
How scFv affinity affects CAR function is an area of active research. One study (Chmielewski et al., 2011) suggests that binding affinity and antigen density is the major determinant of CAR T cell activation and even augmentation through costimulatory signaling domains will not lower this activation threshold. Additionally, Chmielewski et al. (2011) found that a higher affinity scFv as a first generation CAR is insufficient to induce cytokine secretion comparable to that of a second generation CD28 CAR, suggesting that binding affinity enables activation but does not direct the T cell effector response. Further studies (Caruso et al., 2015) revealed that activation of an scFv-based CAR is dependent on the binding affinity of the CAR and the antigen density on the target cell. Caruso et al. (2015) illustrates this with CARs generated from Cetuximab and Nimotuzumab, antibodies with different affinities that bind to similar epitopes on EGFR (Talavera et al., 2009). Exposure to cells expressing various amounts of EGFR showed that the increase in the response of T cell cytotoxicity and cytokine production with the lower affinity Nimotuzumab scFv-based CAR correlated with an increase in the antigen density on the target cell. In comparison, the higher affinity Cetuximab scFv-based CAR T cells responded similarly across all levels of antigen expression on the target cell.
Other studies (Liu et al., 2015) have attempted to fine tune scFv affinity of a CAR T cell to minimize reactivity to cells expressing the targeted antigen but which are not tumors. This on target but off tumor toxicity can be an issue when CAR T cells are designed to recognize antigens which can be found overexpressed on tumor but are also expressed on normal cells. Liu et al. (2015) generated CARs with scFvs of different affinities against ErbB2, a cell surface receptor overexpressed in some types of cancer such as ovarian and breast cancers but can also be expressed on normal tissues. In vivo experiments showed that CARs expressing a low affinity scFv had strong activity against high ErbB2 expressing tumors comparable to that of a higher affinity ErbB2-specific scFv. Furthermore, this same low affinity CAR construct had reduced activity against cells which expressed lower amounts ErbB2 compared to the high affinity scFv CAR. This suggested that a lower affinity scFv CAR could be beneficial by targeting cancers that overexpress the antigen, yet spare those which express the antigen at normal physiological levels. Other research (Lynn et al., 2016) makes the case for choosing higher affinity scFvs over lower affinity ones. Lynn et al. (2016) constructed two different CARs, selecting a high affinity scFv and a low affinity scFv to target tumors overexpressing Folate receptor b (FRb). Their data indicate that only the high affinity scFv could completely clear Frb+ THP1 AML tumor cells in NSG mice. In contrast, their low affinity CAR T cells persisted longer but did not show efficacy against the tumor, as tumor burden in the NSG mice was comparable to that of NSG mice treated with non-Frb specific CAR T cells. This particular observation could have been caused from chronic antigen stimulation in low affinity CAR T cells, in which the CAR T cells were not stimulated to effectively induce T cell effector functions but received enough signal for the CAR T cells to persist. These and other studies suggest that selecting an scFv with an appropriate affinity is crucial in designing CARs targeting antigens that are normally expressed on healthy cells, but which can also be overexpressed on tumor cells. Clearly, an optimal affinity must be attained in the context of targeting overexpressed ligands such that the CAR can still bind its target and activate T cell effector functions, yet limit the on target but off tumor effects of low ligand expression on non-tumor cells. This optimal affinity could be achieved through scFv affinity maturation, selecting for a wide range of affinities and subsequently testing them on cells with varying amounts of the targeted antigen.
TCR-like Antibody CARs
TCR-like antibody based CARs are a class of CARs which express scFvs from antibodies that specifically recognize MHC class molecules and its loaded peptide (Dahan and Reiter, 2012). This specificity can be utilized to target cancers based on recognition of mutated intracellular proteins. If mutated peptide sequences are loaded onto the MHC, they could effectively generate neo-epitopes, which can be used to distinguish a cancerous cell from a normal cell by a CAR that only recognizes the specific MHC/peptide combination. This class of CARs may expand the repertoire of targetable ligands and broaden the range of tumors which can be treated. However, studies (Oren et al., 2014; Willemsen et al., 2005) conducted with TCR-like antibody based CAR constructs have yielded mixed results. Willemsen et al. (2005) generated TCR-like antibody based CARs against a specific MHC/Mage1 peptide combination, creating high affinity and low affinity CARs that are able to induce CAR T cell cytokine secretion and cytotoxicity upon recognition of the MHC/Mage1 peptide combination. However, both CARs also exhibit activity against the MHC molecule when it was not loaded with the specific peptide. These observations corroborated with data from Oren et al. (2014), which compared TCR-like antibody based CARs with different affinities targeting a MHC/Wilms Tumor 1 (WT1) peptide combination. T cells expressing the high affinity CAR variant were shown to have lower viability when the T cells expressed the targeted HLA molecules. This was not observed in CAR T cells that did not express the specific HLA molecules. They further compared the low affinity TCR-like CAR to a TCR specific for the same MHC/WT1 combination, and observed that the TCR response had greater activity than the TCR-like antibody CAR. In this case, the TCR has a lower affinity than the CAR for the MHC/peptide complex. This suggests that other contributing factors such as interactions of the MHC with the TCR and MHC co-receptors could enhance the avidity and sensitivity of a T cell. This may result in greater T cell activity against the targeted cell with a natural TCR compared to a CAR T cell which may be unable to benefit from these interactions. Overall this suggests that though TCR-like antibody based CARs can recognize specific MHC/peptide combinations, they do not share the same relationship with a MHC/peptide complex that a natural TCR has. As such, use of TCR-like antibody based CARs may encounter unexpected auto-reactivity or decreased potency.
Natural Extracellular Binding Domains
CD16 CARs
Some CARs make use of the extracellular binding region of FcγRIIIα (CD16) (Clemenceau et al., 2006; D’Aloia et al., 2016; Kudo et al., 2014). CD16 can be expressed on a variety of immune cells including neutrophils, monocytes, macrophages, and NK cells, functioning to mediate antibody dependent cellular cytotoxicity (ADCC) through binding the Fc region of an antibody. Some researchers (Clemenceau et al., 2006; Kudo et al., 2014) have constructed CARs with the intent of taking advantage of CD16’s ability to bind antibodies, effectively making a universal CAR. Doing so allows the use of specific antibodies to recognize tumor antigens. Then, CAR T cells expressing these CD16-based CAR could interact with the antibody via the Fc region, activate the CAR T cell, and subsequently induce cytotoxicity and cytokine secretion. Theoretically, this system has two advantages with respect to safety. The first is target specificity through antibody design. Antibodies can be designed to target a certain epitope or region of an antigen with different affinities. This could be beneficial in excluding normal tissues which do not have mutated proteins or over express tumor associated antigens. The other is that the antibody has to be administered to a patient and will be lost based on the antibody pharmacokinetics. This provides another layer of control over a CAR T cell response, as selecting the right dose and frequency of administration may prevent excessive immune toxicity. However, a major challenge for this system is the ability of the CAR to bind any antibody by its Fc. Thus, the CAR T cells could become activated to antibody-antigen aggregates that occur regularly as a part of normal immunity against pathogens. These CARs may also interact with the specific antibody that could aggregate at tissue sites or on cells throughout the body without specific antigen. Both these instances could incite unintended immune system activation and off target toxicities.
NK Receptor CARs
One class of natural receptor CARs have been based on NK cells and NK cell receptors. NK cells have robust antitumor activity and many of their receptors can recognize various stress induced or overexpressed ligands on different tumors, thereby activating NK cell cytotoxicity (Moretta and Moretta, 2004; Spear et al., 2013c). Some CARs generated this way were based on the NKp30, DNAM-1, and NKG2D receptors (Lehner et al., 2012; Wu et al., 2015b; Zhang et al., 2006; Zhang et al., 2005; Zhang et al., 2012). NK receptor based CARs were engineered such that the extracellular binding domains of these NK cell receptors remained intact, yet were fused to cytoplasmic activation and costimulatory domains. These NK receptor based CARs have shown efficacy similar to that of CARs expressing an scFv, allowing for recognition of ligands on tumor cells and enabling long term survival in immune intact in vivo models (Spear et al., 2013a; Spear et al., 2013b; Wu et al., 2015b; Wu et al., 2015c; Zhang et al., 2012). The CARs have no extracellular regions that are not normally present on immune cells, so they would not be expected to be immunogenic in vivo. Because these CARs were based on NK cell receptors, they have the capacity to bind multiple ligands and target many different tumors. For example, the NKG2D CAR has been shown to recognize and eliminate myelomas, lymphomas, and ovarian cancers (Barber et al., 2011; Barber et al., 2009; Barber et al., 2008; Sentman and Meehan, 2014; Zhang et al., 2005). Therefore, NK receptor based CARs have the potential to target a wide range of different tumors using one single CAR platform. However, effector T cells expressing these NK receptor based CARs could potentially target non-tumor cells expressing their ligands (Sentman and Meehan, 2014). So, short-term survival of these CAR T cells rather than long-term persistence may be beneficial for such CAR T cells to avoid off tumor effects.
Hinge/Spacer Region
The hinge/spacer region consists of a CAR’s non-antigen binding extracellular region. Research on the role of the hinge/spacer has been observed to modulate CAR function by providing flexibility, extending the length, allowing dimerization to occur, or improving stability. These properties have been suggested to influence the effector cell-target cell interactions, thereby affecting the activation signal strength. Common hinge/spacers regions have made use of immunoglobulin Fc, CD8α and CD28 spacer regions (Lipowska-Bhalla et al., 2012).
One particular type of hinge/spacer class is the use of Fc IgG. These Fc portions are composed of the constant heavy chain domains CH2 and CH3. Most CARs utilizing this spacer region makes use of the Fc CH2-CH3 domains from either IgG1 or IgG4. These types of spacers/hinges have contributed much of what is known about the function of this particular CAR region. Early studies (Guest et al., 2005; Hombach et al., 2000) involving IgG1 as a stalk region first suggested that its addition could affect activation of a T cell and tumor killing or cytokine secretion. In these studies, different scFvs were selected, each of which targeting a different tumor associated antigen. Two types of CARs were generated for each scFv, one which included IgG1 as a spacer and the other without it. The antitumor response for each pair was assessed against tumor cell lines expressing the scFv’s specific antigen. Their findings indicated that there were differences between each scFv CAR’s efficacy in cell killing or cytokine secretion, depending on the antigen being targeted. In some cases, the Fc IgG1 spacer was beneficial in generating a more potent immune response. Other scFv-antigen combinations resulted in better efficacy without this same Fc IgG1 spacer. This discrepancy was suggested to be attributed to the distance of the antigen binding site in relation to the tumor cell membrane. The closer the antigen binding site is on the opposing tumor cell membrane, the more advantageous it may be to have a CAR construct with a longer spacer region to achieve an optimal distance and immunological synapse formation. More recent research (Hudecek et al., 2013; Hudecek et al., 2015) delves into optimizing these hinge lengths and understanding how they can affect the T cell response. Utilizing the Fc IgG4 template of CH2-CH3 and deleting regions within the Fc IgG portion to test varying CAR lengths supported the hypothesis that spacers can potentially play a role in proliferation, activation, and initiation of activation induced cell death (AICD) through overstimulation of the T cell.
Design of the CAR spacer region should be carefully considered. Studies (Hombach et al., 2010; Jonnalagadda et al., 2015) conducted with both Fc spacer regions of native IgG1 or IgG4 sequences maintained partial function with respect to interactions with FcγR receptors. These interactions have the potential to non-specifically activate the CAR T cells without antigen when in the presence of Fc receptor expressing cells, such as monocytes and NK cells. Likewise, the Fc receptor expressing cells can be unintentionally activated through their FcγR receptors when in the presence of CAR T cells, initiating innate immune responses such as secretion of proinflammatory cytokines. This property was observed to inhibit efficacy of CAR T cells in treating tumor bearing mice and may be a potential reason for the limited effectiveness in treating lymphoma patients (Hudecek et al., 2015; Savoldo et al., 2011). To mitigate this effect, the Fc IgG chains used as spacer regions were reengineered with specific mutations within the sequence to disrupt the binding ability of the Fc IgG to bind to its cognate reported Fc receptor (Hombach et al., 2010; Jonnalagadda et al., 2015). These modifications allowed the CAR T cells to avoid off target activation of the CAR T cells and innate immune cells, and improve CAR T cell persistence and antitumor potency in vivo.
Transmembrane Region
The transmembrane domain of the CAR has been less studied than other components, thus its contributions to CAR performance remains unclear. One early study (Romeo et al., 1992) that utilized a CD3ζ transmembrane domain in a CAR construct found that the region was important for formation of dimers with other CD3ζ proteins, as mutations in cysteine and aspartic acid residues within the transmembrane resulted in disruption of dimerization. This lack of dimerization was suggested to impact CAR T cell activation leading to weaker cytolytic activity. These observations implied that the transmembrane region has the capacity to provide stability to the CAR receptor, aiding in associations with other cell surface proteins that can affect CAR T cell function.
The transmembrane region of the NKG2D CAR permits the CAR to associate with Dap10 through interactions between specific amino acids present within the transmembrane region (Zhang et al., 2006). Each NKG2D receptor associates with two Dap10 molecules, and the use of the NKG2D transmembrane in a CAR allows the CAR to associate with endogenous Dap10 in a spatial arrangement that the NKG2D receptor normally has with Dap10 (Lanier, 2009). Thus, it is possible to take advantage of transmembrane domains from proteins to allow a CAR to associate with other beneficial co-receptors and cell surface proteins. Still, more research is needed to further define which proteins may have useful transmembrane domains suitable for optimizing CAR expression and effector functions.
Cytoplasmic Region
The cytoplasmic domain provides the downstream signaling which activates the CAR T cell and directs its response. CARs have become classified into three different generations based on the signaling domains associated with the constructs. The first generation of CARs contained a primary activation signaling motif, utilizing either CD3ζ or FcRγ. Second generation CARs were created soon after and combined the cytoplasmic domain of a secondary costimulating signaling protein with the CD3ζ primary signal region. Subsequently, third generation CARs were constructed, attempting to harness multiple signaling pathways by combining multiple T cell costimulation domains, such as fusing the cytoplasmic domains of CD3ζ, 4-1BB, and CD28 all into one CAR.
First Generation CARs
First generation CARs typically use cytoplasmic portions of FcRγ or a CD3ζ chain, which is sufficient to activate T cells through their immune tyrosine activation motifs (ITAM) (Romeo et al., 1992; Romeo and Seed, 1991). This activation occurs independently of the normal TCR-MHC interaction, as crosslinking antibodies to the extracellular portion of a CAR can initiate signal transduction (Irving and Weiss, 1991). Co-incubation of CAR T cells with tumors expressing the target antigen triggered cytotoxicity and secretion of cytokines, notably IL-2 and IFN-γ (Chmielewski et al., 2013; Eshhar et al., 1993). In vivo models correlated with these readouts, showing improved survival for tumor bearing mice upon CAR T cell treatment (Chmielewski et al., 2013; Hwu et al., 1995). Though first generation CARs could treat tumors, their potency as a therapy was insufficient to completely eradicate the tumors. This spurred the development of later CAR generations to enhance CAR T cell efficacy against tumors.
Second Generation CARs
The need to enhance the potency of CAR T cell therapy resulted in the construction of CARs that could enhance CAR signaling as well as influence T cell effector function. The development of these CARs is centered on the concept of providing a costimulatory signal to the T cell upon binding of a CAR to its ligand. This would ideally allow modulation of persistence, proliferation, memory, trafficking, or cytokine/chemokine secretion profiles. Many of these costimulatory domains are signaling domains which play a role in normal T cell activation and biology. The most widely used costimulatory domains in CAR construction come from CD28 or 4-1BB, but other CAR constructs have utilized the signaling domains from ICOS or OX40. Currently, the second generation CARs are the platform commonly selected when constructing and studying CAR T cells, and are also the ones most often used in clinical trials. In most constructs, the costimulatory domain is located directly after the transmembrane domain and before the primary signaling domain. This close proximity to the cell membrane is thought to be important to allow the costimulation domain to interact with its signaling molecules located in this region of the cell.
CD28
The CD28 costimulatory receptor is most widely known as the signal 2 in T cell activation. Along with 4-1BB, it is one of the most common CAR platforms being developed and used in the clinic. Because CD28 has a high potential to activate the T cells, CAR constructs that contain a CD28 costimulatory domain generally induce greater amounts of cytokine secretion, such as IL-2, IL-10, IFN-γ, and TNF-α than other CAR constructs (Chmielewski et al., 2011; Finney et al., 2004; Hombach et al., 2012; Pule et al., 2005). Additionally, some studies (Long et al., 2015) with a CD28 CAR have shown tonic signaling independent of receptor ligand interactions, which leads to expression of PD-1, TIM-3, and LAG-3 and exhaustion of T cells. However, CD28 containing CARs are effective at tumor cell killing and have been shown to have a higher proliferation rate than other common second generation CARs (Frigault et al., 2015; Hombach and Abken, 2011). In vivo mouse studies (Haynes et al., 2002) using CD28 CARs have shown greater efficacy in either eradicating or inhibiting tumor growth than that of first generation CARs with the same specificity. In clinical trials, CD28 CARs targeting CD19+ lymphomas have shown efficacy in eradicating the tumors (Brentjens et al., 2013; Davila et al., 2014; Kochenderfer et al., 2012). One particular clinical trial (Savoldo et al., 2011) showed the capacity to proliferate and persist longer than a first generation CAR.
4-1BB
4-1BB (CD137) is part of the tumor necrosis factor receptor family and acts as a costimulatory receptor, which helps to drive activation signaling in T cells. 4-1BB signaling results in secretion of IL-2, IFN-γ, and IL-4, cell survival and resistance to activation induced cell death (AICD), promotion of antitumor activity, and augmentation of CD28 mediated signaling (Myers and Vella, 2005; Vinay and Kwon, 1998; Vinay and Kwon, 2012). In vitro studies (Imai et al., 2004) revealed that CARs using the 4-1BB cytoplasmic domain exhibited more cell killing and cytokine secretion upon recognition of the target antigen compared to first generation CARs using the CD3ζ activation domain. Studies (Long et al., 2015; Milone et al., 2009; Song et al., 2011) comparing anti-CD19 CARs with costimulatory domains of either 4-1BB or CD28 showed differences in effector T cell populations. A 4-1BB containing CAR was shown to induce higher amounts of specific lysis compared to the CD28 CAR, while secreting less TH2 cytokines, namely IL-4 and IL-10 (Milone et al., 2009). Additionally, 4-1BB CAR T cells were shown to have mitigated cell exhaustion, expressing less PD-1, TIM-3, and LAG-3 compared to a similarly constructed CD28 based CAR (Long et al., 2015). These in vitro differences translated to in vivo NSG mouse models, as 4-1BB based CARs were observed to have more CAR T cell persistence and increased antitumor efficacy than CAR T cells containing only the CD3ζ signaling domain (Song et al., 2011). Clinically this CAR signaling platform paired with an anti-CD19 scFv has been successful in treating many different CD19+ hematological malignancies including CLL, CML, and ALL, with some patients in sustained remission (Grupp et al., 2013; Maude et al., 2014; Porter et al., 2015; Porter et al., 2011). Many patients have exhibited increases in proinflammatory cytokine secretion 2–3 weeks post infusion, and the presence of CAR T cells could be detected for up to a year post infusion, indicating function and persistence of these CAR T cells (Kalos et al., 2011). The body constantly produces new B cells, so CD19 specific CARs will be activated by recognition of these normal cells, which may allow CD19-specific CAR T cells to remain in vivo for a long period of time. However, secretion of proinflammatory cytokines remains a cause for concern as this can often lead to immune toxicity.
ICOS
ICOS is a member of the CD28 super family but normally only functions after initial T cell activation, acting as a costimulatory receptor. In contrast to CD28, it does not seem to initiate proliferation but instead plays a role in cytokine production of CD4+ TH1 and TH2 T cell subsets (Sharpe and Freeman, 2002). T cells transduced with ICOS based CARs have antitumor activity, including killing target cells and secretion of IFN-γ (Shen et al., 2013). However, major differences have been reported (Guedan et al., 2014) with respect to the TH17 T cell subset. TH17 cells expressing an ICOS based CAR showed increases in cytokine secretion of IL-17A, IL-17F, IL-22 and less IL-2 and TNF-α than a CD28 based CAR. Unsurprisingly, gene expression differed between the second generation CARs targeting human mesothelin, as upregulation of TH17 and TH1 genes were observed in ICOS based CARs compared to CD28 or 4-1BB based CARs. Efficacy of these particular CARs in a mouse NSG in vivo model showed that TH17 cells expressing CARs with an ICOS costimulatory domain could treat tumors expressing mesothelin with similar efficacy as TH17 cells expressing CARs designed with either a 4-1BB or CD28 costimulatory domain. One notable difference was that ICOS based TH17 CAR T cells were able to persist longer in an immunodeficient mouse model than other TH17 CAR T cells.
OX40
OX40, similar to 4-1BB, is part of the TNFR family, and is considered a late costimulatory molecule appearing to augment CD28 activation in T cells. OX40 CAR T cells are reported (Hombach et al., 2012; Pule et al., 2005) to secrete little IL-2 and IL-10, but do secrete IFN-γ comparable to other CAR T cells. Furthermore, OX40 based CAR T cells did not show the same amount of proliferation as CD28 based CAR T cells. Instead, these OX40 based CAR T cells were observed to induce CD62L- memory T cells which can escape AICD (Hombach and Abken, 2011). However, even though second generation OX40 CARs induce unique T cell properties, development of these second generation CAR designs have been curtailed in favor of third generation CARs containing OX40 domains (Hombach and Abken, 2011; Hombach et al., 2012).
Third Generation CARs
Third generation CARs attempted to further enhance potency of CARs by encompassing two costimulatory domains with a primary signaling domain. The idea was to create a CAR which would exhibit a T cell activation profile based on many signaling domains, including cytokine secretion, cytotoxicity, and driving the cell into a specific differentiation state. Two CARs that have exhibited some of these properties make use of CD28/OX40 or CD28/41BB combined costimulation domains. Currently, few of these CARs have made it into clinic, as there have been little advantages in vivo over second generation CARs.
CD28/OX40
In the pursuit of improved T cell effector function against tumors coupled with the knowledge of the success of second generation CARs, the CD28/OX40 CARs were designed to take advantage of the signaling activities of the early costimulatory CD28 molecule and the late costimulatory molecule OX40 to augment CAR function in proliferation, persistence, cytotoxicity, and cytokine secretion. Although the CD28 CAR was effective for CAR design, it does have its pitfalls. Among these was the increased cytokine production of IL-10. In contrast, CD4+ CAR T cells containing the OX40 costimulatory domain were characterized by higher amounts of IL-2, lower amounts of IL-10, and lower proliferation, but had comparable amounts of secreted IFN-γ and tumor cell killing with respect to a CD28 based CAR (Hombach et al., 2012). The fusion of these two costimulatory domains resulted in suppression of IL-10 production, while retaining T cell effector function, proinflammatory cytokine secretion, proliferation, and prevention of apoptosis in certain T cell subsets, namely CD62L- T cells (Hombach and Abken, 2011; Pule et al., 2005). However, in vivo studies (Hombach and Abken, 2011; Hombach et al., 2013) with immune deficient mouse tumor models have given mixed results. In some cases, using these CD28/OX40 based CAR T cells showed better efficacy, for example when treating CEA+ tumors (Hombach and Abken, 2011). However, cytokine induced killer cells, immune cells which have very potent effector functions, expressing the OX40/CD28 CAR did not treat tumors as well as a CD28 based CAR (Hombach et al., 2013). This suggests that certain T cell types may work better with specific CAR constructs over others, and this should be taken into consideration when attempting to manipulate an immune cell to perform specific tasks.
CD28/4-1BB
A different third generation CAR fused the CD28 and 4-1BB signaling domains. This CD28/4-1BB CAR was shown to have greater efficacy in treating in vivo tumor models compared to second generation CARs based on CD28 or 4-1BB and first generation CARs using CD3ζ alone (Tammana et al., 2010). However, cytokine production of IL-2 and IFN-γ showed little differences between the CD28/4-1BB based CAR from its respective second generation CARs upon stimulation with antigen expressing tumor cells. Carpenito et al. (2009) had varying results, observing that CD28/4-1BB CAR T cells treated solid tumors in mice as well as CD28 CAR T cells, but persisted longer in vivo comparable to that of a second generation 4-1BB based CAR (Carpenito et al., 2009). Studies (Zhong et al., 2010) elucidating the signaling of this third generation CAR demonstrated upregulation of Bcl-XL, which was attributed to increased activity of PI3K/AKT signaling. This observed upregulation of Bcl-XL was higher than that of respective second generation CARs. This seemed to further enhance T cell mediated tumor eradication and decrease apoptosis. Other groups (Kunkele et al., 2015) have found that stimulation through the fused CD28/4-1BB signaling domains induced apoptosis in CAR T cells through Fas/FasL interactions, diminishing antitumor efficacy in mouse tumor models in vivo. However, this observation could also be due to other discrepancies in the CAR design between the two studies. Clinically, CD28/4-1BB CARs have not been as successful as second generation CARs, and lymphoma patients treated with a CD28/4-1BB based CARs had partial responses or disease relapse (Till et al., 2012).
CAR-Safety and Efficiency
Inducible Caspase
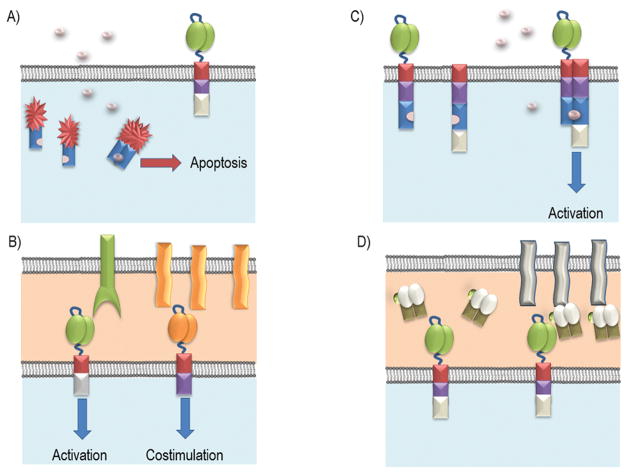
As a way to prevent unwanted CAR mediated signaling, safety mechanisms are being developed to control the activity of CAR T cells. One of these mechanisms revolves around an inducible caspase system, effectively creating a suicide gene system (Figure 3A). These caspase systems use either Caspase 8 or Caspase 9, which are engineered to have domains that require a compound to dimerize the Caspase proteins (Budde et al., 2013; Khaleghi et al., 2012; Straathof et al., 2005). Once dimerization occurs, the apoptotic signaling cascade is initiated. Thus, any CAR T cells that have this suicide gene should undergo apoptosis upon exposure to the dimerization compound. This approach has been used in adoptive T cell therapy clinical trials to resolve graft-versus-host disease (GVHD) and could be used to ensure patient safety upon CAR T cell therapy (Di Stasi et al., 2011).
Figure 3. Approaches to regulate CAR function.
(A) Inducible Caspase System: Caspase 8 or 9 fused to a dimerization protein can be co-expressed with in a CAR T cell. Activation of this inducible Caspase system required addition of a small molecule to effectively dimerize these inducible Caspases and subsequently initiate the cell’s apoptotic cascade. (B) Dual CAR: Regulation system in which the activation domain and the co-stimulation domain are split between two separate CARs. The CAR T cells can be activated alone if the primary activation CAR (Green) can be crosslinked in the presence of high antigen density, but lacks the augmentation that is provided by a co-stimulatory domain. Only when both CARs interact with their respective antigens does the CAR T cell benefit from the combined signaling. (C) ON-Switch CAR: Signaling domains have been split such that the extracellular binding domain is separate from the activation domain. Both separated parts are fused to binding proteins that allow for dimerization in presence of a small molecule. To induce CAR T cell activation, the extracellular domain must bind its target antigen while in the presence of the small molecule. (D) Switchable CAR: This system uses CARs to target one specific epitope. This epitope is then engineered onto an antibody or a Fab (as shown) which can bind to the antigen of interest. In this way, only the addition of the targeting protein containing this unique epitope induces CAR T cell effector functions.
Dual CAR Systems
To regulate off tumor targeting of CAR T cells, some designs make use of a dual CAR system, dissociating the activation signaling domain from the costimulatory signaling domain into two separate CARs with different antigen specificities (Figure 3B). Specificity for the tumor population is attained through the targeting of two distinct tumor associated ligands co-expressed on the tumor. The activation domain is embedded into a CAR specific for one of the ligands, and the costimulatory domain is generated as part of a CAR targeting the other ligand. Ideally, CAR T cells will generate a robust antitumor response only in the presence of both ligands. Thus, this setup could mitigate the off target effects against normal cells that may express only one of the targeted ligands. Studies (Kloss et al., 2013; Wilkie et al., 2012) separating the signaling domains into two CARs has successfully shown that the two CAR system was feasible to create functional CAR T cells and displayed some advantages over a single CAR. Further research (Lanitis et al., 2013) has showed that a robust antitumor T cell response of the two CAR system is comparable to that of second generation CAR outcomes in an in vivo NSG mouse model. Importantly, tumor targets lacking one of the ligands resulted in an attenuated CAR T cell response. However, if the ligand for the primary signaling CD3ζ CAR was highly expressed, then the CAR T cells could be activated by this antigen recognition alone. The avidity of the CARs for their antigens may need to be carefully considered to make this approach effective and selective only for cells that express both antigens.
Switchable CARs
Additionally, CARs that can be switched on and off have been developed. One such type of switchable CAR termed ON-switch CARs has been developed (Wu et al., 2015a). This system revolves around separating the activation domain from the scFv binding domain (Figure 3C). Although, both the scFv and the CD3ζ are attached to the membrane, they are not able to interact and fully activate a T cell even when the scFv has recognized and bound its antigen. Instead, the two pieces of the CAR were engineered with a heterodimerizing domain such that only in the presence of a specific small molecule, such as Rapalog or Gibberellin, can the CAR assemble at the cell membrane and be able to fully stimulate the T cell (Wu et al., 2015a). This setup allows all the potential characteristics of a regularly constructed CAR but gives the ability to control the potency of the CAR T cell through dose titrations of the small molecule, which could aid to curb immune toxicity in patients.
Another type of switchable CAR was created using a two antibody fragment system (Ma et al., 2016; Rodgers et al., 2016). In this system, the CAR contains an scFv that is specific for an antigen such as FITC or a small peptide (Figure 3D). These antigens would then be incorporated onto a second antibody, either by site specific conjugation or engineering the antibody itself to contain the peptide epitope. This second antibody would be specific for a target antigen. Thus, similar to the universal CD16 CAR receptor, CAR T cells should only be activated in the presence of the second target specific antibody, which would be infused when desired and be restricted by their own pharmacokinetics. In this way, the potency of the CAR T cell response could be controlled based on the amount of antibody administered to a patient.
Concluding Remarks
Cell engineering holds great promise to create designer immune cells that can modulate specific disease conditions. CAR technology will allow these cells to target disease tissues in an antigen specific manner. With the use of multicistronic viral vectors, immune cells are now being created that express CARs along with other effector molecules to improve CAR T cell function, such as specific cytokines or blocking Fv regions that can influence immune activity. These innovations, sometimes referred to as fourth generation CARs, will allow therapies to be derived and delivered to disease sites in specific and selective ways. There are likely limits to the size and complexity of a single CAR protein that will still allow it to function effectively. Therefore, inclusion of a second CAR or other effector proteins can make these engineered immune cells even more effective.
As various CAR T cell therapies continue to move through the clinic, understanding why some CAR constructs induce specific responses will enable tailored designs for specific diseases. Each region of a CAR contributes to its overall function and researchers continue to elucidate these complexities. Although much of the knowledge from antibody engineering can be relevant to CAR design, CARs are cell-based receptors and the design needs to function best when cell-cell contact occurs. The ability of CARs to bind their antigen, but also to release antigen and trigger proper signaling cascades in the effector cell is essential to create effective CARs. Additionally, regulation of CAR T cell activity through various novel protein engineering ensures the safety of CAR therapy. Going forward, CAR technology promises to change the therapeutic strategy for patients with cancers and other diseases and act as a major contributor to the new wave of immunotherapies being developed.
Acknowledgments
This work was supported in part by funds from the Center for Synthetic Immunity, the Munck-Pfefferkorn fund of Dartmouth College, and in part by NIH T32 AI007363.
The authors wish to thank Mia Sentman for critical reading of the manuscript.
Abbreviations
- CAR
Chimeric Antigen Receptor
- MHC
major histocompatibility complex
- scFv
single-chain variable fragment
- AICD
Activation induced cell death
- TCR
T cell receptor
- NK cells
Natural Killer cells
Footnotes
Conflict of Interest: CS has patents and financial interests in CAR therapies and cell engineering. CS is a Scientific Founder for Celdara Medical, a consultant, and receives research support from Celdara Medical. AG and BA have filed patent applications involving CAR technologies and cell engineering. These conflicts are managed under the policies of Dartmouth College.
References
- Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene therapy. 2011;18(5):509–516. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183(11):6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Experimental hematology. 2008;36(10):1318–1328. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annual review of medicine. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC, Riddell SR, Press OW. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PloS one. 2013;8(12):e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, Lee DA, Heimberger AB, Champlin RE, Cooper LJ. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer research. 2015;75(17):3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunological reviews. 2014;257(1):91–106. doi: 10.1111/imr.12126. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski M, Hombach AA, Abken H. CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene therapy. 2011;18(1):62–72. doi: 10.1038/gt.2010.127. [DOI] [PubMed] [Google Scholar]
- Chmielewski M, Rappl G, Hombach AA, Abken H. T cells redirected by a CD3zeta chimeric antigen receptor can establish self-antigen-specific tumour protection in the long term. Gene therapy. 2013;20(2):177–186. doi: 10.1038/gt.2012.21. [DOI] [PubMed] [Google Scholar]
- Clemenceau B, Congy-Jolivet N, Gallot G, Vivien R, Gaschet J, Thibault G, Vie H. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood. 2006;107(12):4669–4677. doi: 10.1182/blood-2005-09-3775. [DOI] [PubMed] [Google Scholar]
- D’Aloia MM, Caratelli S, Palumbo C, Battella S, Arriga R, Lauro D, Palmieri G, Sconocchia G, Alimandi M. T lymphocytes engineered to express a CD16-chimeric antigen receptor redirect T-cell immune responses against immunoglobulin G-opsonized target cells. Cytotherapy. 2016;18(2):278–290. doi: 10.1016/j.jcyt.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Dahan R, Reiter Y. T-cell-receptor-like antibodies - generation, function and applications. Expert reviews in molecular medicine. 2012;14:e6. doi: 10.1017/erm.2012.2. [DOI] [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6(224):224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological reviews. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE, Posey AD, Jr, Ang S, Cooper LJ, Platt JM, Johnson FB, Paulos CM, Zhao Y, Kalos M, Milone MC, June CH. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer immunology research. 2015;3(4):356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends in molecular medicine. 2012;18(7):377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, Lee J, Posey AD, Jr, Scholler J, Scholler N, Bonneau R, June CH. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124(7):1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, Embleton MJ, Stern PL, Gilham DE. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28(3):203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, Kershaw MH, Smyth MJ, Darcy PK. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100(9):3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- Hombach A, Heuser C, Gerken M, Fischer B, Lewalter K, Diehl V, Pohl C, Abken H. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene therapy. 2000;7(12):1067–1075. doi: 10.1038/sj.gt.3301195. [DOI] [PubMed] [Google Scholar]
- Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene therapy. 2010;17(10):1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. International journal of cancer Journal international du cancer. 2011;129(12):2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1(4):458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(12):2268–2277. doi: 10.1038/mt.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer immunology research. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu P, Yang JC, Cowherd R, Treisman J, Shafer GE, Eshhar Z, Rosenberg SA. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer research. 1995;55(15):3369–3373. [PubMed] [Google Scholar]
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S, Ostberg JR, Forman SJ, Brown CE. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(4):757–768. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20(2):151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nature reviews Cancer. 2013;13(8):525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- Khaleghi S, Rahbarizadeh F, Ahmadvand D, Rasaee MJ, Pognonec P. A caspase 8-based suicide switch induces apoptosis in nanobody-directed chimeric receptor expressing T cells. International journal of hematology. 2012;95(4):434–444. doi: 10.1007/s12185-012-1037-6. [DOI] [PubMed] [Google Scholar]
- Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nature biotechnology. 2013;31(1):71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer research. 2014;74(1):93–103. doi: 10.1158/0008-5472.CAN-13-1365. [DOI] [PubMed] [Google Scholar]
- Kunkele A, Johnson AJ, Rolczynski LS, Chang CA, Hoglund V, Kelly-Spratt KS, Jensen MC. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer immunology research. 2015;3(4):368–379. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]
- Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunological reviews. 2009;227(1):150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ., Jr Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer immunology research. 2013;1(1):43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, Ensser A, Muller YA, Cerwenka A, Abken H, Parolini O, Ambros PF, Kovar H, Holter W. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PloS one. 2012;7(2):e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(20):8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer immunology, immunotherapy : CII. 2012;61(7):953–962. doi: 10.1007/s00262-012-1254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, Zhou L, Loew A, Young RM, June CH, Zhao Y. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer research. 2015;75(17):3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ, Mackall CL. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn RC, Feng Y, Schutsky K, Poussin M, Kalota A, Dimitrov DS, Powell DJ., Jr High-affinity FRbeta-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016 doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, Fonslow BR, Kochenderfer JN, Wright TM, Schultz PG, Young TS, Kim CH, Cao Y. Versatile strategy for controlling the specificity and activity of engineered T cells. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(4):E450–458. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp SA, June CH. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. The EMBO journal. 2004;23(2):255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends in immunology. 2005;26(8):440–446. doi: 10.1016/j.it.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N, Denkberg G, Elbaz Y, Benchetrit F, Eshhar Z, Stauss H, Reiter Y. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol. 2014;193(11):5733–5743. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science translational medicine. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG, Kim CH, Young TS. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(4):E459–468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992;68(5):889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer discovery. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman CL, Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20(2):156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nature reviews Immunology. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, Zhao YY, Zhao LM, Cui J, Gupta P, Wong AJ, Han SY. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. Journal of hematology & oncology. 2013;6:33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ., Jr In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer research. 2011;71(13):4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunology and cell biology. 2013a;91(6):435–440. doi: 10.1038/icb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. 2013b;2(4):e23564. doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer immunity. 2013c;13:8. [PMC free article] [PubMed] [Google Scholar]
- Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, Lopez-Requena A, Pupo A, Johansen RF, Sanchez O, Krengel U, Moreno E. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer research. 2009;69(14):5851–5859. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL, June CH, Wagner JE, Blazar BR, Zhou X. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Human gene therapy. 2010;21(1):75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, Raubitschek A, Forman SJ, Greenberg PD, Riddell SR, Press OW. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Seminars in immunology. 1998;10(6):481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Molecular cancer therapeutics. 2012;11(5):1062–1070. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, Burbridge SE, Box C, Eccles SA, Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. Journal of clinical immunology. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174(12):7853–7858. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015a;350(6258):aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MR, Zhang T, Alcon A, Sentman CL. DNAM-1-based chimeric antigen receptors enhance T cell effector function and exhibit in vivo efficacy against melanoma. Cancer immunology, immunotherapy : CII. 2015b;64(4):409–418. doi: 10.1007/s00262-014-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MR, Zhang T, DeMars LR, Sentman CL. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene therapy. 2015c;22(8):675–684. doi: 10.1038/gt.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer research. 2006;66(11):5927–5933. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106(5):1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wu MR, Sentman CL. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol. 2012;189(5):2290–2299. doi: 10.4049/jimmunol.1103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]