Abstract
Behavioural pattern separation (BPS), the ability to distinguish among similar stimuli based on subtle physical differences, has been used to study the mechanism underlying stimulus generalisation. Fear overgeneralisation is often observed in individuals with posttraumatic stress disorder and other anxiety disorders. However, the relationship between anxiety and BPS remains unclear. The purpose of this study was to determine the effect of anxiety (threat of shock) on BPS, which was assessed across separate encoding and retrieval sessions. Images were encoded/retrieved during blocks of threat or safety in a 2 × 2 factorial design. During retrieval, participants indicated whether images were new, old, or altered. Better accuracy was observed for altered images encoded during periods of threat compared to safety, but only if those images were also retrieved during periods of safety. These results suggest that overgeneralisation in anxiety may be due to altered pattern separation.
Keywords: Pattern separation, threat of shock, anxiety, startle, generalisation
Behavioural pattern separation (BPS) is a critical feature of episodic memory (Stark, Yassa, Lacy, & Stark, 2013). This process underlies the ability to discriminate similar experiences by forming distinct representations of stimulus features (memory encoding), that can be retrieved later (memory retrieval), and, ultimately, reduce interference among similar inputs (McClelland, McNaughton, & O’Reilly, 1995; O’Reilly & Norman, 2002; Shapiro & Olton, 1994; Treves, Treves, Rolls, & Rolls, 1994; Yassa & Stark, 2011). In laboratory studies of BPS, participants are required to differentiate between a stimulus that they have previously encountered and an altered version of the previously encountered stimulus (e.g., an image that has been rotated or shifted slightly). In real life situations, individuals are required to differentiate between previously encountered stimuli or events (e.g., gunshots vs. fireworks) based on subtle contextual cues. One characteristic of individuals with anxiety disorders is that they show overgeneralisation of fear in real life situations, and in the laboratory (Lissek et al., 2014). The underlying mechanisms of overgeneralisation are not well understood but abnormal pattern separation has been proposed as a candidate mechanism (Kheirbek, Klemenhagen, Sahay, & Hen, 2012). There is a large body of research suggesting that the dentate gyrus of the hippocampus is necessary for BPS, and that it mediates BPS by generating orthogonal output based on sparse inputs (Bakker, Kirwan, Miller, & Stark, 2008; Leal, Tighe, Jones, & Yassa, 2014; Leutgeb, Leutgeb, Moser, & Moser, 2007; Neunuebel & Knierim, 2014; Newman & Hasselmo, 2014; Rolls, 2013; Sahay et al., 2011; Yassa & Stark, 2011). By understanding the link between anxiety and BPS, it may be possible to provide the first steps toward a mechanistic explanation of the fear overgeneralisation experienced by individuals with anxiety disorders. However, it is currently unknown whether anxiety affects BPS in humans. Therefore, the purpose of this study was to determine the effect of induced anxiety on BPS in healthy participants.
In a recent study, Segal, Stark, Kattan, Stark, and Yassa (2012) found that arousal evoked by disturbing pictures prior to stimulus encoding enhanced pattern separation. However, encoding took place immediately after the mild stressor and retrieval was conducted 15 min after the end of encoding. By that time, measures of salivary alpha amylase, a marker of noradrenergic activation, showed that participants’ arousal level had returned to baseline. Memory processes are strongly dependent on the internal and external context, including pharmacological influences, arousal, and mood states (Clark, Milberg, & Ross, 1983; Eich, 1995; Koek, 2011; Smith & Vela, 2001). Although there is evidence that anxiety can facilitate encoding (Kogan & Richter-levin, 2010; McGaugh, 2002, 2004) but impair retrieval (Raio, Brignoni-Perez, Goldman, & Phelps, 2014), it is currently unclear how arousal at retrieval will affect pattern separation. However, evidence suggests that arousal during retrieval may impair memory, suggesting that pattern separation may also be affected (Schwabe & Wolf, 2013).
To address these issues, the present study used a 2 × 2 within-subject design in which each participant encoded stimuli during periods of threat and safety and were subsequently tested for pattern separation also during periods of threat or safety. During the periods of threat participants were at risk for receiving unpredictable presentations of a shock, while during the periods of safety they were told they would not receive any shocks (Davis, Walker, Miles, & Grillon, 2010; Lissek et al., 2007; Mol, Baas, Grillon, van Ooijen, & Kenemans, 2007; Robinson, Vytal, Cornwell, & Grillon, 2013; Vytal, Overstreet, Charney, Robinson, & Grillon, 2014). Participants’ anxiety was assessed with subjective reports and the acoustic startle reflex. The startle reflex was used because it is robustly increased by aversive states and constitutes a reliable online measure of anxiety (Grillon, 2008; Robinson et al., 2013). Performance on pattern separation was investigated using a similar task to that used by Segal et al. (2012). This task, which was designed test the ability to distinguish between old (same items as presented during encoding) and altered (same items presented during encoding but tilted) items during retrieval, has been validated in several behavioural and neuroimaging studies (Bakker et al., 2008; Deuker, Doeller, Fell, & Axmacher, 2014; Holden & Gilbert, 2012; Kirwan & Stark, 2007; Leal et al., 2014; Leutgeb et al., 2007; Segal et al., 2012) and can distinguish “pattern separation” from other aspects of episodic memory such as recognition memory and overall performance. Based on Segal et al. (2012)’s findings we hypothesised that anxiety (threat of shock) at encoding would facilitate pattern separation tested in a safe condition (threat-encoding/safe-retrieval). However, based on findings that arousal during retrieval can impair memory performance (Schwabe & Wolf, 2013), we predicted that this anxiety-induced facilitation at encoding would be lost when pattern separation would be tested in an unsafe condition (threat-encoding/threat-retrieval). Thus, we hypothesised enhanced pattern separation (i.e., the ability to distinguish between old and altered items during retrieval) in threat-encoding/safe-retrieval conditions compared to safe/safe conditions and to threat-encoding/threat-retrieval conditions.
Materials and methods
Participants
Twenty-three participants (17 female) were recruited from the community to take part in this study (age: M = 29.4, SD = 6.5). Individuals were included based on: (1) no current or past history of any Axis I psychiatric disorder as assessed by SCID-I/NP (First, Spitzer, Gibbon, & Williams, 2012), (2) no medical condition that interfered with the objectives of the study as established by a physician, and (3) no use of illicit drugs or psychoactive medications according to history and confirmed by a negative urine screen. One participant misunderstood the instructions and was excluded from the analysis. Another participant failed to respond during the retrieval runs and was also excluded, leaving a total of 21 completers. All participants gave written informed consent approved by the National Institute of Mental Health (NIMH) Combined Neuroscience Institutional Review Board and were compensated for participating.
Procedure
Prior to starting the study, participants were briefed on the experiment and given the pre-experiment questionnaires (see below). Following attachment of the electrodes, the participants underwent the shock workup procedure, to identify an intensity of stimulation rated as “uncomfortable but not painful”. Finally, the participants underwent the startle habituation, to reduce initial startle reactivity.
The experiment consisted of having participants implicitly encode images (see below Images) during an encoding phase and later identify these images presented among new and altered images (old but rotated images) during a retrieval phase. To assess pattern separation we examined performance to the altered, relative to the old images. Greater pattern separation was defined as better performance to the altered images, as indicated by smaller [old minus altered] difference score. The encoding and retrieval phases were conducted during safe periods and during threat periods, when participants anticipated shocks using a within-subject design (see Figure 1).
Figure 1.
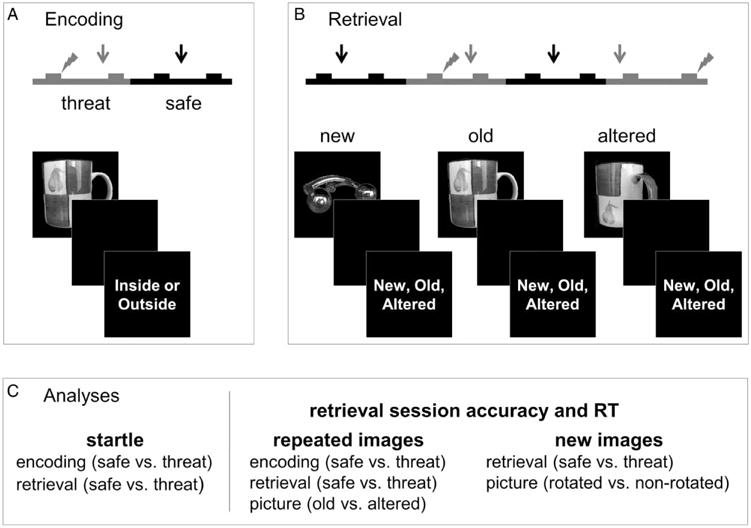
Schematic of experimental design. During encoding (A) and retrieval (B), pictures were presented during safety or threat of shock, resulting in a factorial design with the following conditions: safe/safe, safe/threat, threat/safe, and threat/threat. During retrieval, participants were instructed to identify new, old, and altered (slightly rotated) pictures. Startle probes (arrows) were presented throughout the experiment, and shocks (lightning bolts) were presented during the threat blocks. (C) To measure anxiety, startle was analysed using a 2 (encoding: safe vs. threat) × 2 (retrieval: safe vs. threat) repeated measures ANOVA. To measure pattern separation, repeated images were analysed using a 2 (encoding: safe vs. threat) × 2 (retrieval: safe vs. threat) × 2 (picture: old vs. altered) repeated measures ANOVA. To control for performance effects, new images were analysed using a 2 (retrieval: safe vs. threat) × 2 (picture: rotate vs. non-rotated) repeated measures ANOVA.
There were two encoding runs and two retrieval runs with brief (less than 5 min) breaks between each run. During the breaks, participants completed questionnaires about their cognitive/emotional state during the previous run. Between the second encoding run and the first retrieval run, participants were given the instructions for retrieval. Runs contained alternating blocks of safety and threat. Participants were informed that they would receive occasional and unpredictable shocks in the threat condition but not in the safe condition. Trials began with the following successive events: (1) a 1-s fixation cross, (2) a picture presented for a duration ranging from 1 to 3 s, (3) a brief empty interval (maintenance period) that lasted between 1 and 3 s, (5) a 2 s response prompt, and (6) a variable 6 s ITI (SD = 721 ms; Min = 4000 ms; Max = 8000 ms). Each run during encoding contained a total of four alternating blocks of safety and threat with eight trials per block. Each run during retrieval contained a total of 4 alternating blocks of safety and threat but with 12 trials per block. Block and trial orders were counterbalanced across participants. To induce anxiety, two shocks were presented during either the maintenance period or the ITI of a randomly selected trial during each threat block. To assess anxiety, two startle probes were presented during either the maintenance period or the ITI of a randomly selected trial during each block. Trials containing shocks or probes were discarded prior to analysis.
During encoding, participants were instructed to indicate at the response prompt whether the item presented on each trial typically occurred indoors or outdoors. This was included to ensure that they attended to the stimuli during encoding. They were not informed that there would be a retrieval session afterward. Half of the items were presented in safety blocks, and half of the items were presented in threat blocks. During retrieval, the participants were instructed to indicate whether the item was new, old, or altered. New trials reflected images of items presented only during retrieval. Old trials reflected images of items presented at the same eccentricity (45°) during encoding and retrieval. Altered trials reflected images of items presented at different eccentricities (e.g., 45° → 75°) during encoding and retrieval. There were 32 presentations of each trial type during retrieval. Like encoding, half of the items presented during retrieval were presented during safety blocks, while half were presented during threat blocks. Importantly, half of the repeated items (old and altered) presented in each condition during retrieval were selected from items presented in the safe condition during encoding, while the other half were selected from items presented in the threat condition during encoding. Based on this factorial design, repeated items were grouped into four categories corresponding to their encoding and retrieval condition: safe-encoding/safe-retrieval (SS), safe-encoding/threat-retrieval (ST), threat-encoding/safe-retrieval (TS), threat-encoding/threat-retrieval (TT), resulting in a 2 (encoding: safe vs. threat) × 2 (retrieval: safe vs. threat) × 2 (old vs. altered) design. For all post hoc comparisons we report the false discovery rate (FDR) corrected p-values. Evidence that BPS is affected by anxiety during either encoding or retrieval, would be supported by results that show differences in accuracy (% correct) for old vs. altered stimuli as a function of either the encoding or the retrieval condition, resulting in either two-way or three-way interactions involving the old vs. altered factor.
New items were included as foils, and to rule out performance effects related to the picture rotation. Because new items were not presented in the encoding session, they were grouped into two categories based on their retrieval condition: new/safe-retrieval (NS), and new/threat-retrieval (NT). Additionally, to serve as a negative control for image rotation, and to match the eccentricity distribution of the old items, we presented new items at varying degrees of eccentricity during the retrieval session. Together these manipulations resulted in a 2 (retrieval: safe vs. threat) × 2 (rotated vs. not rotated) design. We hypothesised that threat during retrieval should not affect overall performance; therefore we predicted no effect of threat on new item accuracy. Likewise, because these images were not presented during encoding, the eccentricity of the items in the images does not carry meaningful information. Therefore we also predicted that there should be no effect of image rotation on accuracy as well.
Materials
Images
Images were randomly selected from the Amsterdam Library of Object Images (Geusebroek, Burghouts, & Smeulders, 2005), which contains 1000 images of small objects photographed at varying eccentricities (e.g., stuffed animals, fruits and vegetables, common household objects, etc.). All images in the encoding phase were photographed at 45° eccentricity, as were the old images presented in the retrieval phase. Altered images presented in the retrieval phase were photographed at 15° or 75° eccentricity, the direction of which was chosen randomly. To control for non-specific effects of image rotation, new images presented during the retrieval phase were photographed at 45 (50% of trials), 15 (25% of trials), and 75 (25% of trials) degrees eccentricity. Images were presented using the Presentation software package (version 17.0, Neurobehavioral Systems, Berkeley, CA), on a standard LCD monitor.
Psychometric data
Prior to the experiment and after each block participants were given affective rating scales, which were scored on a 1 to 9 scale: (1) How anxious are you (1 = not anxious, 9 = extremely anxious)? (2) How afraid are you (1 = not afraid, 9 = extremely afraid)? (3) How happy are you (1 = not happy, 9 = extremely happy)? (4) How would you rate the intensity of the electrical stimulation (1 = not painful at all, 9 = uncomfortable but not painful)?
Shock
Threat of shock was used to induce anxiety during the threat blocks (Robinson et al., 2013). Prior to the experiment, shock electrodes were attached to the medial portion of the participant’s left wrist, approximately 3 cm apart. Next the participant underwent a workup procedure, which was used to determine the intensity of stimulation to be given. During this procedure, the participant rated presentations of the shock, and the intensity was gradually increased (max = 5 mA) until the participant reached a level that they rated uncomfortable but not painful.
Acoustic startle stimulus
During the experiment, participants received occasional loud sounds designed to elicit startle reflexes. The sounds were 40 ms presentations of a 103 dB white noise (near instantaneous rise/fall times) over headphones. Prior to the experiment, the participant received nine presentations of the white noise to habituate the startle reflex.
Facial electromyography (EMG)
The acoustic startle response was recorded via facial EMG from 6 mm Ag/AgCl electrodes placed below the right eye over the orbicularis oculi muscle (Blumenthal et al., 2005). EMG was recorded at 1000 Hz and analysed using the Psychlab version 7 software (Contact Precision Instruments, London, UK). The EMG signal was bandpass filtered (30–500 Hz), rectified, and smoothed with a 20 ms time constant. The peak startle/eyeblink reflex was determined in the 20–100 ms after white noise onset. The startle amplitude scores from each participant were transformed to z scores and converted to T scores and then averaged separately within the safe and the threat conditions in order to reduce large differences in the overall magnitude of startle reflex (Blumenthal et al., 2005).
Results
Threat of shock induces a negative affective state during both encoding and retrieval
Startle
The startle magnitude scores were entered into a Phase (encoding, retrieval) × Condition (safe, threat) repeated measures ANOVA (see Figure 2). There were significant main effects for both phase (F(1, 20) = 16.693; p < .001; ) and condition (F(1, 20) = 48.027; p < .001; ), as well as a significant phase × condition interaction (F(1, 20) = 5.398; p = .031; ). These results suggest that threat of shock led to the predicted increase in startle magnitude (i.e., anxiety-potentiated startle or APS), and that both startle magnitude and APS decreased across blocks. Importantly, follow-up t-tests show that although APS was larger during encoding than retrieval (t(20) = −2.323; p = .031; FDR = 0.031; dav = 0.811), there was significant potentiation by threat during both encoding (t(20) = 5.382; p < .001; FDR = 0.003; dav = 1.530) and retrieval (t(20) = 2.792; p = .011; FDR = 0.011; dav = 0.905), indicating successful anxiety induction during both encoding and retrieval.
Figure 2.
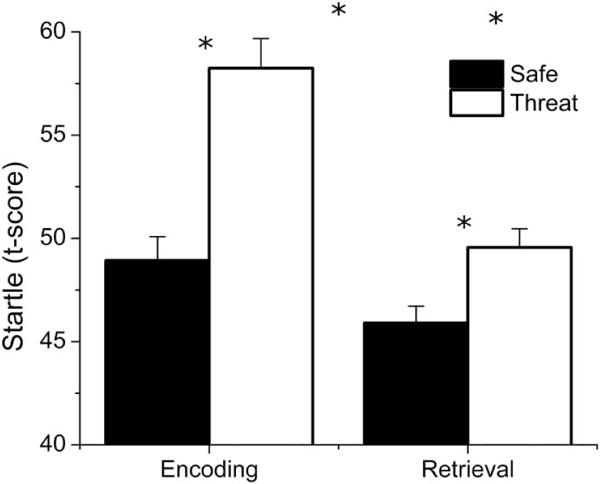
Anxiety-potentiated startle during the encoding and retrieval conditions. Startle potentiation and overall startle magnitude was larger during encoding than during retrieval. However, participants showed significantly larger startle responses during threat blocks than during safety blocks for both encoding and retrieval. (*p < .05, floating “*” indicate significant interactions).
Subjective reports
Participants rated their anxiety, fear, and happiness after each run (see Table 1 for summary). In order to assess the efficacy of our threat manipulation, we averaged these values across blocks separately for the encoding and retrieval phases and for the safe and threat conditions, and performed a 2 condition (safe, threat) × 2 phase (encoding, retrieval) repeated measures ANOVA. For all three rating scales (anxiety, fear, and happiness), the results show a significant main effect for condition (anxiety: F(1, 20) = 35.969; p < .001; ; fear: F(1, 20) = 20.032; p < .001; ; happiness: F(1, 20) = 14.861; p < .001; ) and a condition × phase interaction (anxiety: F(1, 20) = 6.448; p = .02; ; fear: F(1, 20) = 4.885; p = .039; ; happiness: F(1, 20) = 5.077; p = .036; ). Participants reported more anxiety and fear in threat blocks than in safe blocks, and this effect (threat vs. safe) was larger during encoding than retrieval (anxiety: t(20) = 2.539; p = .02; dav = 0.314; fear: t(20) = 2.203; p = .039; dav = 0.197). In contrast, participants reported less happiness in the threat than the safe blocks, and this effect (safe vs. threat) was larger during encoding than retrieval (happiness: t(20) = −2.253; p = .036; dav = 0.268). Although ratings decreased from encoding to retrieval (significant for anxiety and happiness, but trend level for fear), it should be noted that participants were more anxious and afraid, and less happy during the threat blocks than the safe blocks during both phases. Finally, to assess habituation to the shock across the experiment we performed a paired-sample t-test on the subjective ratings of shock intensity between encoding and retrieval, and found larger ratings for encoding compared to retrieval (t(20) = −3.697; p = .001; dav = 0.248).
Table 1.
Mean (sem) for subjective ratings
| Condition | Anxious | Afraid | Happy | Shock |
|---|---|---|---|---|
| Encoding | ||||
| Safe | 2.26 (0.33) | 1.45 (0.22) | 6.02 (0.48) | |
| Threat | 5.69 (0.55) | 4.26 (0.57) | 3.93 (0.53) | 7.69 (0.35) |
| Retrieval | ||||
| Safe | 2.36 (0.37) | 1.45 (0.20) | 5.52 (0.56) | |
| Threat | 5.02 (0.50) | 3.74 (0.57) | 4.02 (0.58) | 7.26 (0.38) |
Threat at encoding and threat at retrieval interact to affect BPS
The effect of condition (safe vs. threat) on BPS (i.e., accuracy to altered items compared to accuracy to old items) was examined with a 2 encoding (safe vs. threat) × 2 retrieval (safe vs. threat) × 2 stimulus (old vs. altered) repeated measures ANOVA on accuracy (see Figure 3A). There were significant main effects for retrieval (F(1, 20) = 5.213; p = .033; ) and stimulus (F(1, 20) = 46.348; p < .001; ), as well as a significant three-way interaction (F(1, 20) = 7.643; p = .012; ).
Figure 3.
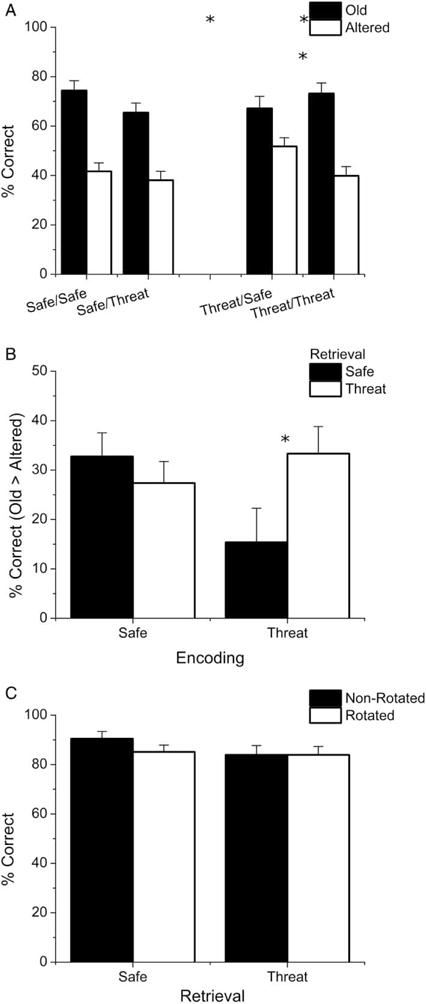
Percent correct during the retrieval session. (A) Results for the old/altered items show a significant encoding condition × retrieval condition × stimulus type interaction. Results for old/altered items encoded during threat show a significant retrieval condition × stimulus type interaction. (B) Pattern separation (% correct [old > altered]) is enhanced (lower scores) for items encoded during threat and retrieved during safety, but this enhancement is lost for items retrieved during threat. (C) Accuracy for new items is not affected by retrieval context, or image eccentricity. (*p < .05, floating “*” indicate significant interactions).
To decompose the three-way interaction, we conducted follow-up two-way repeated measures ANOVAs to determine the effects of condition (safe vs. threat) during retrieval on BPS (old vs. altered) independently for items encoded during threat or safety. For items encoded during safety (Figure 3A, safe/safe, safe/threat), the stimulus main effect was significant (F(1, 20) = 58.795; p < .001; FDR = 0.005; ), indicating that participants were more accurate for old items (old items recognised as old) than for altered (altered items identified as altered) items. There was no main effect for condition during retrieval (p = .074), and no threat by stimulus interaction (p > .1). Although the main effect for condition during retrieval approached significance, this result does not impact the main findings of the current work, and will not be discussed further.
For items encoded during threat (Figure 3A, threat/safe, threat/threat), there was a significant main effect for stimulus type (F(1, 20) = 25.401; p < .001; FDR = 0.005; ), indicating that participants were again more accurate for old than for altered items, but no main effect for condition (p > .1). Importantly, there was a condition × stimulus interaction (F(1, 20) = 4.684; p = .043; FDR = 0.077; ), albeit at trend level when corrected for multiple comparisons. To characterise this interaction, we performed paired-sample t-tests. First we determined whether accuracy for either old or altered items encoded during threat was affected by retrieval condition. Results showed that accuracy for old items was unaffected (Safe vs. Threat: t(20) = −1.062; p = .301; FDR = 0.345; dav = 0.283), but accuracy for altered items was greater for items retrieved during safety (Safe vs. Threat: t(20) = 2.633; p = .016; FDR = 0.048; dav = 0.701). As a follow-up, we created old minus altered difference scores for items encoded during threat, and conducted an additional paired-sample t-test for the retrieval condition: safe vs. threat contrast. Results showed better BPS (i.e., smaller difference scores for “old” vs. “altered”) for items retrieved in safe blocks than those retrieved during threat blocks (see Figure 3B; t(20) = −2.164; p = .043; FDR = 0.0774; dav = 0.619), albeit at trend level when corrected for multiple comparisons. Taken together, these results suggest that threat during encoding facilitates pattern separation, but only if items are retrieved in a safe condition.
Threat during retrieval did not affect accuracy to novel stimuli
To rule out the possibility that the pattern separation results were driven by a bias effect due to picture rotation we conducted a 2 retrieval (threat vs. safe) × 2 stimulus type (rotated vs. not rotated) repeated measures ANOVA on accuracy to the new items (see Figure 3C), and found no significant main effects or interactions (ps > 0.1). These results suggested that simply presenting stimuli in a threatening condition or varying the eccentricity (as is the case with the altered stimuli) had no effect on accuracy.
Threat during encoding or retrieval does not affect RT to previously presented stimuli
To rule out the possibility that the pattern separation results were not driven by a speed-accuracy trade-off, we performed a 2 encoding (safe vs. threat) × 2 retrieval (safe vs. threat) × 2 stimulus (old vs. altered) repeated measures ANOVA on reaction time scores for the old items (see Figure 4A), and found no significant main effects or interactions (ps > 0.1).
Figure 4.
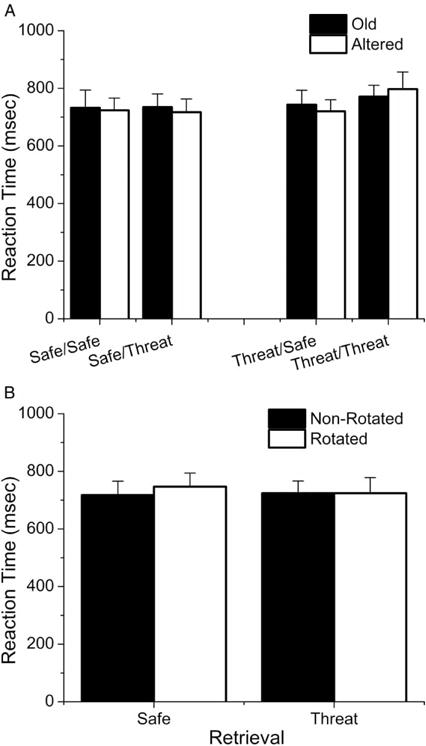
Reaction time during the retrieval session. (A) Reaction time for old/altered items was not affected by either encoding or retrieval context. (B) Reaction time for new items is similarly unaffected by retrieval context or image eccentricity.
Threat during retrieval does not affect RT to novel stimuli
To rule out any other performance related effects during retrieval, we conducted a 2 retrieval (threat vs. safe) × 2 stimulus type (rotated vs. not rotated) repeated measures ANOVA on reaction time scores to the new items (see Figure 4B), and found no significant main effects or interactions (ps > 0.1). Taken together, these results suggest that the effects of threat during encoding and retrieval are specific to pattern separation, and not driven by simple performance or perceptual effects.
Discussion
Pattern separation has been recently implicated in various forms of anxiety-related psychopathology (Das, Ivleva, Wagner, Stark, & Tamminga, 2014; Fujii, Saito, Yanaka, Kosaka, & Okazawa, 2014; Kühn et al., 2014), but little is currently known about the effect of anxiety on pattern separation in humans. The present study investigated the effect of an induced anxiety state, evoked by the threat of unpredictable shock, on BPS during the encoding and retrieval stages of an episodic memory task. Results showed that the anxiety manipulation was effective during encoding and retrieval; the startle reflex (and subjective reports) was robustly increased by shock anticipation, indicating elevated anxiety during the threat compared to the safe condition (Davis et al., 2010; Robinson et al., 2013). The main findings were that (1) pattern separation tested in a safe context was improved when initial encoding was in a threat context compared to a safe context (Threat/Safe > Safe/Safe), (2) this improvement was lost when testing occurred in a threat context (Threat/Safe > Threat/Threat), and (3) this effect was not simply due to performance effects, perceptual processing, or a speed-accuracy trade-off. These results suggest that the mechanism behind this effect may be beneficial during encoding, but detrimental during retrieval.
The current results are consistent with those of Segal et al. (2012) in that pattern separation was facilitated for items encoded during threat, but retrieved during safety. According to Segal et al. (2012), this facilitation in pattern separation for items encoded during threat, but retrieved during safety is mediated by noradrenergic activity during encoding (Segal et al., 2012). This noradrenergic input likely originates in the locus coeruleus (Walling, Nutt, Lalies, & Harley, 2004), and facilitates synaptic plasticity in the dentate gyrus (Hansen & Manahan-Vaughan, 2014; Sara & Bergis, 1991), which contains high levels of noradrenergic receptors (Young & Kuhar, 1980). In addition, locus coeruleus noradrenergic activity can be driven by the basolateral nucleus of the amygdala (Bergado, Frey, López, Almaguer-Melian, & Frey, 2007), an area important for the consolidation of emotional memory (McGaugh, 2002, 2004).
Unlike Segal et al. (2012), we also included a threat condition during retrieval, thus we were able to show that this anxiety-induced enhancement of pattern separation is also dependent upon the retrieval context. However, how this same mechanism (noradrenergic inputs to the dentate gyrus) during retrieval can lead to reduced pattern separationremains to be determined. It has been shown that granule cells in the dentate gyrus send sparse connections to CA3 via the mossy fibres (Neunuebel & Knierim, 2014), which are strongly connected to the postsynaptic cells (Rolls & Kesner, 2006). This allows for a small number of granule cells to activate a random subset of CA3 cells, which then can form an autoassociative network through recurrent connections (Rolls, 2013). The autoassociative nature of this CA3 network is thought to mediate pattern completion (i.e., the retrieval of previously encoded information based on sparse representations) because activation in any node in the network can activate the entire network (Newman & Hasselmo, 2014; Rolls, 2013).
Importantly, noradrenergic activity originating from the locus coeruleus can initiate plasticity in the mossy fibre connections (Hagena & Manahan-Vaughan, 2012), thus incorporating the arousing nature of the context in the representation at encoding. If these noradrenergic inputs are present during both encoding and retrieval, then they could possibly activate the previously encoded memory, leading participants to more often mistake the altered images for old images. According to this hypothesis, the deficit seen in the threat/threat condition relative to the threat/safe condition should be rescued if propranolol is given during retrieval.
Clinical implications
These results may have implications for understanding the impact of anxiety on mnemonic functions. Indeed, it may be adaptive to benefit from strong pattern separation subsequent to stress or trauma because of heightened memory for trauma-related stimuli (Cahill, 1996; Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Segal & Cahill, 2009). Since contextual stimuli are better remembered when encoded during periods of induced anxiety, they are more likely to reactivate emotional memories when encountered at a later time. Robust pattern separation would improve memory for the details of the emotional or traumatic situation and, therefore, would ensure that the range of stimuli that elicit an emotional response is restricted. In other words, arousal increases the likelihood that stimuli are remembered. However, because arousal is also associated with better pattern separation, it limits stimulus generalisation. However, this enhanced pattern separation manifests itself only in a safe-retrieval context. When the retrieval context is unsafe, the benefit of improved pattern separation is lost, suppressing the enhanced ability to distinguish among stimuli, and providing a putative mechanism for overgeneralisation. This could constitute a precipitating mechanism for generalisation of emotional memories in post traumatic stress disorder (PTSD). Individuals with PTSD tend to generalise their fear to a wide range of stimuli. Fear conditioning studies show a lack of discrimination ability in posttraumatic stress disorder and other anxiety disorders (Lissek et al., 2014; Milad et al., 2009), which may be linked to faulty pattern separation. According to the present results, individuals who have experienced a trauma may be most at risk for overgeneralisation when anxious. In a non-anxious state, trauma-reminders may evoke conditioned emotional responses, but efficient pattern separation limits the range of triggering stimuli. However, when in an anxious state, the weakening of pattern separation widens the range of stimuli that can evoke such conditioned emotional responses, increasing anxiety, which in turn further increases overgeneralisation, possibly entering a vicious circle of progressive chronicity.
Strengths & limitations
Among the strengths, we manipulated anxiety in a within-subject design and relied on well-established methods of fear induction and measurement (Grillon & Baas, 2003) and pattern separation (Stark et al., 2013; Yassa & Stark, 2011). The primary limitation of this study is that it relies on purely behavioural measures. Based on our results, we predict that noradrenergic inputs to the dentate gyrus mediate both the facilitation of pattern separation in the threat/safe condition and the loss of this facilitation in the threat/threat condition. Future studies should be conducted employing pharmacological manipulations and neuroimaging methods to directly test this hypothesis.
Conclusions
This study shows that anxiety during encoding enhances pattern separation, but only when tested in a safe condition. Arousal can increase noradrenergic activity in the locus coeruleus, which projects to the dentate gyrus. We hypothesise that this noradrenergic activity has a bi-directional effect on pattern separation, depending on when it occurs. If encoding occurs during a period of heightened anxiety, this noradrenergic input will facilitate pattern separation by strengthening the memory. However, retrieval also occurs during a period of heightened anxiety, this noradrenergic input will facilitate pattern completion by activating nodes in the autoassociative network that makes up the initially encoded representation.
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the National Institute of Mental Health [grant number MH002798] (Protocol 01-M-0185).
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Frey S, López J, Almaguer-Melian W, Frey JU. Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiology of Learning and Memory. 2007;88(3):331–341. doi: 10.1016/j.nlm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Cahill L. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(RC99):1–5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Milberg S, Ross J. Arousal cues arousal-related material in memory: Implications for understanding effects of mood on memory. Journal of Verbal Learning and Verbal Behavior. 1983;22(6):633–649. [Google Scholar]
- Das T, Ivleva EI, Wagner AD, Stark CEL, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophrenia Research. 2014;159(1):193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuker L, Doeller CF, Fell J, Axmacher N. Human neuroimaging studies on the hippocampal CA3 region – Integrating evidence for pattern separation and completion. Frontiers in Cellular Neuroscience. 2014;8:64. doi: 10.3389/fncel.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich E. Mood as a mediator of place dependent memory. Journal of Experimental Psychology General. 1995;124(3):293–308. doi: 10.1037//0096-3445.124.3.293. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV® axis I disorders (SCID-I), clinician version, administration booklet. Washington, DC: American Psychiatric Publishing; 2012. [Google Scholar]
- Fujii T, Saito DN, Yanaka HT, Kosaka H, Okazawa H. Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: A functional MRI study. Hippocampus. 2014;24(2):214–224. doi: 10.1002/hipo.22216. [DOI] [PubMed] [Google Scholar]
- Geusebroek JM, Burghouts GJ, Smeulders AWM. The Amsterdam library of object images. International Journal of Computer Vision. 2005;61(1):103–112. [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology. 2008;199(3):421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. Learning-facilitated long-term depression and long-term potentiation at mossy fiber—CA3 synapses requires activation of β-adrenergic receptors. Frontiers in Integrative Neuroscience. 2012 May;6:1–11. doi: 10.3389/fnint.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N, Manahan-Vaughan D. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cerebral Cortex. 2014;25(7):1889–1896. doi: 10.1093/cercor/bht429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Frontiers in Aging Neuroscience. 2012 May;4:1–6. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nature Neuroscience. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory (Cold Spring Harbor, NY) 2007;14(9):625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W. Drug-induced state-dependent learning: Review of an operant procedure in rats. Behavioural Pharmacology. 2011;22(5–6):430–440. doi: 10.1097/FBP.0b013e328348ed3b. [DOI] [PubMed] [Google Scholar]
- Kogan I, Richter-levin G. Emotional memory formation under lower versus higher stress conditions. Frontiers in Behavioral Neuroscience. 2010;4:183. doi: 10.3389/fnbeh.2010.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Charlet K, Schubert F, Kiefer F, Zimmermann P, Heinz A, Gallinat J. Plasticity of hippocampal subfield volume cornu ammonis 2 + 3 over the course of withdrawal in patients with alcohol dependence. JAMA Psychiatry C. 2014;71(7):806–811. doi: 10.1001/jamapsychiatry.2014.352. [DOI] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Jones CK, Yassa MA. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. 2014;24(9):1146–1155. doi: 10.1002/hipo.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry. 2014;75(11):909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Orme K, Mcdowell DJ, Johnson LL, Luckenbaugh DA, Baas JMP, Grillon C. Emotion regulation and potentiated startle across affective picture and threat-of-shock paradigms. Biological Psychology. 2007;76(1–2):124–133. doi: 10.1016/j.biopsycho.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27(1):1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol N, Baas JMP, Grillon C, van Ooijen L, Kenemans JL. Startle potentiation in rapidly alternating conditions of high and low predictability of threat. Biological Psychology. 2007;76(1–2):43–51. doi: 10.1016/j.biopsycho.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81(2):416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Hasselmo ME. CA3 sees the big picture while dentate gyrus splits hairs. Neuron. 2014;81(2):226–228. doi: 10.1016/j.neuron.2014.01.004. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in Cognitive Sciences. 2002;6(12):505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Raio CM, Brignoni-Perez E, Goldman R, Phelps EA. Acute stress impairs the retrieval of extinction memory in humans. Neurobiology of Learning and Memory. 2014 Feb;112:212–221. doi: 10.1016/j.nlm.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: Perspectives from human threat of shock studies. Frontiers in Human Neuroscience. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Frontiers in Systems Neuroscience. 2013 Oct;7:74–1. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Bergis O. Enhancement of excitability and inhibitory processes in hippocampal dentate gyrus by noradrenaline: A pharmacological study in awake, freely moving rats. Neuroscience Letters. 1991;126(1):1–5. doi: 10.1016/0304-3940(91)90356-x. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress and multiple memory systems: From “thinking” to “doing”. Trends in Cognitive Sciences. 2013;17(2):60–68. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Segal SK, Cahill L. Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology. 2009;34(9):1263–1271. doi: 10.1016/j.psyneuen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiology of Learning and Memory. 2012;97(4):465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M, Olton D. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory systems. Vol. 1994. Cambridge, MA: MIT Press; 1994. pp. 87–117. [Google Scholar]
- Smith SM, Vela E. Environmental context-dependent memory: A review and meta-analysis. Psychonomic Bulletin & Review. 2001;8(2):203–220. doi: 10.3758/bf03196157. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51(12):2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Treves A, Rolls ET, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Vytal KE, Overstreet C, Charney DR, Robinson OJ, Grillon C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry & Neuroscience. 2014;1:1–9. doi: 10.1503/jpn.130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling SG, Nutt DJ, Lalies MD, Harley CW. Orexin-A infusion in the locus ceruleus triggers norepinephrine (NE) release and NE-induced long-term potentiation in the dentate gyrus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(34):7421–7426. doi: 10.1523/JNEUROSCI.1587-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Kuhar MJ. Radiohistochemical localization of benzodiazepine receptors in rat brain. The Journal of Pharmacology and Experimental Therapeutics. 1980;212(X):337–346. [PubMed] [Google Scholar]


