Abstract
Psychopathic offenders are described as emotionally cold, displaying deficits in affective responding. However, research demonstrates that many of the psychopathy-related deficits are moderated by attention, such that under conditions of high attentional and perceptual load psychopathic offenders display deficits in affective responses, but do not in conditions of low load. To date, most studies use measures of defensive reflex (i.e., startle) and conditioning manipulations to examine the impact of load on psychopathy-related processing, but have not examined more direct measures of attention processing. In a sample of adult male offenders, the present study examined time-frequency EEG phase coherence in response to a picture-viewing paradigm that manipulated picture familiarity to assess neural changes in processing based on perceptual demands. Results indicated psychopathy-related differences in the theta response, an index of readiness to perceive and integrate sensory information. These data provide further evidence that psychopathic offenders have disrupted integration of sensory information.
Keywords: Time Frequency, Psychopathy, Sensory Integration, Perception, Emotion
Prominent models of psychopathy attribute these offenders’ failures of conscience, antisocial behavior, and insensitivity to affective information to a core emotion deficit. However, substantial evidence indicates that experimental context moderates these emotion deficits. Baskin-Sommers and colleagues (2011) propose that this context specificity is associated with an early attention bottleneck that filters multidimensional information in serial, rather than simultaneously, thus hindering the fluid processing of information. Across experimental contexts, psychopathic offenders display normal responses to affective information when it is part of their goal-directed task or embedded in a perceptually simple display, yet their reactions to the same stimuli are deficient when their attention is allocated to an alternative goal or complex aspect of the situation (Baskin-Sommers et al., 2013; Decety et al., 2013; Meffert et al., 2013; Newman et al., 2010; Newman and Kosson, 1986; Sadeh and Verona, 2012).
Arguably the strongest evidence for the emotion deficit in psychopathy comes from research examining startle responses during picture viewing. In contrast to non-psychopathic offenders, who display startle potentiation during unpleasant pictures and startle inhibition during pleasant pictures, the startle potentiation to unpleasant pictures appears to be lacking in psychopathic offenders, particularly in offenders high on interpersonal-affective (Factor1) traits (Vaidyanathan et al., 2011). However, Baskin-Sommers and colleagues (2013) demonstrated that by manipulating picture familiarity, psychopathic offenders displayed the classic deficit in emotion-modulated startle during novel pictures, but no deficit in emotion-modulated startle during familiar pictures.
Using explicit instruction or condition manipulations, previous work provides strong evidence of dysfunctional attention-emotion processing in psychopathy. It is possible, though, that an attention bottleneck can also affect perceptual and sensory processing (Kastner and Ungerleider, 2000). Previous research shows that the phase coherence of theta, particularly in parietooccipital and primary sensory cortices, represents a neural index of readiness to perceive and integrate sensory inputs, both across and within sensory modalities (Buzsaki, 2005; Lakatos et al., 2009). Moreover, theta phase coherence is modulated by familiarity, possibly indicating greater dynamic coordination in familiar conditions across sensory domains (Miyakoshi et al., 2010)1. The present study measured theta phase coherence, as an index of readiness to perceive and integrate sensory information, during the picture-viewing paradigm used by Baskin-Sommers and colleagues. If readiness to perceive and integrate sensory information affects the efficient processing of affective information among offenders with psychopathy, then their theta inter-trial coherence (ITC), much like their defensive startle reactivity, should be impacted by the familiarity manipulation.
Methods
Participants
Ninety-nine incarcerated males between the ages of 18 and 45, with an IQ greater than 70, no clinical diagnoses of schizophrenia, bipolar disorder, or psychosis, and who were not currently using psychotropic medications were assessed for psychopathy and its related traits with the Psychopathy Checklist-Revised (PCL-R) (Hare, 2003) (see Table S1).
Task
Thirty-six pictures (12 unpleasant, 12 neutral, 12 pleasant) were selected from the International Affective Picture System (Lang et al., 2008). Affective pictures were matched on arousal. Six of these pictures (2 unpleasant, 2 neutral, 2 pleasant) were chosen randomly and displayed 10 times each during a familiarization block. Following familiarization, participants completed 60 trials of passive picture-viewing (intermixed trials with half displaying familiar and half novel pictures) (see Baskin-Sommers et al., 2013 for details).
Psychophysiological Recording and Reduction
Stimulus presentation and data collection were controlled by a PC-based Matlab script (Brainard, 1997; Pelli, 1997) and Neuroscan Synamps amplifiers and acquisition software. Offline processing was conducted using EEGLab (Delorme and Makeig, 2004).
EEG was recorded from Ag-AgCl electrodes mounted in an elastic cap and located at standard midline positions referenced to the left mastoid. Vertical eye-movement was measured with electrodes placed above and below the left eye. Electrode impedance was kept below 10 KΩ. Offline processing included re-referencing to average mastoids, low pass filtering (2nd order, 30Hz Butterworth low pass filter), epoching (−500ms −1000ms epochs surrounding picture onset), baseline correction, artifact rejection (±75microvolts).
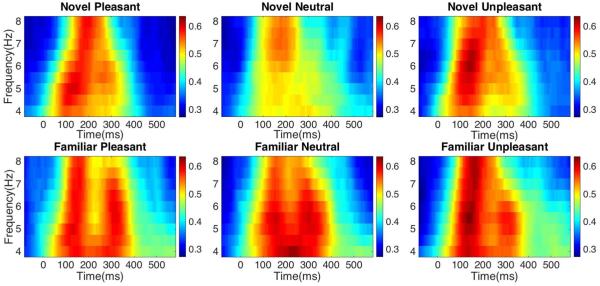
Time frequency analyses focused on theta (4-8 Hz) at Pz. Changes in ITC were extracted using the EEGLab ‘newtimef’ function. Three-second epochs (at three cycles) were convolved using Morlet wavelets to yield Time × Frequency spectrograms for each of the experimental conditions (Figure1). Following visual inspection of the spectrograms, mean ITCs were extracted from the pre-computed matrices between 100-300ms for the theta frequency band. Fisher r-to-z transformations were performed on the ITC data prior to all statistical analyses.
Figure 1.
Time-frequency spectrograms show changes in inter-trial coherence (ITC) following the presentation of novel pleasant, neutral, and unpleasant pictures (top row), and familiar pleasant, neutral, and unpleasant pictures (bottom row) during a passive picture-viewing task. Mean ITC data used for comparison was extracted from the 100-300ms time window.
Results
Psychopathy
Data were examined in a 2 (familiar, novel) by 3 (pleasant, neutral, unpleasant) General Linear Model (GLM) with PCL-R (z-score) as a continuous factor. Interaction contrasts were used to examine valence (unpleasant vs. pleasant) and affect (unpleasant/pleasant vs. neutral) effects.
Consistent with prior research, there was a significant familiarity main effect, F(1,97)=4.71, p=.032, ηp2=.046, with familiar pictures eliciting greater theta phase coherence than novel pictures. There were no significant main effects of emotion (p=.435), psychopathy (p=.386), nor two-way interactions.
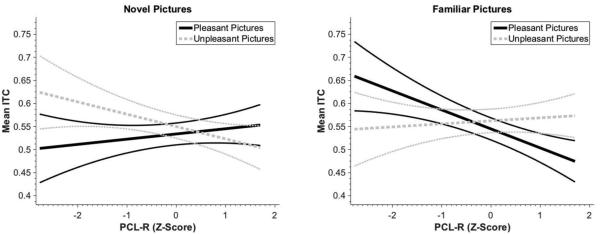
There was a significant Familiarity × Valence × Psychopathy interaction, F(1,97)=7.69, p=.007, ηp2=.073. Psychopathic offenders displayed descriptively less coherent theta response to unpleasant versus pleasant novel pictures (B=−.038, p=.111), but significantly more theta coherence to unpleasant versus pleasant familiar pictures (B=.048, p=.042) (Figure2).
Figure 2.
Theta response as a function of psychopathy. Results for novel trials (left) show that as psychopathy increases, the valence contrast of the theta response (i.e. unpleasant vs pleasant) decreases (B=−.038, p=.111), but during familiar trials (right) as psychopathy increases, the valence contrast of the theta response increases (B=.048, p=.042). Error bands are set at 1 standard error.
Psychopathy Factors
Some researchers have advocated parsing psychopathy into two component factors (Factor1: Interpersonal/Affective; Factor2: Impulsive/Antisocial) so that any unique correlates of these factors may be identified (Patrick, 2007). PCL-R Factor1 and Factor2 were entered simultaneously (z-score) into a GLM. There was a significant Familiarity × Valence × Factor1 interaction, F(1,91)=6.29, p=.014, ηp2=.065, where offenders high on Factor1 displayed significantly higher theta coherence during unpleasant versus pleasant familiar pictures (B=.067, p=.010), but not during novel pictures (B=−.016, p=.538). In contrast, for Factor2, there was a significant Familiarity × Affect × Factor2 interaction, F(1,91)=4.92, p=.029, ηp2=.051, where offenders high on Factor2 displayed descriptively more theta coherence to affective (pleasant and unpleasant) compared to neutral pictures during novel trials (B=.042, p=.061), but not familiar ones (B=−.034, p=.163).
Discussion
The present study used time-frequency analysis whether sensory processing and integration affects the core affective deficits characteristic of psychopathy. Psychopathic offenders, and offenders high on Factor1, showed enhanced emotion-modulation of theta ITC to familiar, but not novel pictures. In contrast, theta coherence for offenders high on Factor2 was greater for both types of affective novel stimuli. These results suggest that the psychopathy and Factor-related dysregulation in processing affective information may stem from problems in the coherence of sensory and perceptual processing, albeit in different ways.
The majority of research on the psychopathy-related attention abnormalities uses instructional, top-down, sets to show that an attention bottleneck limits the allocation of resources when processing complex information (Hoppenbrouwers et al., 2015; Larson et al., 2013). The present data suggest that this bottleneck may also inhibit lower level sensory perception of stimuli (Tombu et al., 2011). During novel pictures, psychopathic offenders (and Factor1) showed less emotion-modulation of theta phase coherence, suggesting that under high perceptual load, these individuals display dysfunction in their readiness to perceive and integrate information. However, when load was minimized during familiar pictures, offenders high on psychopathy displayed enhanced emotion-modulation of theta phase coherence. Given the consistency with the emotion-modulated startle findings, it is possible that the startle deficits in novel pictures may be due, in part, to a diminished readiness to perceive and integrate sensory information from multiple streams (e.g., visual processing of the picture and auditory processing of the startle probe). Moreover, the increased theta coherence during familiar pictures is consistent with work showing that psychopathic offenders over-respond to affective information when emotion is central to their goal or the demands of processing are alleviated (Flor et al., 2002; Newman et al., 2010). This diminished readiness to perceive and integrate affective information may result in poor neural detection and integration of sensory inputs across modalities and contribute to disruption of other neural processes that normally inhibit and regulate responses (Moul et al., 2012).
Unlike the effects for psychopathy or Factor1, offenders high on Factor2 showed enhanced theta coherence to novel affective pictures, but a habituated response to affect during familiar pictures (see Hidalgo-Munoz et al., 2014). Factor2 traits are associated with heightened reward sensitivity and affective reactivity (Buckholtz et al., 2010). It is possible that offenders high on Factor2 are characterized by an underlying neurobiological vulnerability whereby they reflexively attend to and amplify affective information, ultimately leading to dysregulated responses to reward, threat, and emotionally-laden information (Baskin-Sommers and Newman, 2014). Though perception and integration may be dysfunctional in both psychopathy/Factor1 and Factor2 traits, the underlying sensitivities appear distinct, with the former relating more to load-based differences in processing valence and the latter relating more to elevated affective salience.
Combined with previous research, the present study provides evidence that, for psychopathic offenders, their impairment in evaluating and sorting sensory information leads to a disjointed perception of information and degraded representation of affective information. This failure in sensory processing may be central to understanding the underlying mechanisms responsible for the fractionated affective responses associated with psychopathy.
Supplementary Material
Acknowledgements
This work was supported by grant 5R21DA030876 from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For time-frequency analysis, power analyses generally examine the magnitude or strength of processing, whereas coherence methods measure readiness and integration aspects of processing. For the present study, the latter method aligns with the bottleneck model, which suggests psychopathic individuals fail to “answer the call for processing” because they have not integrating all of the components of the stimuli/environment.
References
- Baskin-Sommers AR, Curtin JJ, Newman JP. Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychological Science. 2011;22:226–234. doi: 10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Emotion-modulated startle in psychopathy: clarifying familiar effects. Journal of Abnormal Psychology. 2013;122:458–468. doi: 10.1037/a0030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Newman JP. Psychopathic and externalizing offenders display dissociable dysfunctions when responding to facial affect. Personality disorders. 2014;5:369–379. doi: 10.1037/per0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the Revised Psychopathy Checklist. 2 Multi-Health Systems; Toronto, Ontario, Canada: 2003. [Google Scholar]
- Hidalgo-Munoz AR, Lopez MM, Galvao-Carmona A, Pereira AT, Santos IM, Vazquez-Marrufo M, Tome AM. EEG study on affective valence elicited by novel and familiar pictures using ERD/ERS and SVM-RFE. Medical & Biological Engineering & Computing. 2014;52:149–158. doi: 10.1007/s11517-013-1126-6. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Van der Stigchel S, Slotboom J, Dalmaijer ES, Theeuwes J. Disentangling attentional deficits in psychopathy using visual search: failures in the use of contextual information. Personality and Individual Differences. 2015;86:132–138. [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review in Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Lakatos P, O'Connell MN, Barczak A, Mills A, Javitt DC, Schroeder CE. The leading sense: supramodal control of neurophysiological context by attention. Neuron. 2009;64:419–430. doi: 10.1016/j.neuron.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainesville FL: 2008. [Google Scholar]
- Larson CL, Baskin-Sommers AR, Stout DM, Balderston NL, Curtin JJ, Schultz DH, Kiehl KA, Newman JP. The interplay of attention and emotion: top-down attention modulates amygdala activation in psychopathy. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:757–770. doi: 10.3758/s13415-013-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert H, Gazzola V, den Boer JA, Bartels AA, Keysers C. Reduced spontaneous but relatively normal deliberate vicarious representations in psychopathy. Brain. 2013;136:2550–2562. doi: 10.1093/brain/awt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi M, Kanayama N, Iidaka T, Ohira H. EEG evidence of face-specific visual self-representation. Neuroimage. 2010;50:1666–1675. doi: 10.1016/j.neuroimage.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR. A model of differential amygdala activation in psychopathy. Psychological Review. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biological Psychiatry. 2010;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- Patrick CJ. Getting to the heart of psychopathy. In: Herve H, Yuille JC, editors. The Psychopath: Theory, research, and social implications. Erlbaum; Hillsade, NJ: 2007. pp. 207–252. [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Sadeh N, Verona E. Visual complexity attenuates emotional processing in psychopathy: implications for fear-potentiated startle deficits. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:346–360. doi: 10.3758/s13415-011-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A Unified attentional bottleneck in the human brain. Proceedings of the National Academy of Sciences. 2011;108:13426–13431. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Hall JR, Patrick CJ, Bernat EM. Clarifying the role of defensive reactivity deficits in psychopathy and antisocial personality using startle reflex methodology. Journal of Abnormal psychology. 2011;120:253–258. doi: 10.1037/a0021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.