Abstract
Background
Low levels of energy expenditure (TEE) may contribute to excess weight during childhood, but limited longitudinal data exist.
Objectives
Test whether low TEE during the first six years of life could predict excess weight status at 8y.
Methods
TEE from doubly labeled water, weight, stature, waist circumference and fat mass and fat-free mass (FFM) in children at 0.25, 2, 4, and 6 years of age. This cohort includes individuals at high- (n=27) and low-risk (n=26) for childhood obesity, based upon whether pre-pregnant maternal obesity. A linear mixed effects model was fit to TEE. Individual variation was accounted for as a random effect. Residual TEE was calculated for age and individually averaged across time.
Results
FFM (kg) was highly correlated (R2=0.91) with TEE (kcal/d), and waist circumference, and sex were also significant predictors of TEE. TEE residual tracked within individuals. TEE residuals did not correlate with either BMI or %fat at age 8y.
Conclusion
Using the residual TEE approach to identify high and low TEE during the first 6 years of life did not explain excess weight at 8 years of life in this cohort of children at high and low risk of obesity based upon maternal obesity status.
Keywords: Obesity, children, energy expenditure, body composition, growth, dietary energy
The prevalence of childhood obesity between 1980 and 2010 has tripled. This increase is a result of undue positive energy balance, however, the individual roles of energy expenditure and energy intake in childhood obesity are controversial. For example, Roberts et al. followed 18 infants and found that subjects who became overweight age one year had a significantly lower TEE at 0.25 years than subjects who did not become overweight.1 In contrast, Davies et al followed 33 infants from 0.25 to 2 years of age and found no relationship between TEE at 0.25 years of age and adiposity at 0.75 or 2 years.2 Among older children, DeLany et al. examined the relationship of TEE on adiposity in lean and obese 9–11 year old children and found that TEE with or without adjustment for height was negatively associated with subsequent increased fat mass.3. In contrast, Johnson et al, found no association between TEE and increase in ratio of body fat mass to fat-free mass and black and white children between 5 and 11 years old.4 They did, however, find an inverse relationship between fitness and adiposity suggesting a role for physical activity independent of TEE.
These studies have focused on the first year of life or the later years of childhood. Few studies included the period of adiposity rebound, after which the association between childhood obesity and adult obesity increases.5,6 To address this gap, the Infant Growth Study (IGS) was initiated to examine the roles of energy intake and expenditure in children from 0.25 to 8 years, thus included adiposity rebound.7 Infants born to lean or obese mothers (pre-pregnancy BMI <33 percentile or >66th percentile) were followed from age 0.25 through 8 years. Early IGF findings have been reported, including data on TEE and weight status at 1 and 2 years of age.7–12 Findings included markers of energy intake, but not energy expenditure contributed to excess body weight and adiposity at age 1 year.8 These two reports, however, preceded the age of adiposity rebound.5,6 BMI typically decreases until the age of 4 to 7 years, before beginning to increase through late childhood, adolescence, and into adulthood and the characteristics of the rebound predict adult BMI.5 These findings suggest that it may be important to examine TEE through the period of BMI rebound.
The IGS included measures of TEE at 0.25, 2, 4, 6 years allowing us to test whether low TEE from during these years predicted excess adiposity at age 8 years.
SUBJECTS AND METHODS
Subjects
The IGS enrolled 81 subjects who were classified as low-risk children (lean mothers, (average BMI 19.5±1.1 kg/m2) or high-risk (overweight or obese mothers, average BMI = 30.3±4.2). All subjects were White to minimize the differences in growth pattern due to race.7 Details on study design are reported elsewhere.7–12 The IGS was approved by Internal Review Boards of the institutions and written informed consent was provided by parents.
Subject Exclusion
Of those 81 children, 70 subjects remained in the study at age 8 years. Three measures of adiposity were used in current analysis: BMI percentile, BMI z-score, and percent body fat (% BF) at 8 years of age. Not all subjects completed all measures included in the protocol resulting in more exclusions (Supplemental Figure 1). Eight subjects completed all four TEE measurements, 33 completed three, 22 completed two, 13 completed only one, and five did not complete any. This resulted in 188 TEE measures from 76 participants between ages 0.25 and 6 years, a TEE participation rate of 58%. Eleven subjects did not complete the body composition analysis at age 8 years, a participation rate of 86%. After these exclusions, there were 53 subjects (29 females and 24 males) in the BMI percentile and BMI z-score outcome analysis. Of these 53 subjects, 27 were at high-risk for the development of obesity and 26 were at low-risk. For the %fat outcomes, 19 of the subjects were missing body composition measurements at 8 years and 14 had less than 2 TEE measures, leaving 41 subjects (19 females and 22 males) who were included in the %fat outcome analysis. Of these, 19 were in the high-risk group and 22 in the low-risk group.
Measures
Total Energy Expenditure
TEE was measured using the doubly labeled water (DLW) method as described previously.9 Briefly, a baseline urine was collected and subjects drank 1 g of 10 atom percent 18O water and 0.15 g of 99.9 atom percent 2H water per kg body weight. Urines were collected on days 1 and 7, isotope abundance s were measured by mass spectrometry in the laboratories of the University of Wisconsin, and CO2 production and TEE was calculated as summarized by Thorson.13
Growth and Body Composition
Subject protocol visits included those at 0.25, 2, 4, 6, and 8 years of age. Length/height, weight and waist circumference (WC) were measured as described previously. 7–12 Body composition was measured by Total Body Electrical Conductivity (HP2-TOBEC; EM-SCAN Incorporated, Springfield, IL) at age 0.25 and 2 years,8,9 and by Dual-energy X-ray Absorptiometry (DXA) (2DR 2000; Hologic Inc, Bedford MA) at 4 and 6 years,11 using standardized protocols.14
Statistical Analysis
Analyses were performed using R version 2.8.1 (R Foundation Statistical Computing, Vienna, Austria). A TEE prediction equation was developed using stepwise regression including fat-free mass (FFM), fat mass (FM), waist circumference (WC), BMI, BMI z-score, BMI percentile, length/height, weight, and sex in a linear mixed effects model until all remaining predictors were significant (p<.05).
Residual TEE was calculated for each TEE measurement and the TEE prediction model. TEE residuals were averaged within each subject to determine whether subjects had persistently high TEE (higher TEE than predicted) or low TEE residuals (lower TEE than predicted) by regressing residual against BMI percentile, BMI z-score, and %fat at age 8 years, respectively.
To calculate energy intake, we applied the energy balance equation:
where EI denotes metabolizable energy intake, ES denotes change in body energy stores, and TEE represents total energy expenditure (kcal/d). The value for ES was estimated by calculating the average change in FFM and FM in kg/d denoted by . The changed values of kg of FFM and FM were determined from data collected at the endpoints, and the interval duration of time in days (Δt).15 ES was calculated by converting the average change in body stores using the respective energy density constants:15
For each interval, TEE values at beginning and end were averaged. It was assumed that ES and TEE increased linearly over the time. 15,16
RESULTS
The subject physical characteristics at each age for the children who had at least one TEE measure (n=75) are summarized in Supplemental Table 1. Participants were classified by BMI percentile at age 8 years as underweight, healthy weight, overweight, and obese (<5, 5–85, 85–95, and >95 percentile). Characteristics at age 0.25 years between these BMI categories did not differ.
Linear mixed effects models were developed to predict TEE from the potential factors FFM (kg), FM (kg), WC (cm), BMI (kg/m2), BMI z-score, BMI percentile, height (cm), weight (kg), and sex (1=female) for the 75 subjects with at least one TEE measure. For all models, FFM was the predictor that was most highly correlated with TEE (R2=0.91, syx= 125 kcal/d). One data point which was 8 standard deviations off the line had a large influence on the regression was removed from this and further analyses (Supplemental Figure 2).
After that exclusion, step-wise rejection of potential predictors identified four predictors, which along with their univariate correlations with TEE, were FFM (r2=0.89), p<0.00001), WC (r2=0.42, p<0.00001), sex (r2=0.03, p=0.02), and whether the child was an infant (r2=0.63, p<0.00001). The resulting prediction equation was:
where sex = 1 or 0 for females or males respectively, and infant = 1 for 0.25 years of age or 0 for ages 2, 4 and 6 years. The standard deviation for the prediction (sy,x) was 104 kcal/d. Based on forced step-wise regression, FFM was the major predictor, whereas sex, infant and WC explained only an additional 5, 4, and 3% of the variance, respectively.
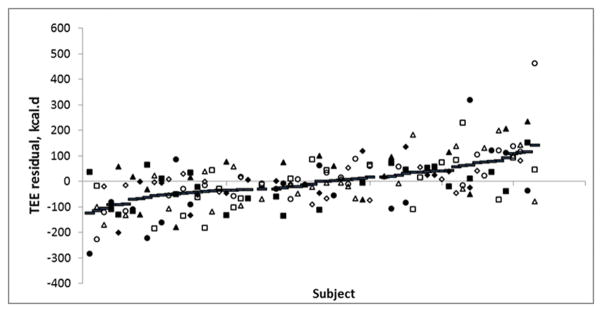
Of the 75 subjects with at least one TEE measurement, there were 98 TEE residuals from 39 low-risk individuals, and 87 from 36 high-risk individual (low= 2±96 kcal/d, high= −4±113 kcal/d), a nonsignificant difference. When ranked according to average TEE residual, within individual residuals ranged from 142 to −124 kcal/d and residuals did cluster within individuals (p<0.05) (Figure 1). We also investigated linear regression models. Average TEE residual across age 0.25 to 6 years was regressed against BMI percentile at age 8 years. There was no significant association between average TEE residual from 0.25 to 6 years of age and BMI percentile at age 8 (R2=0.01, NS). Similarly, no association with average residual was found for BMI z-score at age 8 years (R2=0.004, NS) and percent body fat at age 8 (R2=0.047, NS).
Figure 1.
TEE Residuals by subject, ranked by average TEE residual. Each subject is plotted with their individual TEE residual value as well as their average TEE residual (lines). Open and closed symbols are the low risk and high risk individuals, respectively. Diamonds, squares, triangles and circles indicate ages 0.25, 2, 4, and 6 years, respectively.
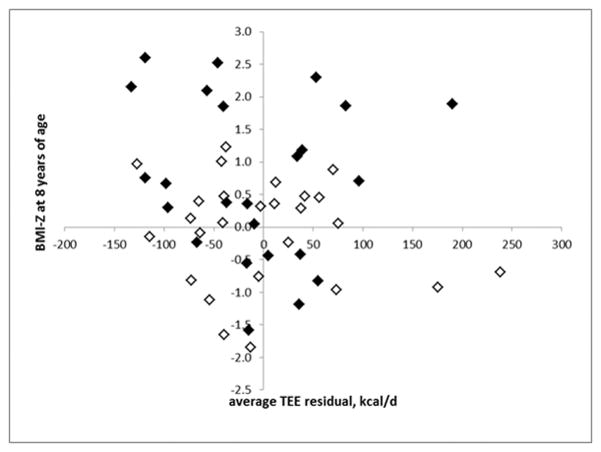
To investigate smaller time intervals, we regressed the TEE residual at each age against the change in BMI during the following year (Figure 2) and there was no correlation (R2=0.005, NS). Similarly, we regressed individual TEE residuals against change in %fat during the subsequent year but this limited us to changes between ages 0.25 and 1 year, and 4 and 6 years because different body composition methods before and after age 2 years. We found no correlation (R2=0.005, NS). This was repeated with the residual expressed as percent of TEE for that individual at that age and neither regression was significant (R2 = 0.018 and 0.012)
Figure 2.
Average TEE Residual vs. BMI Percentile at Age 8 years. There is no significant association (r2 = 0.020) between average TEE residual and BMI Percentile. Open symbols are the low risk group and closed symbols are the high risk group.
Unadjusted total energy expenditure at intervals from 0.25 to 6 years (last age that TEE was measured) was calculated for subjects who were underweight (n=2), typical weight (n=41), overweight (n=3), and obese (n=9) at age 8 years. The subjects who were obese at age 8 years expended more energy than healthy weight subjects, but differences were not significant until after age 2 years (Figure 3).
Figure 3.
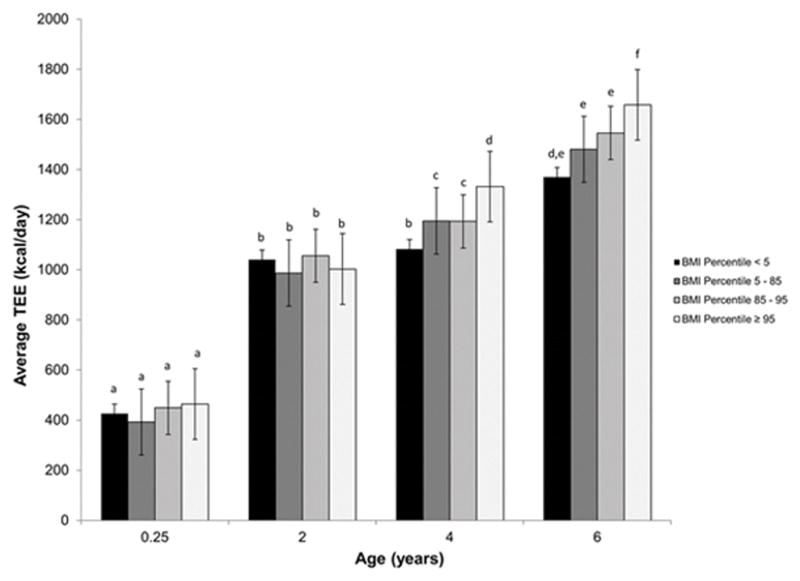
Average TEE vs. age for underweight, healthy, overweight, and obese subjects as classified by BMI percentile at 8 years of age. TEE increases with age and body weight. At 3 months age 2 years, there is no association between average TEE and 8-year BMI percentile. At ages 4 and 6 differences in TEE are found for the difference outcomes at age 8 as indicated by different letters within the age grouping (p>0.05).
Calculated cumulative metabolizable energy intake from 0.25 to 8 years was lowest in the underweight group and highest in the obese group, as expected (Supplemental Figure 3). The obese group consumed approximately 748,000 kcal more over the first 8 years of life than the healthy weight group. This corresponds to an average daily difference of 256 kcal/day.
DISCUSSION
The principal findings from this study were that TEE in children increases with body size and there was no evidence supporting the hypothesis that a low habitual TEE for that body size leads to subsequent increases in BMI or % fat in our study cohort of children followed from 0.25 to 8 years of age. There was no association between adiposity measures expressed as BMI z-score or % body fat at age 8 years and TEE residual from 0.25 to 6 years expressed in kcal/d or as the percentage of the child’s age specific TEE.
As expected, energy expenditure was highly correlated with FFM, and weight was not a significant predictor of TEE when the model also included FFM, reflecting that FFM is more metabolically active than FM.17 Similar to our findings, Zakeri and colleagues reported that FFM explained 75 to 85% of variation in energy expenditure in 5 to 19 year old subjects, while FM explained only 2 to 3% of the variation.18 In addition to FFM, we found WC was a minor, but significant predictor of TEE. This is consistent with the use of WC in adults and adolescents as a proxy measure of central adiposity and a predictor of BMI and FM.19–21 Sex was the other predictor of TEE in this cohort. It explained little of the total variance in slope, but identified a systematic sex difference in which males expended 62±15 kcal/day more than females when controlled for FFM. This is similar to Butte et al. who reported that males had 3.6% (=43 kcal/day for our cohort) higher TEE than females 22,23 and Goran et al. who reported males had a 59 kcal/day higher TEE than females in children aged 4 to 10 years.24 Goran et al. reported this was due a higher resting metabolic rate.24
Our finding that TEE is greater in obese compared to healthy weight children extends the cross-sectional report by Butte et al. who demonstrated that obese children aged 4 to 19 years had a higher, unadjusted 24-hour energy expenditure than in non-overweight children.22 In our cohort, the weight difference between children at age 6 years classified as obese vs. healthy weight at age 8 years was approximately 5 kg. According to the energy costs predicted from cross-sectional data by Butte et al., this difference in weight predicts a higher total daily energy cost of approximately 244 kcal/d assuming similar physical activity level (PAL), height, sex, and pubertal development stage, which is quite similar to the value derived from our longitudinal study.23 Both analyses come to the conclusion that the weight gain was not due to decreased or low energy expenditure.22,23
This absence of correlation between body size adjusted energy expenditure and excess relative weight and fat at 8 years of life is consistent with some of the studies in this controversial research area. Goran and colleagues found that energy expenditure measured at age 5 years, did not predict FM gain or loss in 75 children followed from age 5 to 9 years.24, 25 Instead, they found that the main predictors of growth in were initial fatness at age 5 years, parental weight status, and sex. Johnson et al also reported that energy expenditure at baseline in 115 Black and White children did not explain any of the variance in increasing adiposity over the next 3 to 5 years.4 A study from Davies et al. as well as a subanalysis from the IGS found that weight status at 1 year of life, was not correlated with energy expenditure at 0.25 years of age.2,9 In contrast, Roberts et al. found that six who became overweight by age 1 year had lower TEE at 0.25 years of age than the twelve subjects who did not become overweight.2 This was supported by a larger study by Delany et al found that low energy expenditure at baseline among 114 Black and White prepubertal children predicted increased in percent body fat when measured two years later.3 In that study, however, adjusted TEE only explained a small percent of the variation in excess adiposity. Taken together, these studies indicate that at most a small fraction of excess weight or fat gain is due to low TEE after adjustment for body size.
Following the lead of Butte et al, we calculated excess energy intake associated with the development of obesity in our longitudinal cohort.23 We found the difference in cumulative energy intake from 0.25 to 8 years between those subjects who were obese at age 8 years and those who were healthy weight was 748,000 kcal, an average energy intake of 256 kcal/day more than the healthy weight group. Other findings from the IGS, included positive relationships with various eating behaviors that were thought to be markers of high consumption including, high rates of nutritive suckling, rapid eating, eating in the absence of hunger or energy consumption, with excess weight gain. Roberts et al also reported that the infants who became overweight in the first year of life consumed 42% more energy during test period than the infants that remained at healthy weight.26
Our failure to find a relationship between low TEE and increasing adiposity may explain why physical activity interventions alone in children have been led to small or no reductions in adiposity,27 although there may other compensation in play.28 This, however, does not imply that such interventions are without value because interventions to increase physical activity have improved metabolic markers of health.29 Our study, however, cannot explain why nutritional interventions alone have little impact on the incidence of childhood obesity, but the combination of the two has been more successful.27
There are limitations to our study. The sample was entirely White, the children were recruited based on low or high maternal BMI, and the sample size was relatively small; therefore, our sample should not be assumed to reflect the entire childhood population in the United States. Despite the small sample size and missing measures, we had the ability to detect a difference of 87 kcal/day in average TEE residuals between overweight and healthy weight subjects over 6 years if such a difference existed. Also, TEE measurements were relatively infrequent, so there may have been periods of lower TEE that were not measured and corresponded with weight gain. Finally, our validated body composition measurement methods were different at 0.25–2 years than they were at 4–8 years; however, we only tracked change when the same method was used.
Our study also lacks a direct measure of dietary energy intake. This, however, is not so much a limitation of our study as it is a limitation of methods for directly measuring dietary energy intake. A recent review found parental and childhood dietary reports lacked reliability and accuracy.30 As such, we applied the first law of thermodynamics and calculated dietary energy intake from repeated measures of energy expenditure and change in body energy stores. 15, 16
CONCLUSIONS
Low energy expenditure was not the primary factor leading to excess weight gain in the eight obese children in this sample and energy intake was likely more important. This further complements earlier findings regarding the importance of food intake patterns in the development of obesity. 2,4,8,9,23,25 It should also be noted the eight children who became obese were from the high maternal BMI group and thus are most likely to represent those whose major obesity risk factor is high maternal BMI. These findings suggest that obesity prevention efforts aimed at improving children’s diets are more likely to be effective at obesity prevention than those aimed at increasing physical activity, although increasing physical activity has many other health benefits and should be encouraged, especially when performed in conjunction with nutrition interventions.29
Supplementary Material
Acknowledgments
Funding: This work was funded by National Institutes of Health grants K08 MH01530 and DK068899, Children’s Hospital of Philadelphia Clinical and Translational Research Center (NIH/NCATS grant UL1TR000003, and the Nutrition and Growth Laboratory of Children’s Hospital of Philadelphia.
Abbreviations
- BMI
Body Mass Index
- DLW
Doubly Labeled Water
- FFM
Fat Free Mass
- FM
Fat Mass
- IGS
Infant Growth Study
- TEE
Total Energy Expenditure
- WC
Waist Circumference
Footnotes
Contributor’s Statement
AJ Stunkard was intellectual leader for the creation and design of the longitudinal study. It is regrettable that he has died before this manuscript was completed. RI Berkowitz, VA Stallings, M Faith, and DA Schoeller participated in the concept and design of the study. SRJ Zinkel and DA Schoeller performed the data analysis and were the primary authors. DM Thomas collaborated on the mathematical modeling. All living authors participated in the interpretation of data, drafting of the manuscript and approved the manuscript as submitted.
Financial Disclosure: The authors have no personal financial relationships relevant to this article.
Conflict of Interest: The authors have no conflict of interest to report.
References
- 1.Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers. N Engl J Med. 1988;318:461–466. doi: 10.1056/NEJM198802253180801. [DOI] [PubMed] [Google Scholar]
- 2.Davies PS, Day JM, Lucas A. Energy expenditure in early infancy and later body fatness. Int J Obes. 1991;15:727–731. [PubMed] [Google Scholar]
- 3.DeLany JP, Bray GA, Harsha DW, Volaufova J. Energy expenditure and substrate oxidation predict changes in body fat in children. Am J Clin Nutr. 2006;84:862–70. doi: 10.1093/ajcn/84.4.862. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MS1, Figueroa-Colon R, Herd SL, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106:E50–E54. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 5.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond) 2006;30(Suppl 4):S11–17.7. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- 6.Simmonds M, Burch J, Llewellyn A, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19:1–336. doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stunkard AJ, Berkowitz RI, Stallings VA, Cater JR. Weights of parents and infants: is there a relationship? Int J Obes Relat Metab Disord. 1999;23:159–162. doi: 10.1038/sj.ijo.0800785. [DOI] [PubMed] [Google Scholar]
- 8.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord. 2004;28:503–513. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- 9.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 10.Berkowitz RI, Moore RH, Faith MS, Stallings VA, Kral TV, Stunkard AJ. Identification of an obese eating style in 4-year-old children born at high and low risk for obesity. Obesity (Silver Spring) 2010;18:505–512. doi: 10.1038/oby.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkowitz RI, Stallings VA, Maislin G, Stunkard AJ. Growth of children at high risk of obesity during the first 6 y of life: implications for prevention. Am J Clin Nutr. 2005;81:140–146. doi: 10.1093/ajcn/81.1.140. [DOI] [PubMed] [Google Scholar]
- 12.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring) 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 13.Thorsen T, Shriver T, Racine N, Richman BA, Schoeller DA. Doubly labeled water analysis using cavity ring-down spectroscopy. Rapid Commun Mass Spectrom. 2011;25:1–8. doi: 10.1002/rcm.4795. [DOI] [PubMed] [Google Scholar]
- 14.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champagne, IL: Human Kinetics; 1988. [Google Scholar]
- 15.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92:1326–1331. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jonge L, DeLany JP, Nguyen T, et al. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007;85:73–79. doi: 10.1093/ajcn/85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb P. Energy expenditure and fat-free mass in men and women. Am J Clin Nutr. 1981;34:1816–1826. doi: 10.1093/ajcn/34.9.1816. [DOI] [PubMed] [Google Scholar]
- 18.Zakeri I, Puyau MR, Adolph AL, Vohra FA, Butte NF. Normalization of energy expenditure data for differences in body mass or composition in children and adolescents. J Nutr. 2006;136:1371–1376. doi: 10.1093/jn/136.5.1371. [DOI] [PubMed] [Google Scholar]
- 19.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Br Med J. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maffeis C, Grezzani A, Pietrobelli A, Provera S, Tato L. Does waist circumference predict fat gain in children? Int J Obes Relat Metab Disord. 2001;25:978–983. doi: 10.1038/sj.ijo.0801641. [DOI] [PubMed] [Google Scholar]
- 21.Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tato L. Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res. 2001;9:179–187. doi: 10.1038/oby.2001.19. [DOI] [PubMed] [Google Scholar]
- 22.Butte NF, Puyau MR, Vohra FA, Adolph AL, Mehta NR, Zakeri I. Body size, body composition, and metabolic profile explain higher energy expenditure in overweight children. J Nutr Dec. 2007;137:2660–2667. doi: 10.1093/jn/137.12.2660. [DOI] [PubMed] [Google Scholar]
- 23.Butte NF, Christiansen E, Sorensen TI. Energy imbalance underlying the development of childhood obesity. Obesity (Silver Spring) 2007;15:3056–3066. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- 24.Goran MI, Nagy TR, Gower BA, et al. Influence of sex, seasonality, ethnicity, and geographic location on the components of total energy expenditure in young children: implications for energy requirements. Am J Clin Nutr. 1998;68:675–682. doi: 10.1093/ajcn/68.3.675. [DOI] [PubMed] [Google Scholar]
- 25.Goran MI, Shewchuk R, Gower BA, Nagy TR, Carpenter WH, Johnson RK. Longitudinal changes in fatness in white children: no effect of childhood energy expenditure. Am J Clin Nutr. 1998;67:309–316. doi: 10.1093/ajcn/67.2.309. [DOI] [PubMed] [Google Scholar]; 29 DeLany JP, Harsha DW, Kime JC, Kumler J, Melancon L, Bray GA. Energy expenditure in lean and obese prepubertal children. Obes Res. 1995;3(Suppl 1):67–72. doi: 10.1002/j.1550-8528.1995.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts SB. Nutritional Needs of the Six to Twelve Month Old Infant. In: Heird WC, editor. Carnation Nutrition Education Series. Vol. 2. Glendale Raven Press Ltd; NY: 1991. [Google Scholar]
- 27.Williams AJ, Henley WE, Williams CA, Hurst AJ, Logan S, Wyatt KM. Systematic review and meta-analysis of the association between childhood overweight and obesity and primary school diet and physical activity policies. Int J Behav Nutr Phys Act. 2013;10:101. doi: 10.1186/1479-5868-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thivel D1, Aucouturier J, Metz L, Morio B, Duché P. Is there spontaneous energy expenditure compensation in response to intensive exercise in obese youth? Pediatr Obes. 2014;9:147–54. doi: 10.1111/j.2047-6310.2013.00148.x. [DOI] [PubMed] [Google Scholar]
- 29.Ho M1, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167:759–68. doi: 10.1001/jamapediatrics.2013.1453. [DOI] [PubMed] [Google Scholar]
- 30.Bryant M, Ashton L, Nixon J, et al. Framework of outcome measures recommended for use in the evaluation of childhood obesity treatment interventions: the CoOR framework. Ped Obes. 2014;9:e116–e131. doi: 10.1111/j.2047-6310.2014.220.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.