Abstract
Idiopathic pulmonary fibrosis (IPF) is an irreversible lethal lung disease with an unknown aetiology. IPF patient’s lung fibroblasts express inappropriately high Akt activity, protecting them in response to an apoptosis-inducing type I collagen matrix. FasL, a ligand for Fas, is known to be increased in the lung tissues of patients with IPF, implicated with the progression of IPF. Expression of Decoy Receptor3 (DcR3) which binds to FasL, thereby subsequently suppressing the FasL/Fas-dependent apoptotic pathway is frequently altered in various human disease. However, the role of DcR3 in IPF fibroblasts in regulating their viability has not been examined. We found that enhanced DcR3 expression exists in the majority of IPF fibroblasts on collagen matrices, resulting in the protection of IPF fibroblasts from FasL-induced apoptosis. Abnormally high Akt activity suppresses GSK-3β function, thereby accumulating the nuclear factor of activated T-cells c1 (NFATc1) in the nucleus, increasing DcR3 expression in IPF fibroblasts. This alteration protects IPF cells from FasL-induced apoptosis on collagen. However, the inhibition of Akt or NFATc1 decreases DcR3 mRNA and protein levels, which sensitizes IPF fibroblasts to FasL-mediated apoptosis. Furthermore, enhanced DcR3 and NFATc1 expression is mainly present in myofibroblasts in the fibroblastic foci of lung tissues derived from IPF patients. Our results showed that when IPF cells interacted with collagen matrix, aberrantly activated Akt increased DcR3 expression via GSK-3β/NFATc1 and protected IPF cells from the FasL-dependent apoptotic pathway. These findings suggest that the inhibition of DcR3 function may be an effective approach for sensitizing IPF fibroblasts in response to FasL, limiting the progression of lung fibrosis.
Keywords: IPF, DcR3, FasL, Akt, NFATc1, GSK-3β, apoptosis, collagen
Introduction
Idiopathic pulmonary fibrosis (IPF) is a lethal, irreversible fibrotic lung disease [1–3]. IPF fibroblasts maintain their apoptosis-resistant phenotype via inappropriately high PI3K/Akt activities due to PTEN suppression [4–6]. Akt plays a central role in protecting IPF fibroblasts from collagen matrix induced cell death by regulating several downstream proteins [7–9]. A recent study demonstrated that IPF fibroblasts express reduced levels of Fas (CD95) protein, a member of the tumor necrosis factor receptor superfamily (TNFRSF), as a result of an altered Akt-dependent pathway and protecting IPF cells from Fas-dependent apoptosis [5]. Fas ligand (FasL, CD95L) is a tumor necrosis factor (TNF) ligand that binds to Fas receptors, and the FasL/Fas interaction directs diverse biological responses ranging from proliferation to apoptosis [10–15]. In human lung fibroblasts, the binding of FasL to Fas receptor recruits Fas-associated protein with death domain (FADD) to the cytoplasmic tail of Fas, activating a caspase cascade that promotes apoptosis [13,16]. Enhanced FasL levels have been observed in the bronchoalveolar lavage fluid (BALF) and infiltrating lymphocytes of IPF patients, strongly suggesting that the increase in FasL expression is linked to promoting lung fibrosis [15,17]. Like Fas, decoy receptor 3 (DcR3, tumor necrosis factor receptor superfamily member 6B; TNFRSF6B), is a member of the TNFRSF, and it binds and inhibits FasL, suppressing the FasL/Fas-dependent apoptotic pathway [18]. DcR3 is known to be over-expressed in pancreatic, lung, hepatocellular, and colorectal cancers [19–22], and DcR3 expression is negatively correlated with disease specific survival and qualified as an independent prognostic indicator [23,24].
Interestingly, when IPF fibroblasts attach to collagen matrix, DcR3 expression is greatly increased. Therefore, in this study, we examined whether aberrantly high DcR3 expression protects IPF fibroblasts from FasL-dependent apoptosis in response to collagen matrix. We found that Akt inhibition decreases DcR3 expression in IPF cells, which sensitizes them to FasL-induced apoptosis. We further elucidated that enhanced Akt suppresses GSK-3β activity, facilitating the translocation of NFATc1 (a key transcription factor of DcR3), to the nucleus, enhancing DcR3 mRNA levels. Altered DcR3 expression was also found in the cytoplasm and the cytoplasmic membranes of myofibroblasts, and NFATc1 was mainly present in the nuclear or perinuclear regions in fibroblastic foci of lung tissues from IPF patients. Thus, our results show that when IPF fibroblasts interact with collagen matrix, DcR3 expression is greatly increased as a result of an aberrant Akt/GSK-3β/NFATc1 axis, and this pathological alteration contributes to IPF fibroblasts acquiring a resistance to FasL-induced apoptosis. Our study suggests that IPF fibroblasts utilize various mechanisms to protect themselves from cell death inducing conditions, furthering IPF progression.
Materials and methods
Human subjects
Use of human lung tissues was approved by the Institutional Review Board (IRB) at the University of Minnesota. Details are given in Supplementary Materials and Methods.
Preparation of primary lung fibroblasts and collagen matrix
Control and IPF lung fibroblasts derived from lung tissues of non-IPF and IPF patients were generated as described previously [25,26]. We used cells at passages 3 through 7 in the experiments reported here. The collagen matrices were prepared using 80% of type I collagen solution (Advanced BioMatrix, San Diego, CA, USA), 10% 10x DMEM and 10% 1x DMEM, and pH was adjusted to 7.2 with 0.1M NaOH. Cell culture dishes or 96-well plates were coated with collagen and incubated for 3 h at 37°C to solidify.
Reagents and antibodies
FasL (ALX-522-020-C005) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). LY294002 and FK506 were purchased from Cell Signaling (Beverly, MA, USA) and InvivoGen (San Diego, CA, USA), respectively. DcR3 (catalogue No. 4758; 1:1000 dilution), phosphorylated Akt Serine 473 (pAkt; catalogue No. 4060/clone D9E; 1:1000 dilution), Akt (catalogue No. 9272; 1:1000 dilution), NFATc1 (catalogue No. 8032/clone D15F1; 1:1000 dilution), pan-cadherin (catalogue No. 4073/clone 28E12; 1:1000 dilution), phosphorylated GSK-3β Serine 9 (pGSK-3β; catalogue No. 5587/clone D1A3; 1:1000 dilution) and GSK-3β (catalogue No. 9315/clone 27C10; 1:1000 dilution) antibodies were purchased from Cell Signaling (Beverly, MA, USA). GAPDH (catalogue No. SC-25778/clone FL335; 1:2000 dilution) and lamin A/C (catalogue No. MABE 481/clone EP4520; 1:2000 dilution) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Millipore (Bedford, MA, USA), respectively. For immunohistochemistry, DcR3 (catalog No. 333202/clone B08-35; 1:100 dilution) and NFATc1 (catalog No. 649601/clone 7A6; 1:100 dilution) antibodies were purchased from Biolegend (San Diego, CA, USA).
ELISA and Reverse Transcription (RT)-PCR
Control fibroblasts were cultured in wells of a 24-well plate pre-coated with collagen in 200 Ml of serum free DMEM for 48 h. The culture media was then collected and soluble DcR-3 levels were measured using the DcR3 ELISA kit (Biolegend). For RT-PCR, the total RNA from control or IPF fibroblasts (n=8, each) grown on collagen for 48 h was extracted using TRIGent (Biomatik, Cambridge, Canada). The total RNA fraction was then separated by centrifugation at 12,000 x g for 15 min after adding chloroform. The total RNA was precipitated with isopropyl alcohol and washed with 75% ethanol. One microgram of isolated total RNA was subjected to complementary DNA (cDNA) synthesis using Oligo d(T) (Roche Applied Science, Indianapolis, IN, USA), dNTP (QIAGEN, Valencia, CA, USA) and reverse transcriptase (Sigma-Aldrich, St. Louis, MO, USA). PCR analyses were conducted using a Light Cycler 1.5 (Roche Applied Science) under the following reaction conditions: initial denaturation at 95ºC for 10 min, then amplification by 40 cycles of: 95ºC for 15 s; 60ºC for 7 s for DcR3 or 58ºC for 7 s for GAPDH; 72ºC for 13 s. GAPDH was used as a reference gene. Sequences of primers for DcR3 (TNFRSF6B) and GAPDH were described as follows: DcR3 (Forward: 5’-CAG AAA CAC CCA CCT ACC C-3’; Reverse:5’-GTA GTT CCA GAA CTG CGT GT -3’), or GAPDH (Forward: 5’-TTC ATT GAC CTC AAC TAC ATG GT-3’; Reverse:5’-CCT TCT CCA TGG TGG TGA AGA-3’).
Cell binding assay
For the assay, magnetic beads (Bio-Rad, Hercules, CA, USA) were washed with PBST (PBS with 0.1% Tween 20) three times and conjugated with DcR3 antibody (catalog No. 333202, Biolegend) in PBST for 30 min at room temperature. Control and IPF fibroblasts were cultured on collagen for 48 h, and were harvested and resuspended in PBS. The cells were then incubated with prepared magnetic beads conjugated with DcR3 antibody for 3 h at room temperature. Cells were washed with PBS, magnetized, and resuspended in PBS. The cell suspension was diluted 10 times with Isoton II diluent (Beckman Coulter, Brea, CA, USA) and total cell numbers were counted using the Coulter counter ZM (Beckman).
Cell viability assay and Caspase 3/7 activity assay
Details are given in Supplementary Materials and Methods.
Nuclear and cytoplasmic extraction, and the isolation of plasma membrane, adenovirus, siRNA and constructs
Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific, Waltham, MA, USA) were used for these assays. The protein fraction of plasma membranes was separated using a Mem-PER Plus membrane protein Extraction kit (Thermo Scientific) according to the manufacturer’s protocol. For the over-expression or forced inhibition of Akt, control and IPF fibroblasts were infected with 2 × 106 PFU of an adenovirus expressing hyper-active Akt (Hyper Akt) or dominant negative Akt (Akt DA; Vector BioLabs, Eagleville, PA, USA) for 48h, respectively. Adenovirus expressing GFP was used as a control. For the over-expression of GSK-3β or Fas protein, IPF fibroblasts were transfected with a plasmid expressing HA-tagged GSK-3β (GSK-3β-HA, Addgene, Cambridge, MA, USA) or Fas (Sino Biological, Beijing, China) for 24 h using Lipofectamine 3000 (Life technologies, Carlsbad, CA, USA). The plasmids pcDNA3 (Addgene) or pCMV (Sino Biological) were used as controls, respectively. For silencing of DcR3 or GSK-3β, fibroblasts were transfected with 50 nM DcR3, GSK-3β or scrambled siRNA (Santa Cruz Biotechnology) using Lipofectamine 3000. Cells were then cultured on collagen matrix for 48 h, and Western analysis, RT-PCR or caspase 3/7 activity assays were performed.
Immunohistochemistry
Details are given in Supplementary Materials and Methods.
Statistical analysis
Protein expression data were presented as box-and-whisker plots showing the lowest expression, lower quartile, median, upper quartile and the highest expression using SPSS v.19. Bar graphs and scatter plots were prepared using Microsoft Excel. Group data were expressed as the mean ± S.D. and group comparisons between IPF and control were conducted using Student's t-test. The significance levels are presented as *p<0.05, **p<0.01 and ***p<0.001.
Results
DcR3 expression is enhanced in IPF fibroblasts on collagen matrix
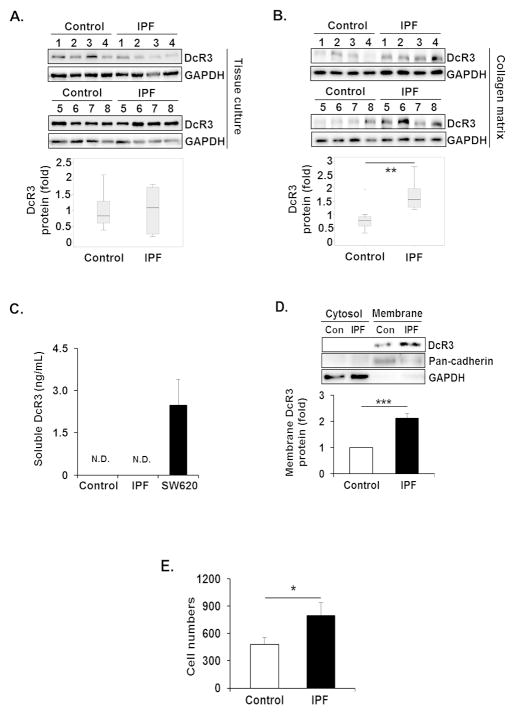
Since DcR3 is known to bind and inhibit FasL, thereby suppressing FasL-induced apoptosis [28], IPF fibroblast desensitization to FasL is possibly due to altered DcR3 expression. Therefore, we first measured the DcR3 protein levels in control and IPF fibroblasts in response to an apoptosis-inducing polymerized type I collagen matrix. When control and IPF fibroblasts were cultured in the absence of collagen matrix, the level of DcR3 protein was relatively similar in both control and IPF fibroblasts (Figure 1A, upper and lower). However, when these cells were cultured on collagen, DcR3 expression was clearly enhanced in the majority of IPF fibroblasts (n=8) compared with that of control fibroblasts (n=8) (Figure 1B, upper). Statistical analysis demonstrated that IPF fibroblasts showed a 2-fold greater DcR3 expression than control fibroblasts (Figure 1B, lower). To confirm that the expression of DcR3 is predominantly increased in IPF fibroblasts on collagen rich matrix, control or IPF fibroblasts were then attached to tissue culture plates and collagen matrix, and DcR3 expression was measured. DcR3 expression was 4.4 fold greater when IPF fibroblasts attached to collagen while the expression of DcR3 was only 1.7 fold increased (Supplementary Figure 1A and B) compared to that from tissue culture plates. These findings showed that DcR3 expression is predominantly increased in IPF cells on collagen, suggesting the possibility that the alteration of DcR3 protein expression participates in protecting IPF fibroblasts from FasL-induced apoptosis.
Figure 1.
DcR3 protein expression is aberrantly high in IPF fibroblasts on collagen matrix. (A) Randomly selected 6 × 105 control and IPF fibroblasts (n=8, each) were grown in tissue culture plates (without polymerized collagen) for 48 h in serum free DMEM. Upper, DcR3 protein expression in control and IPF fibroblasts were examined by Western analysis. Lower, box-and-whisker plots of DcR3 normalized to GAPDH in control and IPF fibroblasts. All values of DcR3 in control and IPF fibroblasts were divided by mean value of DcR3 of the control group to set the average expression of DcR3 in control fibroblasts as 1. (B) The same control and IPF fibroblasts (n=8, each) were grown on polymerized collagen matrix for 48 h in serum free DMEM. Upper, DcR3 protein expression in control and IPF fibroblasts was examined by Western analysis. Lower, box-and-whisker plots of DcR3 normalized to GAPDH in control and IPF fibroblasts. (C) 1 × 105 control and IPF fibroblasts (n=3 each) were cultured on polymerized collagen matrices in 200 Ml of serum free DMEM per well for 48 h. Soluble DcR3 levels were measured by ELISA as described in the Materials and Methods. Human colon cancer cell line, SW620 was used as a positive control. (D) 1.5 × 106 control and IPF fibroblasts (n=4, each) were cultured on polymerized collagen matrices for 48 h in serum free DMEM, and cytosolic and plasma membrane fractions were separated as described in the Materials and Methods. Upper, a representative Western blot for DcR3 protein expression in cytosolic and plasma membrane fractions from control (Con) and IPF fibroblasts. Pan-cadherin and GAPDH were used as plasma membrane and cytosolic protein markers, respectively. Lower, densitometric analysis of DcR3 protein expression on plasma membrane normalized to pan-cadherin. (E) Control (n=3) and IPF fibroblasts (n=4) were cultured on collagen for 48 h in serum free DMEM. Cells were then incubated with magnetic beads conjugated with a DcR3 antibody. Total binding cell number was measured using a Coulter counter. Control fibroblasts expressing low levels of DcR3 protein and IPF fibroblasts expressing high levels of DcR3 were selected for Figure 1C–E. *p<0.05, **p<0.01 and ***p<0.001. N.D. non-detected.
Membrane bound DcR3 is found in IPF fibroblasts on collagen matrix
DcR3 is known to exist in predominantly a soluble form [29,30] but also found in membrane associated forms [31]. Therefore, soluble and membrane bound forms of DcR3 were measured in control and IPF fibroblasts on collagen. Soluble DcR3 was not detected in medium from either control or IPF fibroblasts on collagen matrix (Figure 1C). However, enhanced membrane bound DcR3 was mainly found in IPF fibroblasts while cytoplasmic DcR3 was absent or very low in both cells (Figure 1D). To confirm, these findings control and IPF fibroblasts cultured on collagen matrix were incubated with magnetic beads conjugated with DcR3 antibody, and the number of binding cells was measured. IPF fibroblasts showed significantly increased binding cell number compared to control fibroblasts (Figure 1E). Collectively, these results showed that when IPF cells interacted with collagen, DcR3 was highly expressed in a membrane-bound form.
PI3K/Akt and NFATc1 regulate DcR3 expression in IPF fibroblasts on collagen matrix
Previous studies showed that the PI3K/Akt axis is associated with the regulation of DcR3 protein expression [32,33]. Therefore, IPF fibroblasts were first treated with various doses of a PI3K inhibitor, LY294002, and DcR3 protein levels were measured. DcR3 expression progressively decreased in the presence of increasing doses of LY294002 in IPF fibroblasts (Figure 2A). Likewise, Akt inhibition by dominant negative Akt (Akt DA) also decreased DcR3 expression in IPF fibroblasts compared to that of cells infected with virus expressing empty vector (GFP; Figure 2B). To confirm our findings, control lung fibroblasts were then infected with adenovirus expressing hyper-active Akt (Hyper Akt), and DcR3 levels were measured. As shown in Figure 2C, DcR3 protein expression was increased in control fibroblasts by hyperactive Akt compared to that of empty vector infection (GFP). Collectively, these results demonstrated that the PI3K/Akt axis is responsible for the regulation of DcR3 protein expression.
Figure 2.
Aberrantly high PI3K and Akt regulate DcR3 expression in IPF fibroblasts on collagen matrix. (A) IPF fibroblasts (n=3) were plated on collagen matrices for 24 h, then cells were treated with LY294002 for an additional 24 h. DMSO was used as a vehicle control (VH). Upper, representative Western analysis of DcR3, pAkt, pGSK3β, Akt, GSK3β, and GAPDH protein expression after LY294002 treatment. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. (B) IPF fibroblasts (n=3) infected with adenovirus expressing dominant negative Akt (Akt DA) or empty vector (GFP) were cultured on polymerized collagen matrices for 48 h. Upper, representative Western blot of DcR3, Akt DA, pGSK3β, pAkt, GSK3β, and GAPDH protein expression under the condition as described above. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. GAPDH for DcR3 expression was shown in the lower Western blot panel. (C) Control fibroblasts (n=3) infected with adenovirus expressing hyper active Akt (Hyper Akt) or GFP were plated on collagen matrices for 48 h. Upper, representative Western blot of DcR3, pAkt, pGSK3β, Akt, GSK3β, and GAPDH protein expression. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. Values are the average of three cell lines, presented as relative fold change ± S.D. against VH or GFP group for individual fibroblasts set as 1 fold. IPF fibroblasts expressing high DcR3 protein and control fibroblasts expressing low levels of DcR3 protein were selected for Figure 2A and B, and Figure 2C, respectively. (D and E) Control and IPF fibroblasts shown in Figure 1 (n=8 each) were cultured on tissue culture plates (D) or on collagen matrix (E) for 48 h. Upper, pGSK-3β, pAkt, and GAPDH protein expressions in control and IPF fibroblasts were examined by Western analysis. Lower, box-and-whisker plots of pGSK-3β and pAkt after normalization to GAPDH in control and IPF fibroblasts by densitometric analysis. *p≤0.05, **p<0.01 and ***p<0.001.
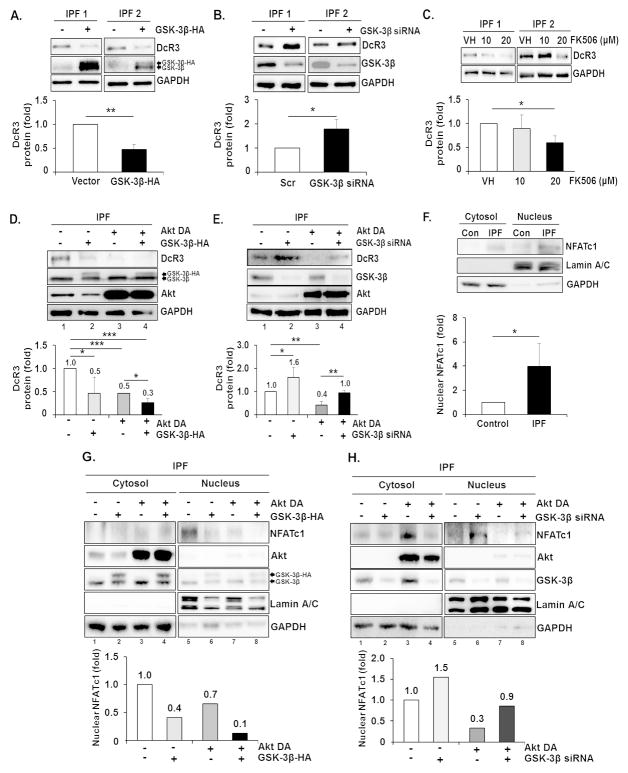
A key transcription factor for DcR3 is nuclear factor of activated T cells (NFATc1). Phosphorylation of NFATc1 by GSK-3β enhances NFATc1 localization to the cytoplasm from the nucleus, resulting in suppression of DcR3 gene expression [33]. Furthermore, enhanced Akt inhibits GSK-3β activity by phosphorylating the Ser 9 residue [34–36]. Thus, DcR3 up-regulation in IPF fibroblasts is possibly due to an altered Akt/GSK-3β/NFATc1 axis. To test this, we next examined GSK-3β activity in control and IPF fibroblasts in the absence or presence of collagen matrix. Overall, pGSK-3β (Ser 9) and pAkt levels were relatively lower in IPF fibroblasts compared to control fibroblasts in the absence of collagen matrix (Figure 2D, upper and lower). However, when IPF fibroblasts were cultured on collagen, the majority of IPF fibroblasts expressed enhanced pGSK-3β and pAkt (Figure 2E, upper and lower). To confirm the function of GSK-3β on DcR3 regulation, GSK-3β was then over-expressed or silenced in IPF fibroblasts, and DcR3 protein expression was measured. While DcR3 expression was decreased by GSK-3β over-expression (Figure 3A), GSK-3β siRNA increased DcR3 protein expression (Figure 3B). These findings showed that enhanced DcR3 expression is due to GSK-3β suppression as a result of Akt activation in IPF fibroblasts on collagen matrix. Next, we examined whether NFATc1 is involved with DcR3 expression by using the NFATc1 inhibitor, FK506, and found that DcR3 protein expression was significantly reduced when cells were treated with 20 MM of FK506 (Figure 3C). These results suggest that activated NFATc1 enhances DcR3 expression in IPF fibroblasts, due to reduced GSK-3β function by inappropriately high Akt activity. To test this, we next over-expressed GSK-3β in IPF fibroblasts infected with a virus that expressed dominant negative Akt (Akt DA) on collagen matrix, and DcR3 protein levels were measured. DcR3 expression was suppressed by the over-expression of GSK-3β (lane 2) or Akt DA (lane 3) compared to that of cells treated with GFP and empty vector (Figure 3D, lane 1, upper and lower). Moreover, DcR3 expression was further suppressed when IPF fibroblasts were treated with both GSK-3β over-expression and Akt DA compared to individual treatments of each (Figure 3D, lane 4, upper and lower). In contrast, the silencing of GSK-3β in IPF fibroblasts over-expressing dominant negative Akt partially restored DcR3 protein levels compared with levels when Akt was inhibited (Figure 3E, lane 4 and 3, respectively). These findings demonstrate that the Akt/GSK-3β axis contributes to the regulation of DcR3 expression at least in part when IPF fibroblasts interact with collagen rich matrix, and the alteration of this axis is responsible for aberrant DcR3 expression.
Figure 3.
Translocation of NFATc1 into the nucleus by Akt/GSK-3β axis is important for DcR3 expression. (A) IPF fibroblasts (n=3) were transfected with empty vector (−) or plasmid expressing HA tagged GSK-3β (+) and subsequently cultured on polymerized collagen matrices for 48 h. Upper, Representative Western blot of DcR3 and GSK-3β protein expression. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH after GSK-3β-HA over-expression. (B) IPF fibroblasts (n=3) transfected with scrambled (−) or GSK-3β siRNA (+) were cultured on polymerized collagen matrices for 48h. Upper, representative Western blot of DcR3, GSK-3β and GAPDH protein expression under the condition as described above. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH after GSK-3β siRNA treatment. (C) IPF fibroblasts (n=3) were plated on collagen matrices for 24 h, and cells were incubated for an additional 24 h in the presence of various doses of FK506 as described. DMSO was used as a vehicle control (VH). Upper, representative Western blot of DcR3 protein expression. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. (D) IPF fibroblasts (n=3) infected with adenovirus expressing Akt DA or GFP were transfected with empty vector or GSK-3β-HA and cultured on collagen matrices for 48 h. Upper, representative Western blot of DcR3, Akt and GSK-3β protein expression. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. Values are presented as relative fold change ± S.D. against the control group set as 1 fold. (E) IPF fibroblasts (n=3) infected with adenovirus expressing Akt DA or GFP were transfected with scrambled or GSK-3β siRNA and cultured on collagen matrices for 48 h. Upper, representative Western blot of DcR3, Akt and GSK-3β protein expression under the conditions as described above. Lower, densitometric analysis of DcR3 protein expression normalized to GAPDH. IPF fibroblasts expressing high levels of DcR3 protein were chosen for Figure 3A–E. (F) 1.5 × 106 control and IPF fibroblasts (n=3 each) were cultured on polymerized collagen matrices for 48 h, and the cytosolic and nuclear fractions were separated as described in the Materials and Methods. Upper, representative Western analysis of NFATc1. Lower, densitometric analysis of NFATc1 expression in the nuclear fraction. Values are presented as relative fold values ± S.D. against NFATc1 expression of control fibroblasts set as 1 fold after lamin A/C normalization. Control fibroblasts expressing low levels of DcR3 protein and IPF fibroblasts expressing high levels of DcR3 protein were selected for this experiment. (G) Upper, representative Western analysis of NFATc1 expression in the cytoplasm and the nucleus of IPF fibroblasts over-expressing Akt DA and/or GSK-3β-HA on collagen matrix at 48 h. Lower, densitometric analysis of NFATc1 expression in the nuclear fraction. Values are presented as relative fold values ± S.D. against control treatment (GFP and pcDNA3 over-expression) set as 1 fold after lamin A/C normalization. (H) Upper, representative Western blot of NFATc1 expression in the cytoplasm and the nucleus of IPF fibroblasts over-expressing Akt DA following GSK3β siRNA transfection. Lower, densitometric analysis of NFATc1 expression in the nuclear fraction. IPF fibroblasts expressing high levels of DcR3 protein were selected for G and H. Lamin A/C and GAPDH were used as nuclear and cytosolic protein markers, respectively. *p≤0.05, **p≤0.01 and ***p<0.001.
NFATc1 is localized to the nucleus of IPF cells by Akt/GSK-3β
We next investigated whether NFATc1 is mainly located in the nucleus of IPF fibroblasts on collagen matrix due to GSK-3β suppression. NFATc1 was found more predominantly in the nucleus of IPF fibroblasts compared to that of control fibroblasts (Figure 3F). These results suggest that IPF fibroblasts activate the NFATc1-dependent signaling pathway by facilitating its localization into the nucleus by GSK-3β inhibition via Akt activation. To confirm this, we altered GSK-3β and/or Akt functions, and measured NFATc1 localization in IPF fibroblasts on collagen. Akt inhibition by dominant negative Akt (Akt DA, Figure 3G, lane 7) or GSK-3β over-expression (GSK-3β-HA, lane 6) clearly decreased nuclear NFATc1. Furthermore, when GSK-3β and Akt DA were co-expressed, nuclear NFATc1 localization was further suppressed (Figure 3G, lane 8 upper and lower). Unlike this observation, enhanced NFATc1 was localized to the nucleus when Akt DA was overexpressed in IPF fibroblasts in which GSK-3β was silenced (Figure 3H, lane 8 upper and lower). Taken together, these results demonstrate that Akt regulates NFATc1 localization between the cytosol and nucleus via GSK-3β, and the suppression of GSK-3β activity as a result of high Akt activity in IPF fibroblasts increased the localization of NFATc1 to the nucleus.
DcR3 mRNA is highly up-regulated in IPF fibroblasts
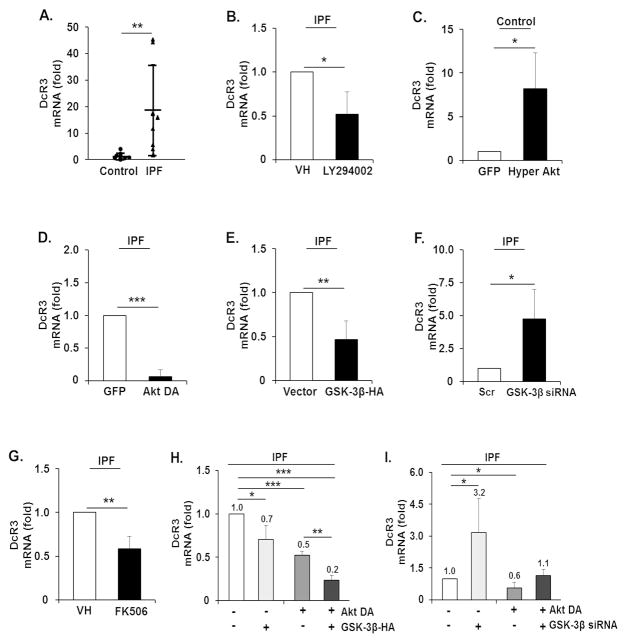
We next examined if the enhanced DcR3 expression is regulated by the PI3K/Akt/GSK-3β/NFATc1 axis at the mRNA level. We initially measured DcR3 mRNA levels (relative to GAPDH) in control and IPF cells (n=8 each) on collagen. The DcR3 mRNA level was significantly higher in IPF fibroblasts compared to that of control cells (Figure 4A). IPF fibroblasts on collagen matrix were then treated with a PI3K inhibitor LY294002, and DcR3 mRNA levels were measured. DcR3 levels were lower in response to LY294002 compared to that of the vehicle control (VH, Figure 4B). The role of Akt in regulating DcR3 mRNA levels was also measured in control fibroblasts by the activation of Akt using hyper-active Akt (Hyper Akt). DcR3 mRNA levels were increased in control fibroblasts by the enhancement of Akt function (Figure 4C). In contrast, forced inhibition of Akt by dominant negative Akt (Akt DA) in IPF fibroblasts produced a significant decrease in DcR3 mRNA levels (Figure 4D). To test the role of GSK-3β in regulating DcR3 mRNA levels, GSK-3β was also over-expressed in IPF fibroblasts, and DcR3 mRNA levels were measured. DcR3 mRNA levels were decreased by GSK-3β over-expression compared to that of cells transfected with a control vector, pcDNA3 (Figure 4E). However, GSK-3β silencing by GSK-3β siRNA in IPF fibroblasts showed an enhanced DcR3 mRNA level compared to that of cells transfected with the scrambled siRNA (Figure 4F). In contrast, when NFATc1 function was inhibited by FK506, DcR3 mRNA levels decreased in IPF fibroblasts (Figure 4G). In order to elucidate the role of Akt/GSK-3β axis in regulating DcR3 mRNA levels, DcR3 mRNA levels in IPF fibroblasts expressing Akt DA and GSK-3β-HA were measured. DcR3 mRNA levels were further suppressed in the presence of Akt DA and GSK-3β-HA (Figure 4H). In contrast, DcR3 mRNA levels remained low when Akt was inhibited, but when GSK-3β was silenced in IPF fibroblasts over-expressing dominant negative Akt, DcR3 mRNA levels were partially increased (Figure 4I, lane 4). Collectively, these results demonstrate that when IPF fibroblasts attach to collagen matrix, enhanced NFATc1 localization in the nucleus due to Akt/GSK-3β increases DcR3 mRNA levels.
Figure 4.
Enhanced DcR3 mRNA levels in IPF fibroblasts are due to the alteration of PI3K/Akt/GSK-3β/NFATc1. (A) 2 × 105 control and IPF fibroblasts used in previous experiments (n=8, each) were grown on collagen matrices for 48 h, and RT-PCR analysis was performed to examine DcR3 mRNA levels. On the scatterplot, each circle and triangle point represents the fold change of normalized DcR3 mRNA from control and IPF fibroblasts, respectively. (B) IPF fibroblasts (n=3) cultured on polymerized collagen were treated with a PI3K inhibitor, LY294002, at 20 MM for 24 h. DMSO was used as a vehicle control (VH). Values are presented as relative fold change ± S.D. against VH set as 1 fold. (C) Control fibroblasts (n=3) infected with adenovirus expressing hyper Akt or GFP were cultured on polymerized collagen matrices for 48 h. (D) IPF fibroblasts (n=3) expressing Akt DA or GFP were cultured on polymerized collagen matrices for 48 h. DcR3 mRNA levels are presented as a relative fold change ± S.D. against GFP set as 1 after GAPDH normalization in C and D. (E) IPF fibroblasts (n=3) transfected with empty vector (pcDNA3) or GSK-3β-HA were cultured on polymerized collagen matrix for 48 h. DcR3 mRNA levels are presented as a fold change ± S.D. against individual empty vector groups set as 1 fold after GAPDH normalization. (F) IPF fibroblasts (n=3) transfected with scrambled (Scr) or GSK-3β siRNA were cultured on polymerized collagen matrix for 48 h. DcR3 mRNA levels are presented as a fold change ± S.D. against the individual Scr group set as 1 fold after GAPDH normalization. (G) IPF fibroblasts (n=3) cultured on polymerized collagen matrix were incubated with 20 MM of FK506 or DMSO (VH) for 24 h. DcR3 mRNA levels are presented as a relative fold change ± S.D. against VH set as 1 fold after GAPDH normalization. (H and I) DcR3 mRNA levels in IPF fibroblasts (n=3) infected with adenovirus expressing Akt DA followed by GSK-3β-HA transfection (H), or with GSK-3β siRNA (I). DcR3 mRNA levels are presented as a fold change ± S.D. against an individual control group set as 1 after GAPDH normalization. IPF fibroblasts expressing increased levels of DcR3 mRNA were used in Figure 4B, D, E, F, G, H, and I, and control fibroblasts showing low levels of DcR3 mRNA were used in Figure 4C. GAPDH mRNA was used as a reference transcript. *p<0.05, **p<0.01 and ***p<0.001.
IPF fibroblasts utilize DcR3 to protect them from FasL-induced cell death via Akt/GSK-3β/NFATc1
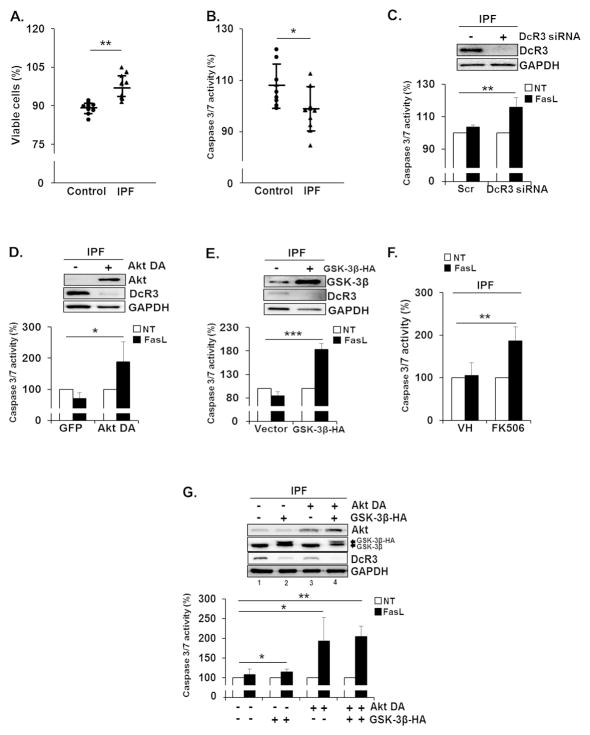
To measure whether enhanced DcR3 expression can protect IPF fibroblasts from FasL-induced apoptosis, cell viability and caspase 3/7 activity in control and IPF fibroblasts on collagen matrix were measured in the presence of FasL. As shown in Figure 5A and B, IPF fibroblasts showed reduced cell death and caspase 3/7 activity compared to control fibroblasts in response to FasL, suggesting that increased levels of DcR3 protected IPF fibroblasts from FasL-induced apoptosis. To confirm the protective role of DcR3, DcR3 was then silenced using siRNA, and caspase 3/7 activity was measured in IPF fibroblasts in response to FasL. Enhanced caspase 3/7 activity was observed in IPF fibroblasts in the presence of DcR3 siRNA compared to those treated with scrambled siRNA (Figure 5C). This finding showed evidence that the presence of DcR3 protects IPF fibroblasts from FasL-induced apoptosis. To further test the role of the Akt/GSK-3β/NFATc1 axis for mitigating apoptosis via DcR3, we next inhibited Akt activity using a dominant negative Akt (Akt DA), and caspase 3/7 activity was measured in the presence of FasL. Caspase 3/7 activity increased when Akt activity was inhibited in IPF fibroblasts (Figure 5D) in response to FasL. Likewise, GSK-3β over-expression or the treatment with NFATc1 inhibitor FK506 augmented caspase 3/7 activity in IPF fibroblasts in response to FasL, compared with that of each control group (Figure 5E and F, respectively). Furthermore, caspase 3/7 activity was greatly increased when Akt DA and GSK-3β-HA were co-expressed in IPF fibroblasts (Figure 5G, lane 4, lower). Since Fas suppression is also thought to be linked to the resistance to FasL-induced apoptosis, and Fas protein is aberrantly low in IPF fibroblasts on collagen matrix (5), we next measured whether the increase in Fas protein sensitizes IPF fibroblasts to FasL-induced apoptosis on collagen. As we expected, enhanced apoptosis was seen when Fas protein was over-expressed in IPF fibroblasts. Furthermore, caspase 3/7 activity was increased when DcR3 was also silenced under this condition (Supplementary Figure 2A and B). Importantly, when Fas was over-expressed in IPF fibroblasts treated with DcR3 siRNA, the fewest viable IPF fibroblasts were found (Supplementary Figure 2C). Our data suggests that IPF fibroblasts are likely to utilize various mechanisms that effectively protect them from FasL-driven apoptosis. Taken together, our results demonstrated that DcR3 suppression is Akt/GSK-3β-dependent, and the inappropriately high Akt increases DcR3 expression via suppression of GSK-3β activity, localizing NFATc1 to the nucleus, thereby protecting IPF cells from FasL-induced apoptosis on collagen.
Figure 5.
Enhanced DcR3 expression is responsible for the resistance of IPF fibroblasts to FasL-mediated apoptosis. 1 × 104 control and IPF fibroblasts (n=8 each) used in previous experiments were grown on collagen for 48 h and treated with 500 (A) or 200 ng/ml of FasL (B) for an additional 24 h. Cell viability and caspase 3/7 activity were measured as described in the Materials and Methods. On the scatterplot, each circle and triangle point represents the percentage of viability (A) or caspase 3/7 activity (B) of each control and IPF cell in response to FasL. (C) IPF fibroblasts (n=3) transfected with scrambled or DcR3 siRNA were cultured on collagen matrix for 48 h. Upper, shown is the representative DcR3 protein expression in IPF fibroblasts used for the caspase 3/7 activity assay. Lower, caspase 3/7 activity in IPF fibroblasts was examined at 8 h in the presence of 200 ng/ml of FasL. (D) IPF fibroblasts (n=3) expressing Akt DA or GFP were cultured on collagen matrix for 48 h. Upper, shown is the representative Akt DA and DcR3 protein expression in IPF cells used for the measurement of caspase 3/7 activity. Lower, caspase 3/7 activity was measured at 8 h after FasL treatment. (E) IPF fibroblasts (n=3) over-expressing empty vector (pcDNA3) or GSK-3β-HA were cultured on collagen matrix for 48 h. Upper, shown is the representative GSK-3β and DcR3 protein expression in IPF cells used for the caspase 3/7 activity assay. Lower, caspase 3/7 activity was measured as described above. (F) IPF fibroblasts (n=3) grown on polymerized collagen matrix for 24 h were incubated with 20 MM of FK506 or DMSO (VH) for 24 h. Caspase 3/7 activity was measured at 8 h after FasL treatment. (G) IPF fibroblasts (n=3) expressing GFP or Akt DA were transfected with a construct expressing empty pcDNA3 vector or GSK-3β-HA and cultured on collagen matrix for 48 h. Upper, shown is the representative Western analysis of Akt, GSK-3β, DcR3, and GAPDH protein expression used for the caspase 3/7 activity assay. Lower, caspase 3/7 activity was measured at 8 h after FasL treatment as described above. Values given in Figure 5C–G were expressed as the percentage against their respective non-treatment groups, set at 100%. For experiments shown in Figure 5C–G, IPF fibroblasts expressing enhanced DcR3 protein expression were selected. *p<0.05, **p<0.01 and ***p<0.001.
DcR3 expression is high in IPF patient lung tissues
Given the fact that DcR3 is regulated by NFATc1, we next examined DcR3 and NFATc1 protein expression in lung tissues from IPF patients. Enhanced DcR3 expression was found in the region of the cytoplasm and potentially in the cytoplasmic membrane of myofibroblasts in the fibroblastic foci of patient tissues compared to that of control lung alveoli (Figure 6A and Supplementary Figure 3A). Additionally, when we examined NFATc1 expression in both IPF and control lung tissues, NFATc1 proteins were mainly observed in the cytosol of control lung tissues while most NFATc1 was found in the peri-nuclear or nuclear fraction of IPF lung tissues (Figure 6B, and Supplementary Figure 3B). Collectively, these results suggest that myofibroblasts found in IPF patients are likely to be resistant to FasL-induced apoptosis due to an enhanced DcR3 level resulting from increased NFATc1 activity.
Figure 6.
Enhanced DcR3 and NFATc1 expression in IPF patient lung tissues. Human lung tissues derived from non-IPF (Control) and IPF patients (n=3 each) embedded in paraffin were placed on slides and incubated with DcR3 (A) or NFATc1 (B) antibody solution as described in the Supplementary Materials and Methods. The images were obtained by microscopy at 40 X magnification. Scale bars represent 50 Mm.
Discussion
Lung injury, primarily of epithelial cells by external and internal insults, is believed to trigger the fibrotic process, and FasL is highly expressed in recruited lymphocytes and alveolar epithelial cells, accelerating alveolar tissue damages [17]. FasL is elevated in BALF in patients with IPF [15,17], suggesting that the alteration of FasL expression is associated with the progression of lung fibrosis by augmenting tissue damage. We found previously that when control fibroblasts were cultured on collagen, Fas levels were increased whereas the expression of Fas protein was abnormally low in IPF fibroblasts [5]. This pathological alteration is thought to cause normal fibroblasts to become highly susceptible to FasL-mediated cell death, while IPF fibroblasts bearing reduced Fas protein levels become resistant to the FasL-mediated apoptotic pathway [37,38]. In this study, we further elucidated that activated Akt increases the localization of NFATc1 in the nucleus, and increased DcR3 mRNA levels, which neutralizeD the FasL effect, protecting them from FasL/Fas-mediated cell death on collagen rich matrix. We found that with low levels of DcR3 control fibroblasts are susceptible to FasL-induced cell death while IPF fibroblasts express increased levels of DcR3 protein, protecting them from FasL-mediated apoptosis. Our study confirmed that aberrantly activated Akt is a critical kinase to participate in conferring an IPF fibroblast phenotype. We previously found that FoxO3a (an Akt down-stream protein) and its target molecules Bim, p27, and p21 are suppressed when IPF cells are cultured on collagen, and this alteration causes IPF fibroblasts to become viable and proliferative [39]. Furthermore, the FoxO3a target gene caveolin-1 (CAV1) is also suppressed, reducing Fas expression, thereby inhibiting Fas-mediated apoptosis in IPF cells [5]. Likewise, autophagic activity is suppressed in IPF cells due to up-regulated Akt and mTORC1 kinases [7,40]. Thus, these findings consistently show that IPF cells do not sense collagen matrix as a stress inducing condition via altered Akt, and they can maintain their abnormal properties in response to this unfavorable environment.
In this study, we have shown that IPF fibroblasts also utilize a DcR3-dependent mechanism when exposed to FasL-rich environments. Enhanced DcR3 is found mainly as a membrane bound form when IPF fibroblasts interact with collagen matrix. It is thought that the soluble or membrane bound form of DcR3 may have different pathological effects. For example, when DcR3 exists as soluble or circulating form, it can inhibit the function of FasL that can potentially bind to themselves or any neighboring cells that express Fas protein. However, in case of the membrane bound form, the inhibition of FasL-induced apoptosis by DcR3 is rather specific to cells expressing DcR3. Thus, membrane-bound DcR3 may be a direct indicator that IPF fibroblasts with abnormally high DcR3 protein protect them from the FasL-mediated apoptotic effect. Based on DcR3’s unique pathological functions, DcR3 has been thought to be a prognostic indicator of certain types of cancer. For example, DcR3 is a prognostic factor for renal carcinoma [24] and gastrointestinal cancer [41]. Since there has been limited knowledge available for finding pathologically relevant proteins over-expressed in IPF fibroblasts, these studies also suggest a possible role of DcR3 as a diagnostic and/or prognostic indicator for IPF. Perhaps, identification and the characterization of additional protein markers along with DcR3 can potentially be useful for the precise diagnosis of IPF. Although our results showed that the aberrant Akt/GSK-3β/NFATc1 signalling pathway causes the enhanced DcR3 expression in IPF fibroblasts, we still cannot rule out the possibility that additional mechanisms may also exist for the abnormal DcR3 expression. Based on the fact that there is a variation in DcR3 expression in IPF fibroblasts we examined, these results suggest that the pathological mechanism we have elucidated is not ubiquitous. Thus, our findings further support the notion that IPF fibroblasts effectively utilize various mechanisms to maintain their persistent phenotype in response to apoptosis inducing environments.
In summary, we showed that abnormal expression of DcR3 participates in the protection of IPF fibroblasts from FasL-dependent apoptosis via the PI3K/Akt/GSK-3β/NFATc1-depedent axis. Based on our results, it may be a feasible and novel approach that the neutralization or inhibition of DcR3 in IPF cells can increase their sensitization to FasL-dependent cell death, limiting the progression of IPF.
Supplementary Material
Acknowledgments
The authors appreciated Mark S. Peterson and Scott M. Miller for their technical and helpful support for the cell binding assay, Western analysis and nuclear and membrane fraction isolation. This work was supported by the National Heart, Lung and Blood Institute Grant R01 HL114662 (RN).
Abbreviations
- AKT
v-AKT murine thymoma viral oncogene homolog 1
- BALF
bronchoalveolar lavage fluid
- Bim
Bcl2 interacting mediator of cell death
- Akt DA
adenovirus expressing dominant negative Akt
- DcR3
decoy receptor3
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- FADD
Fas associated protein with death domain
- Fas
Fas receptor
- FasL
Fas ligand
- FoxO
forkhead box O
- GFP
green fluorescent protein
- GSK-3β
glycogen synthase kinase-3β
- HA
haemagglutinin
- Hyper Akt
adenovirus expressing hyper active Akt
- IPF
idiopathic pulmonary fibrosis
- mTORC1
mammalian target of rapamycin complex 1
- NFAT
nuclear factor of activated T-cells
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homologue
- siRNA
small interfering RNA
- TNFSF
tumor necrosis factor receptor superfamily
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
Author contributions
JI and RSN participated in the study design and analyzed data. JI, KK and PH performed experiments. JI, KK, PH and RSN wrote the manuscript.
References
- 1.Gharaee-Kermani M, Gyetko MR, Hu B, et al. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res. 2007;24:819–841. doi: 10.1007/s11095-006-9216-x. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu JH, Colby TV, Hartman TE. Idiopathic pulmonary fibrosis: current concepts. Mayo Clin Proc. 1998;73:1085–1101. doi: 10.4065/73.11.1085. [DOI] [PubMed] [Google Scholar]
- 4.Nho RS, Hergert P, Kahm J, et al. Pathological alteration of FoxO3a activity promotes idiopathic pulmonary fibrosis fibroblast proliferation on type I collagen matrix. Am J Pathol. 2011;179:2420–2430. doi: 10.1016/j.ajpath.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nho RS, Peterson M, Hergert P, et al. FoxO3a (Forkhead Box O3a) deficiency protects Idiopathic Pulmonary Fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS One. 2013;8:e61017. doi: 10.1371/journal.pone.0061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia H, Khalil W, Kahm J, et al. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol. 2010;176:2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One. 2014;9:e94616. doi: 10.1371/journal.pone.0094616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura M, Gu J, Danen EH, et al. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 9.Tian B, Lessan K, Kahm J, et al. Beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277:24667–24675. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams VM, Straszewski-Chavez SL, Guller S, et al. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 11.Brint E, O'Callaghan G, Houston A. Life in the Fas lane: differential outcomes of Fas signaling. Cell Mol Life Sci. 2013;70:4085–4099. doi: 10.1007/s00018-013-1327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Park SM, Tumanov AV, et al. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel SK, Cosgrove GP, Cha SI, et al. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagimoto N, Kuwano K, Miyazaki H, et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 15.Kuwano K, Kawasaki M, Maeyama T, et al. Soluble form of fas and fas ligand in BAL fluid from patients with pulmonary fibrosis and bronchiolitis obliterans organizing pneumonia. Chest. 2000;118:451–458. doi: 10.1378/chest.118.2.451. [DOI] [PubMed] [Google Scholar]
- 16.Moodley YP, Caterina P, Scaffidi AK, et al. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–495. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano K, Miyazaki H, Hagimoto N, et al. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol. 1999;20:53–60. doi: 10.1165/ajrcmb.20.1.2941. [DOI] [PubMed] [Google Scholar]
- 18.Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 19.Bai C, Connolly B, Metzker ML, et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci U S A. 2000;97:1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen HW, Gao SL, Wu YL, et al. Overexpression of decoy receptor 3 in hepatocellular carcinoma and its association with resistance to Fas ligand-mediated apoptosis. World J Gastroenterol. 2005;11:5926–5930. doi: 10.3748/wjg.v11.i38.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji S, Hosotani R, Yonehara S, et al. Endogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinoma. Int J Cancer. 2003;106:17–25. doi: 10.1002/ijc.11170. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Han B, Sheng H, et al. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 23.Ho CH, Chen CL, Li WY, et al. Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis. 2009;30:1443–1451. doi: 10.1093/carcin/bgp135. [DOI] [PubMed] [Google Scholar]
- 24.Macher-Goeppinger S, Aulmann S, Wagener N, et al. Decoy receptor 3 is a prognostic factor in renal cell cancer. Neoplasia. 2008;10:1049–1056. doi: 10.1593/neo.08626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia H, Diebold D, Nho R, et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia H, Nho RS, Kahm J, et al. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 27.Xia H, Seeman J, Hong J, et al. Low alpha(2)beta(1) integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the beta-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth W, Isenmann S, Nakamura M, et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- 29.Connor JP, Felder M, Kapur A, et al. DcR3 binds to ovarian cancer via heparan sulfate proteoglycans and modulates tumor cells response to platinum with corresponding alteration in the expression of BRCA1. BMC Cancer. 2012;12:176. doi: 10.1186/1471-2407-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan C, Patskovsky Y, Yan Q, et al. Decoy strategies: the structure of TL1A:DcR3 complex. Structure. 2011;19:162–171. doi: 10.1016/j.str.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Zhang C, Chen C, et al. Correlation between expression of DcR3 on tumor cells and sensitivity to FasL. Cell Mol Immunol. 2007;4:455–460. [PubMed] [Google Scholar]
- 32.Chen PH, Yang CR. Decoy receptor 3 expression in AsPC-1 human pancreatic adenocarcinoma cells via the phosphatidylinositol 3-kinase-, Akt-, and NF-kappa B-dependent pathway. J Immunol. 2008;181:8441–8449. doi: 10.4049/jimmunol.181.12.8441. [DOI] [PubMed] [Google Scholar]
- 33.Weissinger D, Tagscherer KE, Macher-Goppinger S, et al. The soluble Decoy Receptor 3 is regulated by a PI3K-dependent mechanism and promotes migration and invasion in renal cell carcinoma. Mol Cancer. 2013;12:120. doi: 10.1186/1476-4598-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 35.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mottet D, Dumont V, Deccache Y, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 37.Buhling F, Wille A, Rocken C, et al. Altered expression of membrane-bound and soluble CD95/Fas contributes to the resistance of fibrotic lung fibroblasts to FasL induced apoptosis. Respir Res. 2005;6:37. doi: 10.1186/1465-9921-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynes MW, Edelman BL, Kostyk AG, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 39.Nho RS, Im J, Ho YY, et al. MicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am J Physiol Lung Cell Mol Physiol. 2014;307:L632–642. doi: 10.1152/ajplung.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im J, Hergert P, Nho RS. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am J Physiol Lung Cell Mol Physiol. 2015;309:L552–561. doi: 10.1152/ajplung.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong J, Ao R, Wang Y, et al. Prognostic and clinicopathological differences of DcR3 in gastrointestinal cancer: evidence from meta-analysis. Int J Clin Exp Med. 2014;7:3096–3105. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.