Abstract
Introduction
Bariatric surgery is the most effective method for producing sustained weight loss, improving obesity-associated comorbidities and reducing inflammation in the morbidly obese population. The red cell distribution width (RDW) is a novel marker of inflammation that is usually reported as part of a complete blood count. In this study, we tested our hypothesis that red cell distribution width might represent a novel biomarker predictive of excess body-mass index loss (EBMIL) following laparoscopic Roux-en-Y gastric bypass (LRYGB).
Methods
Five hundred and forty-seven included LRYGB patients from a single institution were individually reviewed, noting both pre-operative RDW and percent excess BMI loss at six months and one year post-LRYGB (%EBMIL180 and %EBMIL365, respectively). Bivariate and multivariate linear regression analysis was conducted between age, gender, initial body-mass index (BMI0) and RDW and each of the two endpoints, to assess the independence of RDW as a predictor of post-operative success.
Results
The median RDW was 13.9 (13.3 – 14.6)%, and median EBMIL180 and EBMIL365 were 55.4 (45.2 – 66.7)% and 71.3 (58.9 – 87.8)%, respectively. After controlling for age, gender and BMI0, RDW was associated with %EBMIL365 (B = −1.4 [−2.8 – −0.002]%, P = .05), but not %EBMIL180 (B = −0.6 [−1.6 – 0.5]%, P = .30. Upon Kruskal-Wallis analysis, patients with a pre-operative RDW > 15.0% had significantly lower %EBMIL than those in the <13.0% (P < .001) and 13.0–15.0% (P < .01) strata.
Conclusions
RDW is predictive of EBMIL at one year following LRYGB. This represents a novel pre-operative biomarker that may provide clinically useful prognostic information.
Keywords: bariatrics, biomarkers, outcomes, laparoscopy, gastric bypass
Introduction
Morbid obesity is associated with chronic inflammation, a state that can be quantified with well-known biomarkers including C-reactive protein and erythrocyte sedimentation rate.1, 2 Bariatric surgery is the most effective treatment for morbid obesity, resulting in more weight loss, improvement in comorbidities and reduction in polypharmacy than any non-operative therapy.3–5 Of the commonly performed bariatric operations, the Roux-en-Y gastric bypass is perhaps the most effective, consistently leading to sustained weight loss in the compliant patient.6 It is typically performed laparoscopically, with few complications and rapid recovery. In anticipation of this operation, routine pre-operative blood work is often conducted to assess the patient’s iron stores, as post-operative anemia is a common, recognized sequela.7
The red cell distribution width (RDW) is a measurement that is usually reported as part of the complete blood count (CBC). The RDW has traditionally been used to assist in the differential diagnosis of anemia.8 Its value reflects the variability in volume in circulating erythrocytes, and is calculated by dividing the mean corpuscular volume (MCV) into the standard deviation of the MCV of circulating erythrocytes.8 More recently, RDW has been recognized as a marker of both chronic inflammation and oxidative stress.8, 9 Its elevation has been noted in many inflammatory diseases such as cancer and rheumatologic conditions, as well as in hemorrhage, among other pathologic states.8 In this study, we tested the hypothesis that, perhaps as a surrogate for systemic inflammation, the preoperative RDW is a biomarker that predicts weight loss after laparoscopic Roux-en-Y gastric bypass (LRYGB).
Materials and Methods
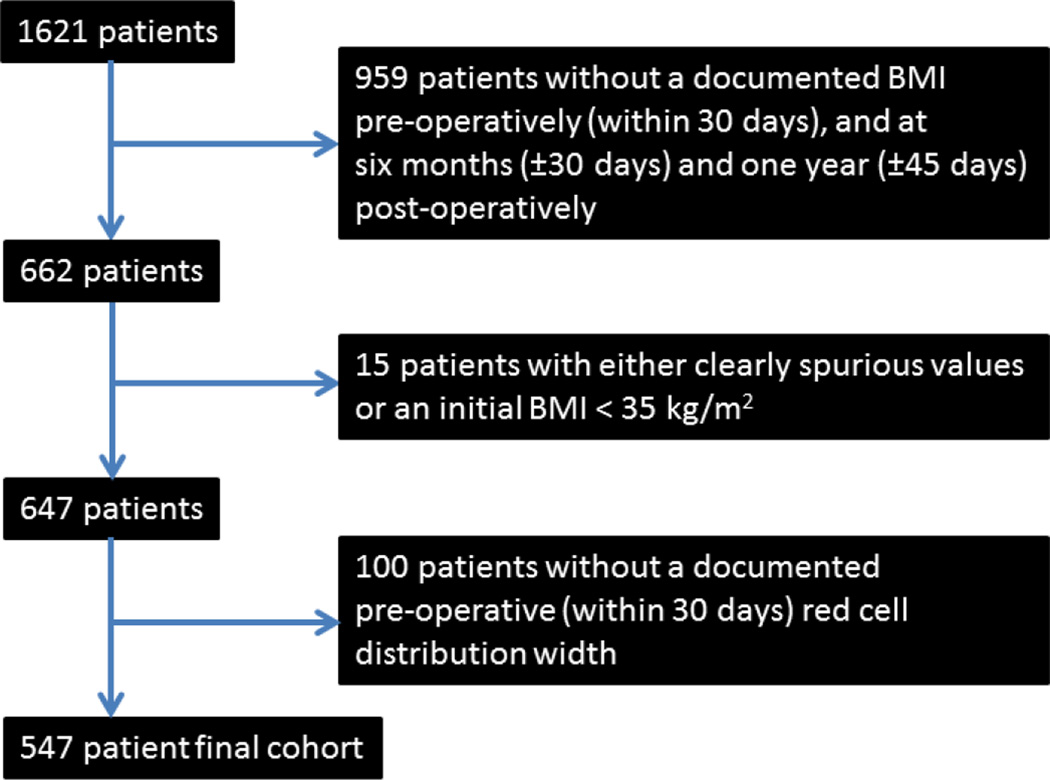
This was a single institution retrospective study using the Synthetic Derivative de-identified database at Vanderbilt University.10 As no patient identifiers were available, this study was approved with waiving of informed consent by the Vanderbilt University Institutional Review Board. The primary endpoints were percent excess BMI loss (over 25 kg/m2) at 180 ± 30 days and 365 ± 45 days post-operatively (%EBMIL180 and %EBMIL365, respectively), defined as 100%*(BMI0 − BMIt)/(BMI0−25 kg/m2), where BMI0 is the preoperative BMI and BMIt is the postoperative BMI at either 180 or 365 days from surgery.11, 12 Between 2004 and 2013, we identified 547 consecutive patients who had primary LRYGB for morbid obesity (BMI > 35 kg/m2) with RDW obtained within thirty days prior to surgery, and BMI documented preoperatively (BMI0), 180 days postoperatively and 365 days postoperatively. Patients with missing data or re-operation during the follow up period were excluded from the analysis. Derivation of the study cohort is outlined in Figure 1. Gender and age were also noted for each patient as these variables, in addition to BMI0, represented three critical factors for which a priori statistical adjustment was felt necessary, to determine the presence of an independent association of RDW with EBMIL. Variables related to operative technique (e.g. pouch size, Roux limb length, etc.) were not considered in this analysis.
Figure 1.
Derivation of the 547 patient study cohort
The strength of associations of BMI0, gender, age and RDW with each of the two endpoints were assessed first using bivariate linear regression analysis, and subsequently using standard least squares multivariate linear regression analysis. As RDW was independently associated with %EBMIL365, further stratification was performed as patients were grouped into three RDW classifications: <13.0%, 13.0–15.0% and >15.0%. Differences in %EBMIL365 among these classifications were assessed using the Kruskal-Wallis test with Dunn’s post-hoc test of multiple comparisons. Central tendency was expressed by median (interquartile range). All statistical analysis was performed using GraphPad Prism 5 (La Jolla, CA). The value P ≤ .05 was used as the criterion for statistical significance.
Results
Pre-operative variables for the 547 cohort are summarized in Table 1. Notably, the median RDW was 13.9 (13.3 – 14.6)%, and median EBMIL180 and EBMIL365 were 55.4 (45.2 – 66.7)% and 71.3 (58.9 – 87.8)%, respectively. Upon bivariate linear regression analysis, both BMI0 (B = −1.1 [−1.2 – −1.0]%, P < .001) and RDW (B = −3.4 [−4.6 – −2.2]%, P < .001) were significantly associated with %EBMIL180, while male gender (B = −8.9 [−13.2 – −4.6]%, P < .001), BMI0 (B = −1.4 [−1.5 – −1.2]%, P < .001) and RDW (B = −4.6 [−6.2 – −3.0]%, P < .001; Figure 2A) were significantly associated with %EBMIL365 (Table 2).
Table 1.
Baseline characteristics and outcome measures from the 547 patient cohort
| Pre-operative Variable (n = 547) | Median (Interquartile Range) or % (Number) |
|---|---|
| Male Gender | 20% (n = 112) |
| Age at Surgery (years) | 47.6 (38.8 – 55.8) |
| Initial Body-Mass Index (BMI0; kg/m2) | 47.3 (42.6 – 53.4) |
| Red Cell Distribution Width (%) | 13.9 (13.3 – 14.6) |
| Outcomes | |
| EBMIL, six months post-operatively (%) | 55.4 (45.2 – 66.7) |
| EBMIL, one year post-operatively (%) | 71.3 (58.9 – 87.8) |
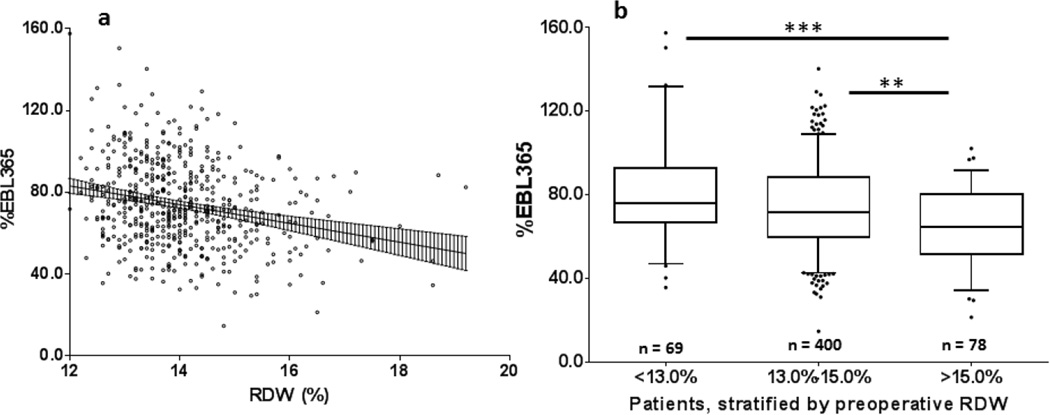
Figure 2.
Graphical depictions of the relationship between red cell distribution width (RDW) and percent excess body-mass index loss at one year postoperatively (%EBMIL365)
a- Simple linear regression analysis of %EBMIL365 vs. RDW revealed a significant relationship between the variables (P < .001, Pearson r2 = .06, n = 547). Linear regression line shown with 95% confidence band
b- Box-and-whisker plots of %EBMIL365, by preoperative RDW stratum. Whiskers represent 5th and 95th percentiles, box represents interquartile range, median represented by horizontal line within box, outliers represented as individual points. Kruskal-Wallis statistic = 18.6, P < .001, n = 547; **P < .01, ***P < .001.
Table 2.
Bivariate linear regression analysis of pre-operative factors with excess BMI loss at both six months and one year post-operatively.
| Pre-operative Variable |
B (95% CI), EBMIL, 6 months |
P value, EBMIL, 6 months |
B (95% CI), EBMIL, 1 year |
P value, %EBMIL, 1 year |
|---|---|---|---|---|
| Male Gender | −1.5 (−4.8 – 1.8)% | .37 | −8.9 (−13.2 – −4.6)% | <.001 |
| Age at Surgery | −0.05 (−0.2 – 0.07)% | .39 | −0.1 (−0.3 – 0.04)% | .15 |
| BMI0 | −1.1 (−1.2 – −1.0)% | <.001 | −1.4 (−1.5 – −1.2)% | <.001 |
| RDW | −3.4 (−4.6 – −2.2)% | <.001 | −4.6 (−6.2 – −3.0) | <.001 |
Upon multivariate analysis, age at surgery (B = −0.2 [−0.3 – −0.1]%, P < .001) and BMI0 (B = −1.2 [−1.3 – −1.0]%, P < .001) were independently associated with %EBMIL180, while male gender (B = −7.6 [−11.3 – −4.1]%, P < .001), age at surgery (B = −0.3 [−0.4 – −0.2]%, P < .001), BMI0 (B = −1.4 [−1.5 – −1.2]%, P < .001) and RDW were all independently associated with %EBMIL365 (B = −1.4 [−2.8 – −0.002]%, P = .05). Full results of the multivariate analysis are reported in Table 3. The multiple linear regression expression for prediction of %EBMIL365 was:
Estimated %EBMIL365 = 167.2 + −0.29(age, yrs.) + 7.68(if female) + −1.36(BMI0, kg/m2) + −1.42(RDW, %).
Upon Kruskal-Wallis analysis, patients with a pre-operative RDW > 15.0% had significantly lower %EBMIL than those in the <13.0% (P < .001) and 13.0–15.0% (P < .01) strata (Figure 2B).
Table 3.
Multivariate linear regression analysis of pre-operative factors with excess BMI loss at both six months and one year post-operatively. Multivariate regression for %EBMIL, six months: r2 = .36, n = 547, F = 76.2, P < .001; Multivariate regression for %EBMIL, one year: r2 = .33, n = 547, F = 67.4, P = <.001.
| Preoperative Variable |
B (95% CI), EBMIL, 6 months |
P value, EBMIL, 6 months |
B (95% CI), EBMIL, 1 year |
P value, %EBMIL, 1 year |
|---|---|---|---|---|
| Male Gender | −0.4 (−3.1 – −2.3)% | .76 | −7.6 (−11.3 – −4.1)% | <.001 |
| Age at Surgery | −0.2 (−0.3 – −0.1)% | <.001 | −0.3 (−0.4 – −0.2)% | <.001 |
| BMI0 | −1.2 (−1.3 – −1.0)% | <.001 | −1.4 (−1.5 – −1.2)% | <.001 |
| RDW | −0.6 (−1.6 – 0.5)% | .30 | −1.4 (−2.8 – −0.002)% | .05 |
Discussion
RDW has been identified as a useful biomarker, predicting outcomes in numerous disease states, including peripheral vascular disease, diabetes, autoimmune diseases13, 14, chronic obstructive pulmonary disease and renal failure.22, 23 RDW has been most extensively studied in cardiovascular disease where it has demonstrated prognostic utility in coronary artery disease and heart failure following interventions.15–18 For example, RDW elevation is associated with increased mortality after percutaneous coronary intervention and transcatheter aortic valve implantation.19, 20 Not surprisingly, obesity, a disease state associated with increased mortality, is associated with increased RDW; as such, controlling for initial BMI was necessary in this study.
Pathophysiologically, it is accepted that RDW summarily reflects the inflammatory state, oxidative stress and nutritional deficiencies.21, 22 The mechanism of impaired weight loss in patients with elevated RDW is not clear, and can only be speculated. Chiefly, a higher RDW may reflect a higher burden of pre-operative comorbidity, as these patients may be less capable of adhering to the diet and exercise regimen required, more likely to take medications that inhibit successful weight loss and more susceptible to physiologic stress of the operation itself. Next, LRYGB is associated with malabsorption of vitamin B12 and other critical nutrients known necessary for successful weight loss.7 Patients with an RDW reflecting a baseline anemia and nutritional deficiency may have a more difficult time adapting to the hormonal and nutritional pertubations experienced after bypass, and concomitantly a more unfavorable profile of post-prandial gut hormone release.23–25 Finally, the anisocytosis reflected in the elevation of RDW leads to impaired microcirculation, diminished peripheral tissue oxygenation, promoting sustained inflammation and oxidative stress in the post-operative state.21 Vaya and colleagues report that in obese patients, elevations in RDW do not reflect systemic inflammation, as the values are not associated with other established inflammatory markers. Rather, RDW may reflect severity of pre-operative hyposideremia.2 While the mechanism of this association requires further investigation, it may provide clinical utility for bariatric patients independent of gender, age and BMI0.
Excess body-mass index loss after bariatric surgery is governed by pre-operative, intra-operative and post-operative considerations. Intra-operative and post-operative considerations include length of the Roux limb and those factors related to the ability for the patient to adhere to appropriate diet and exercise recommendations, as well as the presence of an appropriate support system to avoid recidivism. However, much of the variance in post-operative EBMIL in LRYGB patients can be captured from pre-operative patient variables.5 Multiple pre-operative variables have been shown to predict short-term operative success. Initial BMI has been the pre-operative factor most consistently predictive, however, comorbidities such as hypertension, diabetes and psychiatric disorders, as well as race, gender and the presence of pre-operative weight loss have all been reported as well.3, 6, 26–28In this study, 547 LRYGB patients over a ten year period from a single institution were retrospectively reviewed to test our hypothesis that RDW may predict EBMIL. We aimed to determine the association between RDW and EBMIL, however, there was a need to control for the baseline demographics of gender, age and BMI0 such that any significance in our suspected correlation could have wide applicability and validity. In simple bivariate linear correlations, %EBMIL180 was inversely related to both higher BMI0 and higher RDW; %EBMIL365 was similarly inversely related to both higher BMI0 and higher RDW, but also to male gender. Upon multivariate analysis, RDW was no longer associated with EBMIL180, but remained so with EBMIL365 (B = −1.4% per unit RDW, P = .05).
Clinically, stratification of the preoperative RDW in bariatric surgery patients may be a simpler way to predict post-operative weight loss. As such, differences EBMIL365 was analyzed among patients grouped into classes based on RDW: <13%, 13–15% or >15%. From this analysis, pre-operative RDW >15% portends significantly lower EBMIL365 relative to the other two groups; conversely, patient with RDW <13% do not necessarily benefit relative to those patients within the 13–15% range.
Our results must be taken in the context of several limitations. These findings result from retrospective data collection of patients who adhered to appropriate six-month and one-year postoperative follow-up, potentially introducing a selection bias. External validation is also required; as such, we report the multiple linear regression expression for the prediction of EBMIL365 derived from our 547 patient cohort. Additionally, the preoperative RDW values may have been unduly influenced by factors that are wholly unrelated to the success of the operation, including a transient inflammatory condition, recent hemorrhage or blood transfusion.8 Next, the endpoint of %EBMIL365 reflects a time frame during which active weight loss is generally ongoing and thus, provides only a short-term measure of the success of the operation. Finally, we have not considered the correlation between RDW and reduction of comorbities or polypharmacy, perhaps more important endpoints to some patients than weight loss. Further studies are warranted to both overcome the limitations of this report and to better define the mechanism of impaired weight loss as a function of chronic inflammation as indicated by RDW as a surrogate measure.
Acknowledgments
Dr. Colleen Brophy, M.D., Professor of Surgery, Vanderbilt University
Vanderbilt University Dept. of Biostatistics
Funding:
Vanderbilt RedCAP: CTSA Award UL1 TR000445 from NCATS/NIH
Footnotes
This manuscript is anticipated as a poster at the 2016 Annual Meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES).
Disclosures:
Eric Wise, Kyle Hocking, Adam Weltz, Anna Uebele, Jose Diaz, Stephen Kavic and Mark Kligman have no conflicts of interest to disclose.
References
- 1.Careaga M, Moize V, Flores L, Deulofeu R, Andreu A, Vidal J. Inflammation and iron status in bariatric surgery candidates. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2014 doi: 10.1016/j.soard.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Vaya A, Alis R, Hernandez-Mijares A, Sola E, Camara R, Rivera L, et al. Red blood cell distribution width is not related with inflammatory parameters in morbidly obese patients. Clinical biochemistry. 2014;47(6):464–466. doi: 10.1016/j.clinbiochem.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Still CD, Wood GC, Chu X, Manney C, Strodel W, Petrick A, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity. 2014;22(3):888–894. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA : the journal of the American Medical Association. 2013;309(21):2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 5.Benoit SC, Hunter TD, Francis DM, De La Cruz-Munoz N. Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obesity surgery. 2014;24(6):936–943. doi: 10.1007/s11695-014-1197-y. [DOI] [PubMed] [Google Scholar]
- 6.Wise ES, Hocking KM, Kavic SM. Prediction of excess weight loss after laparoscopic Roux-en-Y gastric bypass: data from an artificial neural network. Surgical endoscopy. 2015 doi: 10.1007/s00464-015-4225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Beek ES, Monpellier VM, Eland I, Tromp E, van Ramshorst B. Nutritional deficiencies in gastric bypass patients; incidence, time of occurrence and implications for post-operative surveillance. Obesity surgery. 2015;25(5):818–823. doi: 10.1007/s11695-014-1456-y. [DOI] [PubMed] [Google Scholar]
- 8.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Critical reviews in clinical laboratory sciences. 2014:1–20. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 9.Dong X, Liao Y, Chen K, Fang Y, Li W, Chen J, et al. Elevated red blood cell distribution width in benign prostatic hyperplasia patients with metabolic syndrome. International journal of clinical and experimental medicine. 2015;8(1):1213–1219. [PMC free article] [PubMed] [Google Scholar]
- 10.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical pharmacology and therapeutics. 2008;84(3):362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Laar A. Bariatric Outcomes Longitudinal Database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obesity surgery. 2012;22(12):1843–1847. doi: 10.1007/s11695-012-0736-7. [DOI] [PubMed] [Google Scholar]
- 12.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015;11(3):489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Peng YF, Zhang Q, Cao L, Liu Y, Chen D, Sun YK, et al. Red blood cell distribution width: a potential maker estimating disease activity of ankylosing spondylitis. International journal of clinical and experimental medicine. 2014;7(12):5289–5295. [PMC free article] [PubMed] [Google Scholar]
- 14.Hu ZD, Chen Y, Zhang L, Sun Y, Huang YL, Wang QQ, et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clinica chimica acta; international journal of clinical chemistry. 2013;425:202–205. doi: 10.1016/j.cca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. Journal of the American College of Cardiology. 2007;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 16.Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Watanabe H, et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. Journal of cardiology. 2015 doi: 10.1016/j.jjcc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Khaki S, Mortazavi SH, Bozorgi A, Sadeghian S, Khoshnevis M, Mahmoodian M. Relationship Between Red Blood Cell Distribution Width and Mortality of Patients with Acute Myocardial Infarction Referring to Tehran Heart Center. Critical pathways in cardiology. 2015;14(3):112–115. doi: 10.1097/HPC.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 18.Gurel OM, Demircelik MB, Bilgic MA, Yilmaz H, Yilmaz OC, Cakmak M, et al. Association between Red Blood Cell Distribution Width and Coronary Artery Calcification in Patients Undergoing 64-Multidetector Computed Tomography. Korean circulation journal. 2015;45(5):372–377. doi: 10.4070/kcj.2015.45.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poludasu S, Marmur JD, Weedon J, Khan W, Cavusoglu E. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thrombosis and haemostasis. 2009;102(3):581–587. doi: 10.1160/TH09-02-0127. [DOI] [PubMed] [Google Scholar]
- 20.Collas VM, Paelinck BP, Rodrigus IE, Vrints CJ, Van Craenenbroeck EM, Bosmans JM. Red cell distribution width improves the prediction of prognosis after transcatheter aortic valve implantation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2015 doi: 10.1093/ejcts/ezv152. [DOI] [PubMed] [Google Scholar]
- 21.Bujak K, Wasilewski J, Osadnik T, Jonczyk S, Kolodziejska A, Gierlotka M, et al. The Prognostic Role of Red Blood Cell Distribution Width in Coronary Artery Disease: A Review of the Pathophysiology. Disease markers. 2015;2015:824624. doi: 10.1155/2015/824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita B, Strodthoff D, Fritzenwanger M, Pfeil A, Ferrari M, Goebel B, et al. Altered red blood cell distribution width in overweight adolescents and its association with markers of inflammation. Pediatric obesity. 2013;8(5):385–391. doi: 10.1111/j.2047-6310.2012.00111.x. [DOI] [PubMed] [Google Scholar]
- 23.Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2015 doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. International journal of obesity. 2013;37(11):1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 25.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of surgery. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 26.Alger-Mayer S, Polimeni JM, Malone M. Preoperative weight loss as a predictor of long-term success following Roux-en-Y gastric bypass. Obesity surgery. 2008;18(7):772–775. doi: 10.1007/s11695-008-9482-2. [DOI] [PubMed] [Google Scholar]
- 27.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. The Cochrane database of systematic reviews. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obesity surgery. 2012;22(1):70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]