Abstract
Background
The transparent ocular structure enables quantitative analysis of microvasculature of retina, a neuronal tissue affected by multiple sclerosis.
Objective
To determine whether the retinal blood flow velocity and flow volume at the macula are impaired in patients with relapsing remitting multiple sclerosis (RRMS).
Methods
Seventeen RRMS patients and 17 age- and gender-matched healthy subjects were assessed. A Retinal Function Imager was used to measure the blood flow velocity of retinal arterioles and venules, and to calculate the total perifoveal blood flow volume.
Results
The blood flow velocities of the retinal arterioles (3.34 ± 0.89 mm/s) and venules (2.61 ± 0.6 mm/s) were significantly lower in MS patients than normal subjects (arteriole: 4.10 ± 0.87 mm/s; venule: 3.22 ± 0.65 mm/s, both P = 0.01). In addition, the total perifoveal blood flow volume in arterioles (3.74 ± 1.64 nl/s) and venules (3.81 ± 1.60 nl/s) were significantly lower in MS patients than in normal subjects (arteriole: 4.87 ± 1.41 nl/s, P = 0.02; venule: 4.71 ± 1.64 nl/s, P = 0.04).
Conclusion
The impaired retinal microcirculation in RRMS patients indicates microvascular dysfunction in MS.
Keywords: Multiple sclerosis, retinal microcirculation, retinal function imager, retinal nerve fiber layer, blood flow velocity, and blood flow volume
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative central nervous system disorder.1 Although inflammation within focal demyelinating lesions is prominent, widespread and subtle forms of inflammation were also found in the normal-appearing white and gray matter by histopathologic studies.2 These widespread, subtle inflammatory changes were correlated with markers of neural damage and loss,3 which subsequently results in disability.3 The subtle inflammation is difficult to monitor by routine MRI because they do not cause obvious blood-brain barrier damage that can be detected as gadolinium enhanced lesions. In addition, they may not cause any significant clinical symptoms or signs. Inflammation leads to mild alterations in the vascular function, such as endotheliopathy,4 resulting in decreased perfusion and neuronal damage. Studying cerebral microvascular alterations via MRI with special techniques and contrast injection revealed hypoperfusion in NAWGM.5,6
The visual pathways are frequently affected by MS, even in patients who have no visual symptoms.7 It has been suggested that MS causes primary retinopathy, which reflects global central nervous system atrophy.8 Especially, diffuse subtle inflammation throughout the retina were observed in postmortem studies besides localized inflammatory cellular infiltrates surrounding retinal veins.9 In addition, the presence of microcystic macular edema in patients with MS demonstrated by Optical Coherence tomography (OCT) studies may indicate an impaired blood-retinal-barrier and mild retinal inflammation.10 The vasculature in the eye and brain are anatomically and physiologically similar.11 Aside from the common occurrence of retinal periphlebitis,12 and increased retinal vessel rigidity suggesting vascular dysregulation,13 there is a lack of information to support the hypothesis that retinal microcirculation alteration exists in MS. The goal of the present study was to characterize the retinal microcirculation in patients with MS by directly measuring the blood flow velocity and flow volume in retinal arterioles and venules.
Methods
MS patients were referred from the MS Center of Excellence at the University of Miami to the neuro-ophthalmology clinic at the Bascom Palmer Eye Institute. The diagnosis of MS was made based on the Revised McDonald Criteria. Neurological disability was assessed according to the Expanded Disability Status Scale. The study was approved by the institutional review board for human research at the University of Miami and informed consent was obtained from each subject. All subjects were treated in accordance with the tenets of the Declaration of Helsinki. A total 17 MS patient and 17 age- and gender-matched healthy subjects were included (Table 1). The majority of the patients had disease onset of less than 5 years. Three patients were diagnosed as RRMS for more than 5 years. Five patients were on immunomodulating therapy: interferon β1-α (Avonex; Biogen, Cambridge, MA) (1 patient), Copaxone (Copaxone; Teva, Petah Tiqvah, Israel) (2 patients), dimethyl fumarate (Tecfidera, Biogen, Cambridge, MA) (1 patient) and fingolimod (Gilenya, Novartis, Basel, Switzerland) (1 patient). None of these medications is known to cause changes in vascular function, except for fingolimod, which is known to be associated with endothelial dysfunction.14 No patients received systemic corticosteroids within 3 months prior to the study. None of the eyes had a history of optic neuritis. Patients with high refractive errors of more than +6.0 or -6.0 diopters (due to the limit of the imaging device) or with any history of eye disease were excluded. Each MS patients had a screening ophthalmic examination by the investigator, including intraocular pressure (IOP) measurements, a slit-lamp examination and optical coherence tomography imaging (Cirrus, Carl Zeiss Meditec, Dublin, CA). High contrast visual acuity was tested, and all MS patients had best corrected visual acuity of 20/20. Color vision and visual field tests were normal. Large meals lower blood pressure,15 the ingestion of alcohol elevates blood pressure.16 In addition, physical exercise has an effect on blood pressure.17 These factors may impact blood pressure, which may result in possible measurement bias of retinal blood flow velocity. When scheduling study visits, patients and normal subjects were asked to avoid large meals and ingesting alcohol on the study day. They were also advised to avoid physical exercise for 24 h prior to the examination. During the study visit, we verbally confirmed the instructions with each of the study subjects. Subjects with history of cerebral vascular disease, hypertension and diabetes or kidney disease were excluded.
Table 1. Demography of MS patients and normal controls.
| MS Patient | Control | P value | |
|---|---|---|---|
| Number | 17 | 17 | |
| M:F | 6:11 | 6:11 | |
| Eye | 15 OD 2 OS | 17 OD | |
| Age (year) | 35.5 ± 9.2 | 35.5 ± 9.8 | 0.99 |
| Systolic blood pressure (mmHg) | 118.6 ± 9.9 | 112 ± 11.6 | 0.08 |
| Diastolic blood pressure (mmHg) | 77.9 ± 6.0 | 71.4 ± 8.2 | 0.01 |
| Mean arterial pressure (mmHg) | 91.5 ± 7.0 | 84.9 ± 8.9 | 0.02 |
| heart rate (per min) | 77.7 ± 8.0 | 75.5 ± 10.9 | 0.51 |
| Disease duration (year) | 4.8 ± 7.1 | ||
| EDSS | 1.8 ± 1.8 | ||
| RNFL (μm) | 90.9 ± 12.3 | ||
| IOP | 15.4 ± 1.5 |
OD: right eye; OS: left eye
The Retinal Function Imager (RFI, Optical Imaging Ltd, Rehovot, Israel) has been previously described in detail.18 Briefly, RFI is an advanced ophthalmic multimodal imaging modality, which uniquely measures retinal microvascular functions, including blood flow velocity in retinal small vessels, fluorescein free angiogram and retinal oximetry. A stroboscopic light source and a high resolution digital camera are used to rapidly take a series of retinal images; robust image processing software is then applied to automatically yield the measurements.18 RFI is cleared by the US Food and Drug Administration (FDA).19 The system uses hemoglobin in red blood cells as the intrinsic motion contrast agent for measuring the velocity of the red blood cells in retinal arterioles and venules. No contrast agent is needed and therefore the method is non-invasive.19 The measurements of blood flow velocities are reproducible with ∼11% variability.20
One eye of each subject was imaged using the RFI device. Each subject was asked to relax for 15 min before imaging. The pupil was dilated with 1% Tropicamide. The fovea of the subject was imaged at 20 degrees with a calibrated field of view of 4.6×4.6 mm2. The calibration of the field of view was done using a model eye with a fine print with a 1 × 1 mm2 grid patched onto the fovea, which was imaged with the RFI device. Multiple image sessions were performed. The RFI's proprietary software was used to determine retinal blood flow velocity in the arterioles and venules. The measured vessels covered the second, third and fourth branches of the retinal vessels. A blood flow velocity map (Fig. 1) of the arteries and venules was determined in all four quadrants in the entire field of view. The blood flow volume was calculated within a 2.3 mm circle (half of the total image width of 20 degrees) centered on the fovea. The diameter of the vessels which crossed the 2.3 mm circle was determined by counting the pixels of the full width and half of the maximum in the intensity profile, which was perpendicular to the vessel. The width in the pixel was then converted to micrometers. Using measured velocities and corresponding vessel diameters, the blood flow volume of each vessel crossing the 2.3 mm circle was calculated. The blood flow volume was computed based on the previously published equation.21 The total perifoveal flow volume (arteriole and venule, separately) in the circled perifoveal zone was the sum of all measured blood flow in the circle (Fig. 1). In addition, using the Cirrus OCT, a standard 200 × 200 scan protocol (with the optic disk centered) was used to measure peripapillary RNFL thickness of the MS patients.
Figure 1. Quantitative retinal blood flow velocity map of microvessels and retinal blood flow volume.
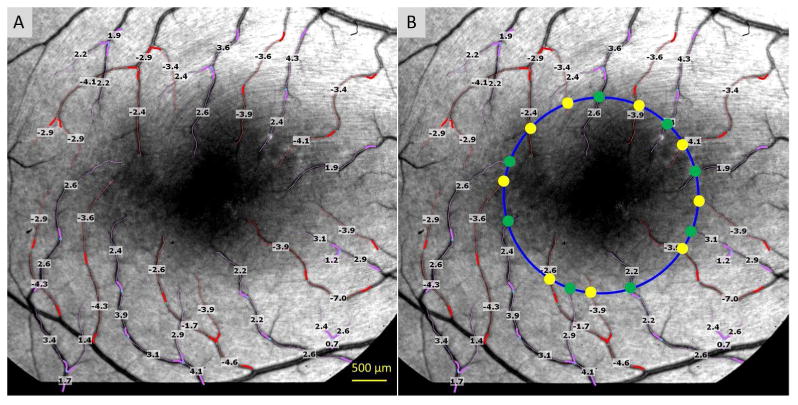
The retina of a healthy subject imaged using the RFI device with a field of view of 20 degrees centered on the fovea (dark area in the center) is shown (A and B). The arterioles are marked in red and overlaid with the measured blood flow velocities (mm/s); the venules and their respective velocities are marked in pink. The measured vessels cover the second, third and fourth branches of the retinal vessels. A negative value indicates blood flow away from the heart. In this case, the arteriolar flow moved towards to the fovea. A positive value indicates blood flow towards to the heart. In this case, the vessels are venules. A 2.3 mm circle (blue) is centered on the fovea (the dark area); vessel diameters were measured (as half width of full maximum intensity) at the locations (yellow and green dots) at the circle (B). Using the velocity and vessel diameter data, blood flow volumes were calculated for each arteriole (yellow dot) or venule (green dot). The total perifoveal flow volume of the arteriole is the sum of all arteriolar flow rates (all yellow dots). The total perifoveal flow volume of the venule was the sum of all venular flow rates (all green dots). The total perifoveal flow volume of this healthy subject was 4.68 nl/s in the arterioles and 4.50 nl/s in the venules.
A statistical software package (STATISTICA, StatSoft, Inc., Tulsa, OK) was used for descriptive statistics and data analysis. A paired t-test was used to test the differences between groups and vessels. Pearson's correlation coefficient was used to determine the relation between pairs. Stepwise regression analysis adjusted for blood pressure metrics was completed to compare velocities and flow volume between MS patients and controls. For each matched case-control pair the difference between dependent variables was calculated and the parallel differences of blood pressure metrics were allowed inclusion as candidate independent variables in a forward stepwise regression analysis. In addition, conditional case-control logistic regression analyses were performed with case-control status as the dependent variable and the following independent variables as candidates in a forward stepwise manner: velocity, flow volume and blood pressure metrics.22 Odds ratios were calculated.
Results
The baseline characteristics of MS patients are listed in Table 1. There were no significant differences in age (P = 0.99), gender (P = 1), systolic blood pressure (P = 0.08) and heart rate (P = 0.51) between the MS group (n = 17) and the control group (n = 17). The diastolic blood pressure (P = 0.01) and mean arterial blood pressure (P = 0.02) were higher in the MS group. The blood flow velocities in arterioles (3.34 ± 0.89 mm/s) and venules (2.61 ± 0.60 mm/s) in MS patients were significantly lower than controls (arteriole: 4.10 ± 0.87 mm/s, P = 0.01; venule: 3.22 ± 0.65 mm/s, P = 0.01, Fig. 2). In addition, the total perifoveal blood flow volume in the arterioles (3.74 ± 1.64 nl/s) and venules (3.81 ± 1.60 nl/s) of MS patients were significantly lower than that of the control (arteriole: 4.87 ± 1.41 nl/s, P = 0.02; venule: 3.74 ± 1.64 nl/s, P = 0.04, Fig. 2). The blood flow velocity was higher in the arterioles than the venules in both the MS (P = 0.001) and control (P = 0.001) groups (Fig. 2A). In contrast, blood flow volume of the arterioles was similar to that in the venules in both the MS (P = 0.42) and control group (P = 0.36) (Fig. 2B).
Figure 2. Scatterplot of retinal blood flow velocity and perifoveal flow volume of MS patients compared with age- and gender-matched controls.
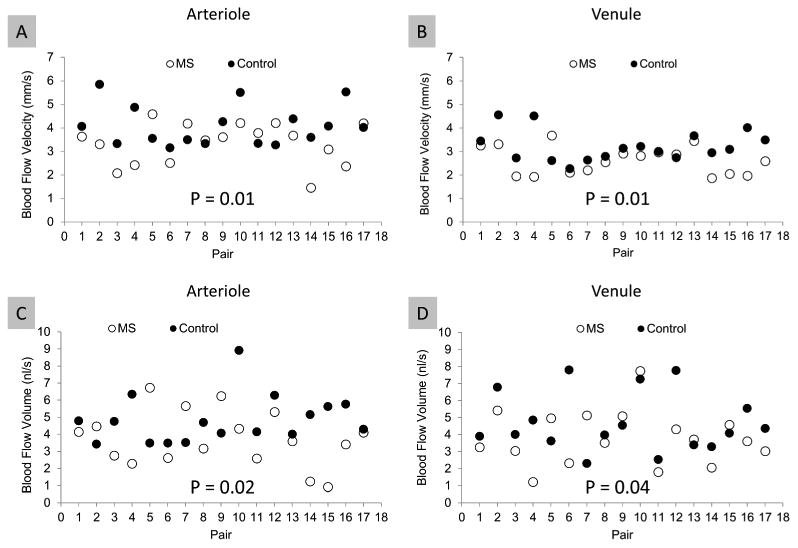
One eye of each subject was imaged with the RFI device, and blood flow velocities in arterioles and venules in the perifoveal region were measured. The blood flow volume of the perifoveal region was also calculated based on the vessel diameter crossing a circle of 2.3-mm, centered at the fovea. The mean blood flow velocities in MS subjects were significantly lower than controls in both the arterioles (A) and venules (B). Similarly, the mean perifoveal blood flow volume of both arterioles (C) and venules (D) were significantly lower in MS subjects than in controls. In both groups, the blood flow velocity was higher in arterioles than venules (A and B, P = 0.001). In contrast, the perifoveal blood flow volume of the arterioles was similar to that in the venules in both groups (C and D, P = 0.36).
The mean vessel diameter of the arterioles crossing the 2.3 mm circle was 18.0 μm (SD 2.1 μm), which showed a tendency of narrowing in MS patients compared to controls (19.1 ± 2.6 μm, mean ± SD, P = 0.05). Stepwise regression adjusted for blood pressure metrics in analyzing velocities and flow volume between groups showed that these measured blood pressure metrics as independent variables did not enter the model and did not have any influence on the differences in blood flow velocities and flow volume between groups (all P > 0.3). The univariate odds ratios are listed in Table 2. After including blood flow velocity in venules, no other variables were statistically significant in the regression model (all P > 0.05). Therefore, the velocity in venules alone was the discriminator with an odds ratio of MS 10.7 times higher for a 1 unit decrease.
Table 2. Odds ratios of measured parameters.
| Variable | Odds Ratio | P value |
|---|---|---|
| Arteriolar velocity | 2.77 | 0.02 |
| Venular velocity | 10.74 | 0.004 |
| Arteriolar flow volume | 1.5 | 0.059 |
| Venular flow volume | 1.69 | 0.062 |
| SBP | 0.94 | 0.079 |
| DBP | 0.9 | 0.02 |
| MAP | 0.91 | 0.033 |
| HR | 0.98 | 0.55 |
In MS patients, there were strong correlations of the blood flow velocity (r = 0.72, P = 0.001) and blood flow volume (r = 0.57, P = 0.02, Fig. 3) between the arterioles and venules. Similarly for the control, the blood flow velocity was strongly correlated (r = 0.83, P = 0.001) between the arterioles and venules. There was no significant difference (r = 0.40, P = 0.11) between the blood flow volumes of arterioles and venules in the control group. Both blood flow velocities in the arterioles (r = 0.16, P = 0.52) and venules (r = 0.12, P = 0.64) of the MS group were not correlated with RNFL (Fig. 4). Both blood flow velocities in the arterioles and venules were not correlated with expanded disability status scale (EDSS, arteriole, r = 0.20, P = 0.43; venule, r = 0.13, P = 0.61) and disease duration (arteriole, r = 0.15, P = 0.56; venule, r = 0.12, P = 0.63). In contrast, RNFL was negatively correlated with EDSS (r = -0.67, P = 0.003) and the disease duration (r = -0.58, P = 0.01, Fig. 4).
Figure 3. Relationships among vascular parameters and clinical manifestations in MS patients.
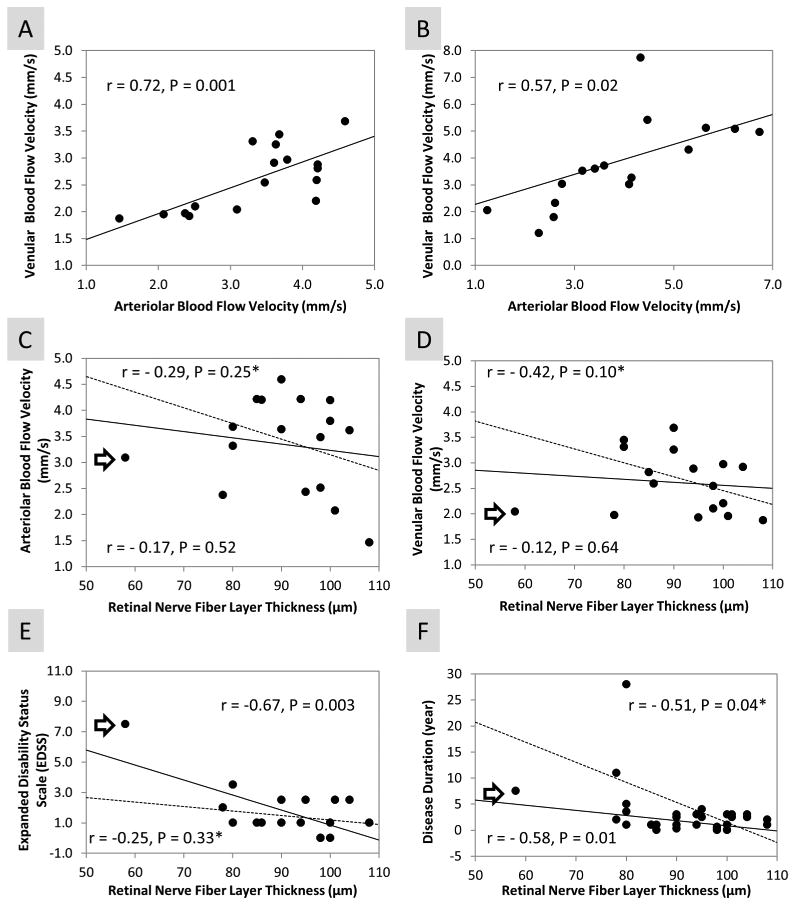
There were correlations of the blood flow velocity (A) and blood flow volume (B) between the arterioles and venules in MS patients. Both blood flow velocities in the arterioles and venules of the MS group were not correlated with RNFL (C and D). In contrast, RNFL thickness was negatively correlated with EDSS (E) and disease duration (F). In addition, an outlier (furthest left patient, marked by arrow) was removed for computing the relationships among RNFL and other measurements (C, D, E and F). The relationships (dashed lines) were included and marked by an asterisk (*). Similar relationships (C, D and F) were found in the MS group (n = 16) after the outlier was removed, compared to the whole MS group (n = 17). However, in the trimmed group, RNFL was no longer significantly related to EDSS (E).
Discussion
Retinal vasculature sits embedded in the retinal sublayers. Previous studies have indicated that retinal vasculopathies are evident,23 which may be associated with retinal inflammation.24 Postmortem studies showed localized inflammatory cellular infiltrates surrounding retinal veins.23An increased retinal inner nuclear layer imaged with optical coherence tomography has been suggested to potentially represent inflammation of the retina in MS.24 On the other hand, the adhesion of activated T lymphocytes observed within the blood-brain barrier is known to result in endotheliopathy and corresponding vascular dysregulation.25 Direct measurement of retinal blood flow velocity and flow volume may provide direct evidence for microcirculation impairment in MS. The decreased blood flow velocity and flow volume found in the present study could be the consequence of direct inflammatory damage to vessels or vascular dysregulation in MS.
To the best of our knowledge, this is the first study exploring retinal microcirculation in MS. Our results showed decreased retinal blood flow velocity and flow volume in patients with RRMS, indicating that microvascular abnormalities are present. However, we did not establish the relationships between RFI measurements and clinical characteristics such as EDSS, RNFL thickness and disease duration, which indicates that impaired microcirculation may be a primary issue, not secondary to underlying neuronal injury as is seen in most retinal diseases. Therefore, impaired microcirculation may be a harbinger of the frequently noted findings of RNFL axonal loss and GCL ganglion loss and may not be a result of disease progression. Impaired microcirculation could be further developed as a predictor of neurodegeneration, and further longitudinal studies will be needed to validate this viewpoint. Alternatively, the changes of blood flow velocity and volume may be simply due to the results of mechanical alternation in retinal architecture from neurodegeneration. However, this is unlikely because none of the eyes had a history of optic neuritis, and the relationship between the retinal blood flow metrics and RNFL thickness was not established. Our findings are compatible with slower blood flow in the cerebral arteries of patients with MS measured by Arterial Spin Labeling Magnetic Resonance Imaging26 and prolongation of the mean transit time, a measure of the average time contrast takes to transit from the arterial to venous circulation in normal appearing white matter, as described in the dynamic susceptibility contrast MRI study.6 The retinal hypoperfusion is in alignment with the observation of cerebral hypoperfusion in NAWGM in MS.27,28 The cerebral hypoperfusion is considered a major step that induces a status defined as energy failure in MS.29 According to a recent large scale clinical study on 1249 patients, MS lesions tend to occur in areas with lower perfusion.30
Various techniques have been used to study the ocular blood perfusion in MS patients with conflicting reports.31-33 Modrzejewska et al.31 found that the lowering of systolic and mean velocities and resistance indices of blood flow in the central retinal artery (CRA) and short posterior ciliary artery (SPCA) in MS eyes with past ON, and also in the contralateral unaffected eyes. However, Akcam et al.32 recently reported no change of blood flow velocity in central retinal artery, posterior ciliary arteries, ophthalmic artery and superior ophthalmic vein in MS patients with significant RNFL thinning, with or without a history of ON. These conflicting results indicate that MS vascular dysfunction is mainly located in the retinal microvasculature rather than the retrobulbar large vessels. Wang et al. used optical coherence tomography angiography (OCTA) to estimate a flow index (FI), a measure that comprises the area of large vessels, vessel density and indirect velocity of the capillaries.33 They found no changes of the FI in the perifoveal region in MS patients. The results by Wang et al. may not contradict our results because the FI may not exclusively represent the blood flow velocity or flow volume.33 In fact, the FI may represent the microvascular morphology (e.g., network) rather than the microcirculation in the retina; this may obscure the intrinsic microcirculation trends due to their respective global characterization. The RFI is an advanced ophthalmic imaging modality which can be used to directly quantify the blood flow velocity in microvessels, including pre-capillary arterioles and post-capillary venules. With the measurement of vessel diameters, the blood flow volume can be quantified; this may be applicable for studying the microcirculation, including the blood flow velocity and flow volume in the retina of patients with MS. As expected, we found the values of these microcirculation measurements using the RFI device helped to predict impaired microcirculation in MS patients. The sensitivity and specificity of the measurements were similar to that reported in a previous study which predicted slow coronary blood flow in patients with coronary artery disease, but normal cardiac angiogram.34
This study did not find a correlation between the retinal nerve fiber layer thickness (an established biomarker of MS) and the retinal microcirculation, which is echoed by a recent study using OCT angiography by Spain et al.35 Spain et al. did not find a relationship between the FI of the optical nerve head and RNFL thickness in MS patients. We speculate that the structural changes may be a cumulative process and the changes of the circulation may directly relate to the ongoing autoimmune inflammation or vascular dysregulation. The impairment of the retinal microcirculation may occur before or during the inflammatory blood brain barrier impairment. We noted that the mean vessel diameter of the arterioles crossing the 2.3 mm circle showed a tendency of vessel narrowing in MS patients compared to controls. Given the single point measurement with the small sample size in the present study, this may not necessarily indicate that vessel contraction occurred in MS. Measuring retinal thickness will reveal retinal structural alternation, which may not necessarily reflect retinal microvascular dysfunction revealed by the direct measurement. If retinal microvascular dysfunction occurs prior to retinal structural changes, measuring retinal thickness may not unveil the time course of retinal microvascular dysfunction and its interaction with the mechanical alternation in retinal architecture. The time course and interaction cannot be tested in the present study because it is a small cross-sectional study. Further longitudinal studies with MS patients at different disease stages and under various therapies may address the question.
In conclusion, the impaired retinal microcirculation in RRMS patients indicates microvascular dysfunction in MS. Longitudinal studies are needed to determine whether the impaired microcirculation is a primary issue and a predictor of underlying neurodegeneration in MS.
Acknowledgments
We would thank Mr. Jeffery Hernandez's help in recruiting the MS patients.
Study funding: Grant/financial support: Supported by National Multiple Sclerosis Society, NIH R01EY020607, R01EY020607S, NIH Center Grant P30 EY014801, a grant from Research to Prevent Blindness (RPB).
Footnotes
Disclosure: Dr. Jianhua Wang is a member of scientific advisory board of Optical Imaging Ltd. All other authors have no proprietary interest in any materials or methods.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 3.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12:154–169. doi: 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steen C, D'haeseleer M, Hoogduin JM, et al. Cerebral white matter blood flow and energy metabolism in multiple sclerosis. Mult Scler. 2013;19:1282–1289. doi: 10.1177/1352458513477228. [DOI] [PubMed] [Google Scholar]
- 6.Law M, Saindane AM, Ge Y, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231:645–652. doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- 7.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–1494. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- 8.Saidha S, Sotirchos ES, Oh J, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz-Perez S, Martinez-Lapiscina EH, Gabilondo I, et al. Retinal periphlebitis is associated with multiple sclerosis severity. Neurology. 2013;81:877–881. doi: 10.1212/WNL.0b013e3182a3525e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochkorov A, Gugleta K, Kavroulaki D, et al. Rigidity of retinal vessels in patients with multiple sclerosis. Klin Monbl Augenheilkd. 2009;226:276–279. doi: 10.1055/s-0028-1109291. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78:672–680. doi: 10.1212/WNL.0b013e318248deea. [DOI] [PubMed] [Google Scholar]
- 15.Visvanathan R, Horowitz M, Chapman I. The hypotensive response to oral fat is comparable but slower compared with carbohydrate in healthy elderly subjects. Br J Nutr. 2006;95:340–345. doi: 10.1079/bjn20051633. [DOI] [PubMed] [Google Scholar]
- 16.Puddey IB, Beilin LJ. Alcohol is bad for blood pressure. Clin Exp Pharmacol Physiol. 2006;33:847–852. doi: 10.1111/j.1440-1681.2006.04452.x. [DOI] [PubMed] [Google Scholar]
- 17.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Debuc DC, Rundek T, et al. Automated segmentation and fractal analysis of high-resolution non-invasive capillary perfusion maps of the human retina. Microvasc Res. 2013;89:172–175. doi: 10.1016/j.mvr.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klefter ON, Lauritsen AO, Larsen M. Retinal hemodynamic oxygen reactivity assessed by perfusion velocity, blood oximetry and vessel diameter measurements. Acta Ophthalmol. 2015;93:232–241. doi: 10.1111/aos.12553. [DOI] [PubMed] [Google Scholar]
- 20.Chhablani J, Bartsch DU, Cheng L, et al. Segmental reproducibility of retinal blood flow velocity measurements using retinal function imager. Graefes Arch Clin Exp Ophthalmol. 2013 doi: 10.1007/s00417-013-2360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koutsiaris AG, Tachmitzi SV, Batis N, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 22.Hosmer D, Lemeshow S. Apllied logistic regression. New York: John Wiley & Sons; 1989. pp. 187–213. [Google Scholar]
- 23.Green AJ. Getting beyond the ganglion cell: morphometric adjustments for retinal optical coherence tomography in multiple sclerosis. JAMA Neurol. 2013;70:13–15. doi: 10.1001/2013.jamaneurol.430. [DOI] [PubMed] [Google Scholar]
- 24.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res. 2006;28:230–235. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 26.Paling D, Thade PE, Tozer DJ, et al. Cerebral arterial bolus arrival time is prolonged in multiple sclerosis and associated with disability. J Cereb Blood Flow Metab. 2014;34:34–42. doi: 10.1038/jcbfm.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saindane AM, Law M, Ge Y, et al. Correlation of diffusion tensor and dynamic perfusion MR imaging metrics in normal-appearing corpus callosum: support for primary hypoperfusion in multiple sclerosis. AJNR Am J Neuroradiol. 2007;28:767–772. [PMC free article] [PubMed] [Google Scholar]
- 28.Inglese M, Adhya S, Johnson G, et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab. 2008;28:164–171. doi: 10.1038/sj.jcbfm.9600504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paling D, Golay X, Wheeler-Kingshott C, Kapoor R, Miller D. Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol. 2011 doi: 10.1007/s00415-011-6117-7. [DOI] [PubMed] [Google Scholar]
- 30.Holland CM, Charil A, Csapo I, et al. The Relationship between Normal Cerebral Perfusion Patterns and White Matter Lesion Distribution in 1,249 Patients with Multiple Sclerosis. J Neuroimaging. 2011 doi: 10.1111/j.1552-6569.2011.00585.x. [DOI] [PubMed] [Google Scholar]
- 31.Modrzejewska M, Karczewicz D, Wilk G. Assessment of blood flow velocity in eyeball arteries in multiple sclerosis patients with past retrobulbar optic neuritis in color Doppler ultrasonography. Klin Oczna. 2007;109:183–186. [PubMed] [Google Scholar]
- 32.Akcam HT, Capraz IY, Aktas Z, et al. Multiple sclerosis and optic nerve: an analysis of retinal nerve fiber layer thickness and color Doppler imaging parameters. Eye (Lond) 2014;28:1206–1211. doi: 10.1038/eye.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Jia Y, Spain R, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014;98:1368–1373. doi: 10.1136/bjophthalmol-2013-304547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbel Y, Sternfeld A, Barak A, et al. Inverse correlation between coronary and retinal blood flows in patients with normal coronary arteries and slow coronary blood flow. Atherosclerosis. 2014;232:149–154. doi: 10.1016/j.atherosclerosis.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Spain R, Che Z, Jia Y, Tan O. Decreased optic nerve head blod flow index hights abnormal retinal microcirculation in Multiple Sclerosis. Americian Academy of Neurology Annual Meeting. 2015:P5.225. [Google Scholar]


