Abstract
BACKGROUND
Antiretroviral medications that are used as prophylaxis can prevent acquisition of human immunodeficiency virus type 1 (HIV-1) infection. However, in clinical trials among African women, the incidence of HIV-1 infection was not reduced, probably because of low adherence. Longer-acting methods of drug delivery, such as vaginal rings, may simplify use of antiretroviral medications and provide HIV-1 protection.
METHODS
We conducted a phase 3, randomized, double-blind, placebo-controlled trial of a monthly vaginal ring containing dapivirine, a non-nucleoside HIV-1 reverse-transcriptase inhibitor, involving women between the ages of 18 and 45 years in Malawi, South Africa, Uganda, and Zimbabwe.
RESULTS
Among the 2629 women who were enrolled, 168 HIV-1 infections occurred: 71 in the dapivirine group and 97 in the placebo group (incidence, 3.3 and 4.5 per 100 person-years, respectively). The incidence of HIV-1 infection in the dapivirine group was lower by 27% (95% confidence interval [CI], 1 to 46; P = 0.05) than that in the placebo group. In an analysis that excluded data from two sites that had reduced rates of retention and adherence, the incidence of HIV-1 infection in the dapivirine group was lower by 37% (95% CI, 12 to 56; P = 0.007) than that in the placebo group. In a post hoc analysis, higher rates of HIV-1 protection were observed among women over the age of 21 years (56%; 95% CI, 31 to 71; P<0.001) but not among those 21 years of age or younger (-27%; 95% CI, −133 to 31; P = 0.45), a difference that was correlated with reduced adherence. The rates of adverse medical events and antiretroviral resistance among women who acquired HIV-1 infection were similar in the two groups.
CONCLUSIONS
A monthly vaginal ring containing dapivirine reduced the risk of HIV-1 infection among African women, with increased efficacy in subgroups with evidence of increased adherence. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT01617096.)
More than half of the 35 million persons currently living with human immunodeficiency virus type 1 (HIV-1) infection are women. A majority of these women reside in sub-Saharan Africa,1 a region that has some of the highest incidences of HIV-1 infection in any population worldwide.2–4 The use of antiretroviral medications as pre-exposure prophylaxis is a promising approach to the prevention of HIV-1 acquisition.5 Several clinical trials of the antiretroviral tenofovir showed such protection against HIV-1.2,6–8 However, in three trials involving African women, adherence to tenofovir-containing pills and vaginal gels was low, and HIV-1 protection was not shown.3,4,9 Across trials of tenofovir-based prophylaxis, a sizable proportion of participants were not adherent, a finding that emphasizes the need for additional options, particularly ones that women can control and longer-acting approaches that do not require daily or coitally dependent use.5
Vaginal rings can provide sustained and controlled release of medications. For example, rings containing exogenous hormones are licensed for contraception and estrogen replacement.10 For HIV-1 prevention, an antiretroviral-containing vaginal ring could provide long-acting HIV-1 protection while reducing systemic exposure to the active pharmaceutical ingredient and delivering the anti–HIV-1 agent at the site of viral transmission. Dapivirine is a non-nucleoside HIV-1 reverse-transcriptase inhibitor that has activity against a broad range of HIV-1 subtypes. In two phase 1 trials,11,12 genital biopsy tissue samples obtained from women using dapivirine vaginally in the form of a ring, films, and gels were substantially less susceptible to HIV-1 when challenged ex vivo than were tissue samples obtained from placebo-treated women. A monthly vaginal ring containing dapivirine was found to be safe and acceptable in phase 1 and 2 studies, with typical plasma levels of the drug that were lower by a factor of 1000 than levels in women receiving oral dapivirine.11–1 5 We conducted a phase 3, randomized, double-blind, placebo-controlled trial of the dapivirine vaginal ring among African women.
METHODS
Study Population
From August 2012 through June 2015, we enrolled and followed healthy, sexually active, non-pregnant, HIV-1–seronegative women between the ages of 18 and 45 years at 15 research sites in Malawi, South Africa, Uganda, and Zimbabwe (Tables S1 and S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org).16 The primary objectives were to determine the efficacy and safety of the dapivirine vaginal ring as compared with a placebo ring; after insertion, the ring is used for 4 weeks and then replaced with another. Community members from each site provided input into trial design and conduct. The trial protocol, which is available at NEJM.org, was approved by the ethics review committee at each site (Table S3 in the Supplementary Appendix). All participants provided written informed consent.
Study Procedures
At enrollment, women were assigned in a 1:1 ratio, with the use of fixed-size block randomization, stratified according to site, to receive either a silicone elastomer vaginal matrix ring containing 25 mg of dapivirine or a placebo vaginal ring. Both the dapivirine and placebo rings were manufactured by QPharma under contract with the International Partnership for Microbicides. The dapivirine and placebo rings were indistinguishable, and with the exception of staff members at the central statistical and data management center, investigators and participants were unaware of the randomization assignments until completion of the trial. Women were taught how to insert and remove the vaginal ring and counseled to wear it for the entire month.
Women returned for monthly follow-up visits, which included HIV-1 serologic testing, safety monitoring, and individualized adherence counseling (Table S4 in the Supplementary Appendix). At each visit, a new ring was provided, and the ring that had been used during the previous month was collected. Women were tested monthly for pregnancy, and the study ring was withheld from women who became pregnant; they resumed use of the study ring when no longer pregnant or lactating. All participants received a package of HIV-1 prevention services, including counseling with respect to HIV-1 risk reduction, partner HIV-1 testing, treatment of sexually transmitted infections in participants and partners, and free condoms. (Details regarding the trial design are provided in the Supplementary Appendix.)
Objective Assessment of Adherence
Plasma samples that were collected quarterly were tested for the presence of dapivirine with the use of a validated ultra-performance liquid chromatography–tandem mass spectrometry assay (Clinical Pharmacology Analytical Laboratory), with a lower limit of quantification of 20 pg per milliliter.17 To aid in distinguishing cases in which the ring was removed during the month and then reinserted before a clinic visit, the detection of a plasma dapivirine level of more than 95 pg per milliliter (a level nearly always achieved within 8 hours of continuous use) was used to define adherence.13,14 While the trial was ongoing, plasma samples were assayed and results were reviewed by the trial leaders. To preserve blinding, samples from both the dapivirine group and the placebo group were tested, and results were summarized only as the percentage of samples with dapivirine detected, overall and for each site. After the first year of the trial, testing for residual dapivirine in used rings was initiated with the use of acetone extraction and high-pressure liquid chromatography (Parexel). Women were defined as being adherent if the returned ring contained less than 23.5 mg of dapivirine (i.e., with >1.5 mg released).13,14
Primary End Points
The primary efficacy end point was HIV-1 infection, identified with the use of a standard seroconversion algorithm (Fig. S1 in the Supplementary Appendix). The study ring was temporarily withheld while confirmatory testing was pending and was permanently discontinued if testing confirmed HIV-1 acquisition. Archived plasma samples from visits before seroconversion were tested for HIV-1 RNA on polymerase-chain-reaction (PCR) assay, and participants with detectable HIV-1 RNA at the time of enrollment were excluded from the primary analysis. Participants completed a final study visit 4 weeks after the last product-use visit to assess for delayed HIV-1 seroconversion, and women who tested positive for HIV-1 at that visit and who had detectable HIV-1 RNA at the last product-use visit were included in the primary analyses because HIV-1 infection had occurred during the product-use period.
The primary safety end point was a composite of any serious adverse event, any grade 3 or 4 adverse event, and any grade 2 adverse event that was assessed by the trial clinicians as being related to dapivirine.
Study Oversight
The National Institutes of Health funded the trial. The authors designed the trial, gathered and analyzed the data, prepared the manuscript, and were responsible for the decision to submit the manuscript for publication. The International Partnership for Microbicides supplied the vaginal rings, was the regulatory sponsor, and participated in the design of the trial, the interpretation of the results, and the preparation of the manuscript. The ring manufacturer, QPharma, had no role in the design or implementation of the trial. The authors vouch for the accuracy and completeness of the data and analyses.
Statistical Analysis
The trial was designed with power of 90% to detect a risk of HIV-1 infection that was 60% lower in the dapivirine group than in the placebo group, with a one-sided alpha level of 0.025. Like other trials of new HIV-1 prevention interventions,6, 7,1 8 this trial was powered so that the lower boundary of the 95% confidence interval would exclude a 25% reduction in risk, with the primary analysis comparison for the trial planned against a standard null of 0% (Table S5 in the Supplementary Appendix). Under these assumptions, a minimum of 120 HIV-1 acquisition events would be required to achieve the statistical power posited in the design of the trial. An end-point–driven design was used, and the trial continued until the target number of HIV-1 end points had been accrued and all participants had been followed for a minimum of 12 months, in accordance with regulatory guidance regarding compilation of safety information for new HIV-1 prevention strategies.19
An annual incidence of HIV-1 infection of 3.9% in the placebo group was assumed, and a sample size of 3476 women was planned. After the trial started, another HIV-1 prevention trial that was conducted at several sites in our trial showed an HIV-1 incidence of more than 5% per year.3 As a result, in October 2013, the sample size for this trial was recalculated to approximately 2600 women, and the statistical analysis plan was modified accordingly. At the same time, the analysis plan was further modified for a fully powered analysis that would exclude all data from 2 of the 15 sites, since these 2 sites had shown lower-than-anticipated participant retention and lower product adherence (with adherence levels of <50%, according to measurement of plasma dapivirine levels) than at the other sites. Further enrollment at the 2 sites was discontinued, but enrolled participants were permitted to continue in follow-up. The sample-size recalculation and plan to exclude data from the 2 sites were approved by the independent data and safety monitoring board and reviewed by regulatory agencies. Interim statistical monitoring, which was performed on the basis of data from all 15 sites, used the Lan–DeMets spending approach to adjust the O’Brien–Fleming sequential monitoring boundaries.20,21
The primary analysis of HIV-1 protection was performed according to the intention-to-treat principle with the use of Cox regression, stratified according to site, to estimate the relative rates of time until HIV-1 acquisition. Two analyses were defined: one included data from all 15 sites and a second excluded data from the 2 sites at which enrollment had been discontinued early (i.e., 13 sites were included). Prespecified subgroup analyses were planned. When it was determined that age was significantly related to the efficacy of HIV-1 protection, a post hoc analysis was designed to characterize more fully that relationship by dividing the population into age-categorized thirds containing approximately equal numbers of participants with HIV-1 infection, thus balancing the statistical power for the exploratory subgroups. All analyses were conducted with the use of SAS software, version 9.4 (SAS Institute), and R software, version 2.15.1 (R Project for Statistical Computing). A P value of less than 0.05 was considered to indicate statistical significance, and all P values are two-sided.
RESULTS
Study Participants
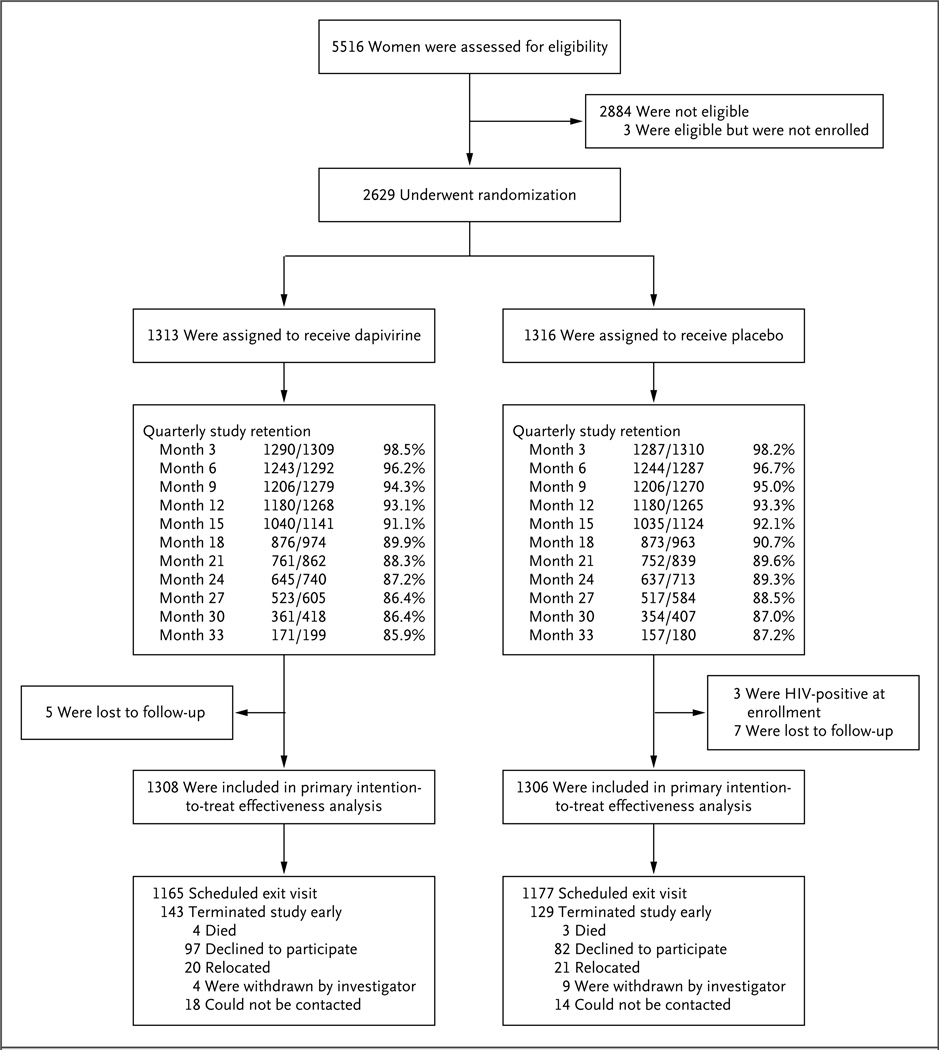
Of 5516 women who underwent screening, 2629 were enrolled: 1313 in the dapivirine group and 1316 in the placebo group (Fig. 1). The median age was 26 years (interquartile range, 22 to 31). Less than half (41%) were married, and 85% had completed some secondary schooling. Nearly all (99.5%) reported having had a primary sex partner during the 3 months before trial enrollment, and 17% reported more than one partner; 57% reported the use of a condom with the most recent sex act. Transactional sex in the previous year was reported by 6%, and anal sex during the previous 3 months by 2%. Nearly two thirds (64%) reported that their primary partner was aware that they would be using a vaginal ring in a research trial. Characteristics were similar in the two groups (Table 1).
Figure 1. Enrollment and Outcomes.
Of the 5516 women who underwent screening, 2629 were enrolled, 2884 were not eligible, and 3 were eligible but did not enroll. The most common reason for ineligibility was seropositivity for HIV-1. Participants were enrolled at 15 study sites: 9 in South Africa, 3 in Zimbabwe, 2 in Malawi, and 1 in Uganda. Of the 2629 women who enrolled, 1426 (54%) were from South Africa, 678 (26%) from Zimbabwe, 272 (10%) from Malawi, and 253 (10%) from Uganda. The per-site enrollment numbers are provided inTable S1 in the Supplementary Appendix. Trial retention was defined as the provision of an HIV-1 test result, and women who withdrew early from the study were counted as having missed visits thereafter. Participants attended 91% of scheduled study visits (97% after accounting for early withdrawals from the study). Interruptions in the use of the study rings owing to pregnancy and breast-feeding accounted for 2% of study follow-up time, and protocol-required, safety-related temporary interruptions of product use accounted for less than 1%.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Dapivirine Group (N = 1313) |
Placebo Group (N = 1316) |
|---|---|---|
| Age — yr | ||
| Mean | 27.2±6.1 | 27.3±6.3 |
| Median (range) | 26 (18–44) | 26 (18–45) |
| Secondary school education or higher — no. (%) | 1101 (84) | 1124 (85) |
| Earns own income — no. (%) | 605 (46) | 581 (44) |
| Currently married — no. (%) | 527 (40) | 547 (42) |
| Partner aware of ring use — no. (%) | 835 (64) | 845 (64) |
| Two or more male sex partners in the past 3 mo — no. (%) | 212 (16) | 227 (17) |
| No. of episodes of vaginal intercourse in the past 3 mo | 26.4±24.3 | 26.5±24.9 |
| Condom use during last vaginal sex — no. (%) | 775 (59) | 733 (56) |
| Anal sex in the previous 3 mo — no. (%) | 25 (2) | 29 (2) |
| Transactional sex in past yr — no. (%)† | 73 (6) | 89 (7) |
| Contraceptive method — no. (%) | ||
| Intrauterine device | 162 (12) | 163 (12) |
| Oral contraceptive pills | 143 (11) | 144 (11) |
| Depot medroxyprogesterone acetate | 515 (39) | 555 (42) |
| Norethisterone enanthate | 200 (15) | 181 (14) |
| Hormonal implant | 258 (20) | 243 (18) |
| Sexually transmitted infection — no. (%) | ||
| Chlamydia trachomatis | 175 (13) | 141 (11) |
| Neisseria gonorrhoeae | 58 (4) | 51 (4) |
| Trichomonas vaginalis | 91 (7) | 90 (7) |
Plus–minus values are means ±SD. There were no significant differences between the two groups at baseline.
The incidence of transactional sex was measured by means of audio computer-assisted self-interview.
Follow-up and Adherence
The rate of retention of participants for assessment of incident HIV-1 infection was 85% or more during follow-up (Fig. 1), with 2614 participants (99.4%) completing at least one post-randomization HIV-1 test and 4280 total person-years of follow-up accrued for assessment of HIV-1 incidence. The median follow-up was 1.6 years (interquartile range, 1.1 to 2.3), and the maximum follow-up was 2.6 years; 1024 women contributed more than 2 years of follow-up. The most common reason for not dispensing the study ring was pregnancy, which occurred at an incidence of 3.9 per 100 person-years in the dapivirine group and 4.0 per 100 person-years in the placebo group (P = 0.82).
In the dapivirine group, the drug was detected in 82% of plasma samples at levels of more than 95 pg per milliliter (Fig. S2 in the Supplementary Appendix). Detection increased during the first year of use and was relatively stable thereafter. In the subgroup of visits in which returned rings were available, 84% contained less than 23.5 mg of dapivirine, and dapivirine levels in plasma and in returned rings were correlated (Fig. S3 in the Supplementary Appendix). In general, for visits at which plasma dapivirine levels were less than 95 pg per milliliter, the residual dapivirine levels in used rings were similar to levels in unused dapivirine rings, whereas residual dapivirine levels in used rings were lower for visits at which plasma dapivirine levels were more than 95 pg per milliliter. However, a range of residual dapivirine levels was observed, with low levels observed for some visits with low plasma dapivirine levels and high levels observed for some visits with plasma dapivirine levels of more than 95 pg per milliliter.
Effect of Dapivirine Vaginal Ring on HIV-1 Acquisition
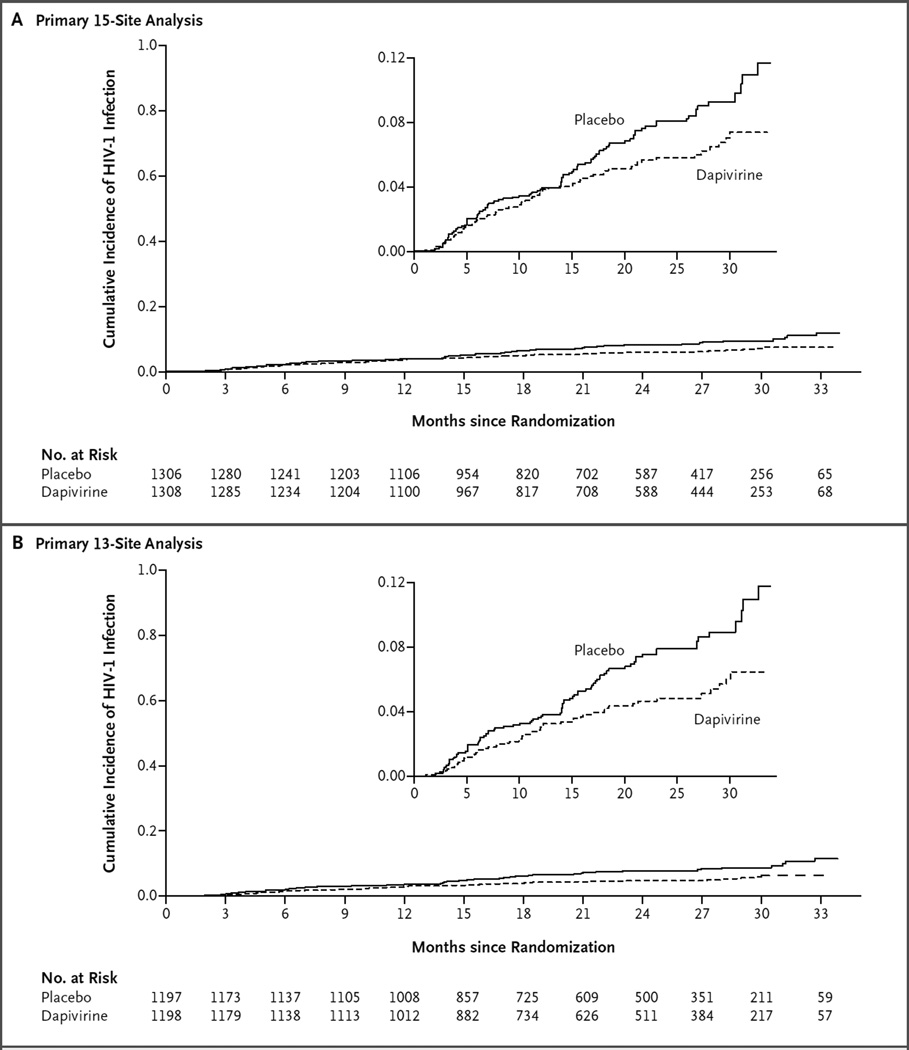
Across all 15 sites in the trial, a total of 168 incident HIV-1 infections occurred during the product-use period: 71 in the dapivirine group and 97 in the placebo group (incidence, 3.3 and 4.5 per 100 person-years, respectively). The incidence of HIV-1 infection in the dapivirine group was lower by 27% (95% confidence interval [CI], 1 to 46; P = 0.05) than that in the placebo group (Fig. 2A). At the two sites that were excluded from the primary analysis because of lower-than-expected protocol and product adherence when the groups were still masked, 29 HIV-1 infections were reported: 17 in the dapivirine group and 12 in the placebo group. After the exclusion of the data from these sites, there were a total of 139 infections among 2395 participants: 54 in the dapivirine group and 85 in the placebo group. The incidence of HIV-1 infection in the dapivirine group was lower by 37% (95% CI, 12 to 56; P = 0.007) than that in the placebo group (Fig. 2B). An efficacy of HIV-1 protection of less than 25% was not ruled out in either analysis (P = 0.88 for 15 sites and P = 0.30 for 13 sites).
Figure 2. Cumulative Incidence of HIV-1 Infection in Two Analyses.
Shown is the cumulative probability of HIV-1 acquisition, as estimated by means of Kaplan–Meier methods, in analyses performed in two populations. Panel A shows the results for the overall 15-site analysis, in which the HIV-1 incidence was 3.3 per 100 person-years (95% confidence interval [CI], 2.6 to 4.2) in the dapivirine group and 4.5 per 100 person-years (95% CI, 3.7 to 5.5) in the placebo group. Panel B shows the results after the exclusion of data from 2 sites because of lower-than-expected protocol and product adherence, in which the HIV-1 incidence was 2.8 per 100 person-years (95% CI, 2.1 to 3.6) in the dapivirine group and 4.4 per 100 person-years (95% CI, 3.5 to 5.5) in the placebo group. Insets show the same data on an enlarged y axis. Excluded from the analyses were data for 3 participants in the placebo group who had HIV-1 infection before enrollment and for 3 participants (1 in the dapivirine group and 2 in the placebo group) who became infected after the product-use period.
In subgroup analyses, HIV-1 protection was generally similar to that seen overall (Fig. S4 in the Supplementary Appendix). However, the efficacy of HIV-1 protection differed significantly according to age, with an efficacy of 61% (95% CI, 32 to 77; P<0.001) among women 25 years of age or older and 10% (95% CI, −41 to 43; P = 0.64) among those under the age of 25 years (P = 0.02 for interaction).
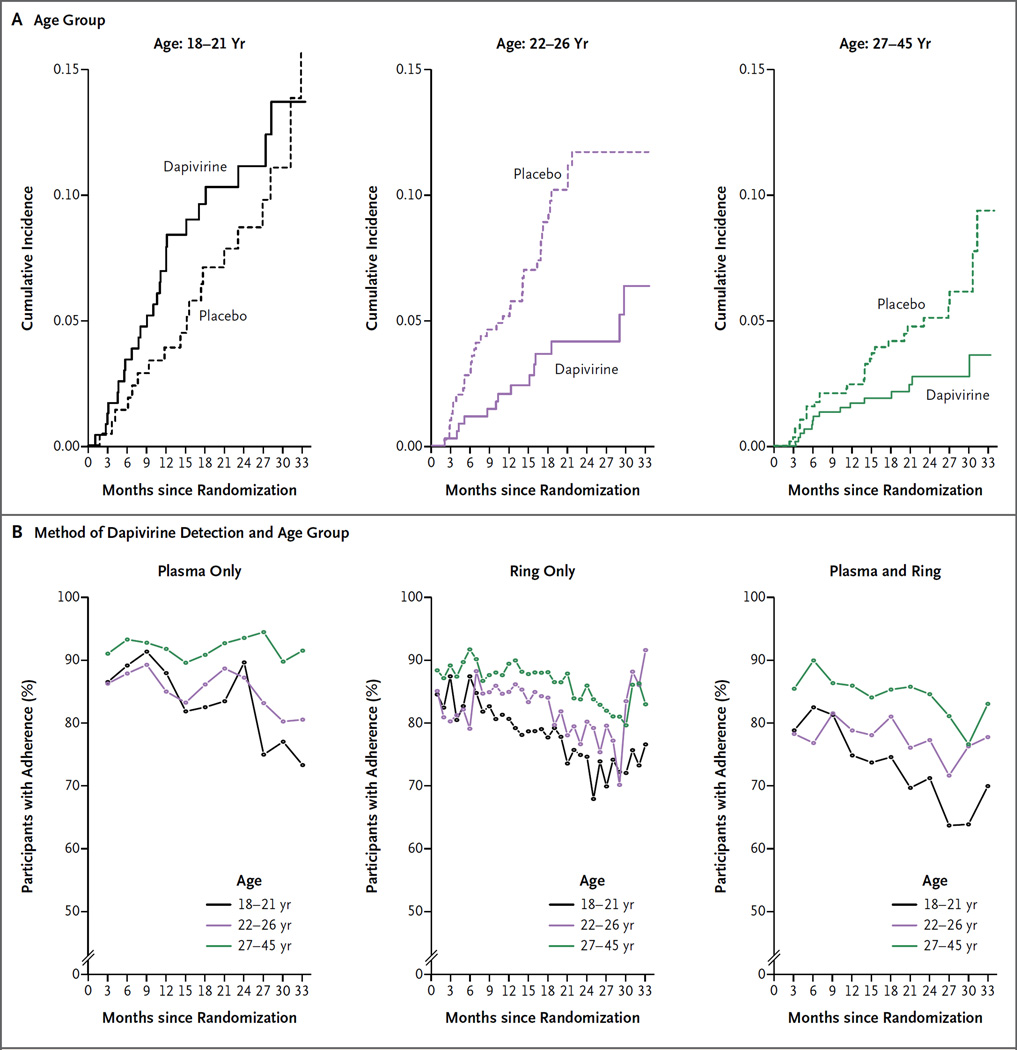
To better characterize the relationship between age and HIV-1 protection seen in the pre-specified subgroup analysis (age <25 vs. ≥25 years), an exploratory analysis was conducted post hoc. Age-categorized subgroups with balanced statistical power were created after dividing the 2395 participants into three groups with approximately equal numbers of those with HIV-1 infection as follows: ages 18 to 21 years, 451 participants with 44 HIV-1 infections (incidence in the placebo group, 5.4 per 100 person-years; 95% CI, 3.2 to 8.4); ages 22 to 26 years, 752 participants with 51 HIV-1 infections (incidence in the placebo group, 6.1 per 100 person-years; 95% CI, 4.3 to 8.3); and ages 27 to 45 years, 1192 participants with 44 HIV-1 infections (incidence in the placebo group, 3.0 per 100 person-years; 95% CI, 2.0 to 4.4) (Fig. 3A). Lack of HIV-1 protection, along with lower adherence, was seen in participants who were 18 to 21 years of age, with an efficacy of HIV-1 protection of −27% (95% CI, −133 to 31; P = 0.45) (Fig. 3B). For women who were older than 21 years of age, the efficacy of HIV-1 protection was 56% (95% CI, 31 to 71; P<0.001), and the rate of adherence was more than 70% overall, as defined by dapivirine detection in plasma, in returned rings, and in the composite of those two measures.
Figure 3. Cumulative Incidence of HIV-1 Infection, According to Age Group and Method of Dapivirine Detection.
Panel A shows that the efficacy of HIV-1 protection in the dapivirine group was −27% (95% CI, −133 to 31) for those 18 to 21 years of age, 56% (95% CI, 19 to 76) for those 22 to 26 years of age, and 51% (95% CI, 8 to 74) for those 27 to 45 years of age, as compared with placebo. The combined efficacy of HIV-1 prevention for participants over the age of 21 years was 56% (95% CI, 31 to 71; P<0.001). Panel B shows the percentage of plasma samples with a dapivirine value of 95 pg per milliliter (the adherence cutoff), the percentage of used rings with a residual dapivirine level of less than 23.5 mg (also the adherence cutoff), and a composite of dapivirine levels in plasma and residual rings. In the plasma-and-ring analysis, adherence levels were higher by a factor of 1.6 (P = 0.07) among women 22 to 26 years of age and by a factor of 4.1 (P<0.001) among women 27 to 45 years of age than among women 18 to 21 years of age, as calculated by means of a generalized linear mixed-effects model with random intercepts and slopes and logit link after the exclusion of data from the first quarter because of small numbers. Returned rings were not available for the first calendar year of the trial, so the curves do not include all participants at all time points.
Safety
There were no significant between-group differences in the frequency of the primary safety end points (Table 2, and Table S6 in the Supplementary Appendix) or in other adverse events commonly detected in the trial population (Fig. S5 in the Supplementary Appendix). Incident sexually transmitted infections occurred at a similar rate in the two groups (Table S7 in the Supplementary Appendix). Finally, among participants who acquired HIV-1 infection, there was no significant between-group difference in the numbers of participants with non-nucleoside reverse-transcriptase inhibitor mutations suggesting antiviral resistance (8 of 68 participants [12%] in the dapivirine group and 10 of 96 [10%] in the placebo group, P = 0.80) (Table S8 in the Supplementary Appendix).
Table 2.
Adverse Events.*
| Adverse Event | Dapivirine Group (N = 1313) |
Placebo Group (N = 1316) |
|---|---|---|
| no. (%) | ||
| Primary safety end point* | 180 (14) | 186 (14) |
| Any serious adverse event | 52 (4) | 48 (4) |
| Death | 4 (<1) | 3 (<1) |
| Any grade 4 event | 22 (2) | 23 (2) |
| Any grade 3 event | 151 (12) | 162 (12) |
| Any grade 2 event assessed as related | 7 (1) | 9 (1) |
The primary safety end point of the study was defined as any serious adverse event, any grade 3 or 4 adverse event, and any grade 2 adverse event assessed by the treating clinician as being related to the study product. P = 0.80 for the overall comparison by the chi-square test.
DISCUSSION
In this multicountry trial, a vaginal ring containing 25 mg of dapivirine that was renewed every month showed evidence of HIV-1 protection, demonstrating the efficacy of a sustained-release approach to delivering antiretroviral prophylaxis. As seen in other studies of HIV-1 prophylaxis,5,7 the protective effect of the dapivirine vaginal ring, as compared with placebo, was significant but not as high as hypothesized in the design of the trial. Greater HIV-1 protection was observed among subgroups of women who had evidence of higher rates of adherence than among those with lower rates of adherence. HIV-1 protection was not observed for women between the ages of 18 and 21 years, and objective markers of adherence were lower in this subgroup than in those older than 21 years.
Strong relationships between adherence and HIV-1 protection have been seen in studies of HIV-1 prophylaxis, with several studies among African women showing low adherence.3,4,9 In our trial, a majority of participants had objective evidence of adherence to the use of the dapivirine ring. We predefined dapivirine levels in plasma and in used rings to indicate adherence on the basis of phase 1 and 2 studies. However, our definitions could have led to an overestimation of adherence because participants were categorized as being adherent even though they may have used the ring for only a portion of the month (and possibly only for a few hours before a clinic visit). Further pharmacokinetic analysis may help define whether there is a threshold of use of dapivirine that provides protection against HIV-1, analogous to investigations that followed the initial clinical trials of tenofovir-based strategies.22,23 Some studies of other treatment and prevention interventions have shown that use and resultant benefits often decline over time.2,22 In contrast, we found that adherence appeared to increase after the first months of use, which may indicate that some time was needed for participants to become comfortable with the ring, and HIV-1 protection was sustained during follow-up of 24 months or more. Qualitative analyses showed that nonadherence to HIV-1 prophylaxis strategies in clinical trials may be related to characteristics of the participant (e.g., a younger age), the product (e.g., side effects), and the research process (e.g., concern about unproven safety and efficacy).24,25 Notably, studies of the oral antiretroviral agent tenofovir have shown higher adherence in open-label studies after a demonstration of safety and efficacy than had been seen in the initial blinded, placebo-controlled trials.26–28
Both behavioral and biologic effects may have contributed to a lack of HIV-1 protection in women between the ages of 18 and 21 years in this trial. Adherence to use of the ring appeared to be lower in this group than in women above 21 years of age; lower adherence to HIV-1 treatment and use of contraceptives has been reported among persons from 18 to 21 years of age.29,30 The genital tract of women in this age group may be more susceptible to HIV-1 infection and potentially also more difficult to protect with antiretroviral prophylaxis strategies.31,32 Further research is needed to understand the unique prevention needs of young women, including open-label evaluations that may allay concern about the efficacy of HIV-1 prevention, safety, or use of placebo. In addition, studies are needed to assess whether HIV-1 protection could be achieved in younger women with levels of adherence that were greater than those seen in this trial. The dapivirine ring significantly reduced HIV-1 incidence by more than half among women over the age of 21 years; women between the ages of 22 and 26 in the placebo group had an annualized incidence of HIV-1 of more than 6%, a finding that emphasizes the great need for prevention strategies for this population.
The concept of a topical microbicide for vaginal or rectal use for HIV-1 prevention was first proposed more than two decades ago.33 Microbicide products — including gels, films, foams, and rings — have been evaluated in multiple studies among at-risk women and men. Microbicides can provide personal control over HIV-1 prevention and offer the ability to be used discreetly. Affordability is an important challenge to HIV-1 prophylaxis interventions, and topical delivery of antiretroviral drugs may allow minimal toxicity monitoring, which would improve cost-effectiveness. Only one previous trial, a phase 2 evaluation of a vaginal gel containing tenofovir, showed evidence of HIV-1 protection,2 but this result was not confirmed in subsequent studies.3,9 The results of an ongoing second phase 3 trial of the dapivirine vaginal ring (the Ring Study; ClinicalTrials .gov number, NCT01539226) will be important for improving our understanding of this intervention.
African women bear a disproportionate burden of the global HIV-1 epidemic. In the placebo group in our trial, the annualized HIV-1 incidence was more than 4%, despite monthly HIV-1 testing and risk-reduction counseling, testing of male partners, screening and treatment of sexually transmitted infections, and provision of free condoms. Our results show that a vaginal ring containing an antiretroviral drug can provide some protection against HIV-1 acquisition.
Supplementary Material
Acknowledgments
Supported by grants (UM1AI068633, UM1AI068615, and UM1AI106707) from the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the women who participated in this study for their motivation and dedication and the communities that supported this work; Dr. Roberta Black of the National Institute of Allergy and Infectious Diseases for her oversight; and the members of the multinational data and safety monitoring board of the National Institute of Allergy and Infectious Diseases, Division of AIDS — Shrikant Bangdiwala, Alan R. Fleischman, Joan F. Hilton, Alejandro Llanos-Cuentas, Olavo H.M. Leite, V.I. Mathan, Michael Proschan, Carlos Seas, Panpit Suwangool, Gabriel Thimothe, and W.D. Francois Venter — for their expertise and guidance.
APPENDIX
The authors’ full names and academic degrees are as follows: Jared M. Baeten, M.D., Ph.D., Thesla Palanee-Phillips, Ph.D., Elizabeth R. Brown, Sc.D., Katie Schwartz, M.P.H., Lydia E. Soto-Torres, M.D., M.P.H., Vaneshree Govender, M.B., B.Ch., Nyaradzo M. Mgodi, M.B., Ch.B., Flavia Matovu Kiweewa, M.B., Ch.B., Gonasagrie Nair, M.B., Ch.B., M.P.H., Felix Mhlanga, M.B., Ch.B., Samantha Siva, M.Med.Sci., Linda-Gail Bekker, M.B., Ch.B., Ph.D., Nitesha Jeenarain, B.Pharm., Zakir Gaffoor, M.Med.Sci., Francis Martinson, M.B., Ch.B., Ph.D., Bonus Makanani, M.B., B.S., Arendevi Pather, B.Pharm., Logashvari Naidoo, M.B., Ch.B., Marla Husnik, M.S., Barbra A. Richardson, Ph.D., Urvi M. Parikh, Ph.D., John W. Mellors, M.D., Mark A. Marzinke, Ph.D., Craig W. Hendrix, M.D., Ariane van der Straten, Ph.D., M.P.H., Gita Ramjee, Ph.D., Zvavahera M. Chirenje, M.D., Clemensia Nakabiito, M.B., Ch.B., Taha E. Taha, M.B., B.S., Ph.D., Judith Jones, M.S., Ashley Mayo, M.S.P.H., Rachel Scheckter, M.P.H., Jennifer Berthiaume, M.P.H., Edward Livant, M.P.H., Cindy Jacobson, Pharm.D., Patrick Ndase, M.B., Ch.B., M.P.H., Rhonda White, B.S., Karen Patterson, M.P.H., Donna Germuga, B.S.N., Beth Galaska, M.I.D., Katherine Bunge, M.D., Devika Singh, M.D., M.P.H., Daniel W. Szydlo, M.Sc., Elizabeth T. Montgomery, Ph.D., Barbara S. Mensch, Ph.D., Kristine Torjesen, M.D., M.P.H., Cynthia I. Grossman, Ph.D., Nahida Chakhtoura, M.D., Annalene Nel, M.B., Ch.B., Ph.D., Zeda Rosenberg, Sc.D., Ian McGowan, M.D., D.Phil., and Sharon Hillier, Ph.D., for the MTN-020– ASPIRE Study Team
The authors ’ affiliations are as follows: the Departments of Global Health (J.M.B., B.A.R., P.N.), Medicine (J.M.B.), Epidemiology (J.M.B., M.H.), and Biostatistics (E.R.B., B.A.R.), University of Washington, and the Statistical Center for HIV/AIDS Research and Prevention, Fred Hutchinson Cancer Research Center (E.R.B., M.H., B.A.R., J.B., K.P., D.W.S.) — both in Seattle; Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg (T.P.-P.), HIV Prevention Research Unit, South African Medical Research Council (V.G., S.S., N.J., Z.G., A.P., L.N., G.R.), and Center for AIDS Programme of Research in South Africa (G.N.), Durban, and the Desmond Tutu HIV Center, University of Cape Town, Cape Town (L.-G.B.) — all in South Africa; FHI 360, Durham, NC (K.S., A.M., R.S., R.W., K.T.); National Institute of Allergy and Infectious Diseases (L.E.S.-T., D.G.), National Institute of Mental Health (C.I.G.), and Eunice Kennedy Shiver National Institute of Child Health and Human Development (N.C.), National Institutes of Health, Bethesda, the Departments of Medicine (M.A.M., C.W.H.) and Epidemiology (T.E.T.), Johns Hopkins University, Baltimore, and the International Partnership for Microbicides, Silver Spring (A.N., Z.R.) — all in Maryland; University of Zimbabwe–University of California San Francisco Collaborative Research Program, Harare, Zimbabwe (N.M.M., F. Mhlanga, Z.M.C.); Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda (F.M.K., C.N.); University of North Carolina Project, Lilongwe (F. Martinson), and Malawi College of Medicine–Johns Hopkins University Research Project, Queen Elizabeth Central Hospital, Blantyre (B.M., T.E.T.) — both in Malawi; the Departments of Medicine (U.M.P., J.W.M., I.M.) and Obstetrics, Gynecology, and Reproductive Sciences (K.B., S.H.), University of Pittsburgh, and Magee–Women’s Research Institute (J.J., E.L., C.J., B.G., D.S.) — both in Pittsburgh; the Women’s Global Health Imperative, RTI International (A.S., E.T.M.), and the Department of Medicine, University of California (A.S.) — both in San Francisco; and the Population Council, New York (B.S.M.)
REFERENCES
- 1.The Gap report. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. ( http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf) [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S122–S129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 9.Rees H, Delany-Moretlwe S, Lombard C, et al. FACTS 001 phase III trial of peri-coital tenofovir 1% gel for HIV prevention in women CROI 2015; Presented at the Conference on Retroviruses and Opportunistic Infections; Boston. 2015. Feb 23–26, abstract. [Google Scholar]
- 10.Thurman AR, Clark MR, Hurlburt JA, Doncel GF. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health. 2013;5:695–708. doi: 10.2147/IJWH.S34030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen BA, Panther L, Marzinke MA, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr. 2015;70:242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunge KE, Dezzutti CS, Rohan LC, et al. A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmaco-dynamics of a novel dapivirine vaginal film. J Acquir Immune Defic Syndr. 2015 Nov 11; doi: 10.1097/QAI.0000000000000897. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS. 2014;28:1479–1487. doi: 10.1097/QAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 14.Nel A, Kamupira M, Woodsong C, et al. Safety, acceptability, and pharmacokinetic assessment (adherence) of monthly dapivirine vaginal microbicide rings (Ring-004) for HIV prevention; Presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI; Seattle. 2012. Mar 5–8, abstract. [Google Scholar]
- 15.Devlin B, Nuttall J, Wilder S, Wood-song C, Rosenberg Z. Development of dapivirine vaginal ring for HIV prevention. Antiviral Res. 2013;100(Suppl):S3–S8. doi: 10.1016/j.antiviral.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Palanee-Phillips T, Schwartz K, Brown ER, et al. Characteristics of women enrolled into a randomized clinical trial of dapivirine vaginal ring for HIV-1 prevention. PLoS One. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seserko LA, Emory JF, Hendrix CW, Marzinke MA. The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretro-viral agent dapivirine in human plasma. Bioanalysis. 2013;5:2771–2783. doi: 10.4155/bio.13.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 19.Guidance for industry: vaginal microbicides — development for the prevention of HIV infection. Silver Spring, MD: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2014. [Google Scholar]
- 20.Lan K, Gordan K, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 22.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66:340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure pro-phylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corneli A, Perry B, McKenna K, et al. Participants’ explanations for non-adherence in the FEM-PrEP clinical trial. J Ac-quir Immune Defic Syndr. 2015 Nov 3; doi: 10.1097/QAI.0000000000000880. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015;387:53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeten J, Heffron R, Kidoguchi L, et al. Near elimination of HIV transmission in a demonstration project of PrEP and ART; Presented at the Conference on Retroviruses and Opportunistic Infections; Seattle. 2015. Feb 23–26, abstract. [Google Scholar]
- 28.Bekker L-G, Hughes J, Amico R, et al. HPTN 067/ADAPT Cape Town: a comparison of daily and nondaily PrEP dosing in African women; Presented at the Conference on Retroviruses and Opportunistic Infections; Seattle. 2015. Feb 23–26, abstract. [Google Scholar]
- 29.Blanc AK, Tsui AO, Croft TN, Trevitt JL. Patterns and trends in adolescents’ contraceptive use and discontinuation in developing countries and comparisons with adult women. Int Perspect Sex Reprod Health. 2009;35:63–71. doi: 10.1363/ipsrh.35.063.09. [DOI] [PubMed] [Google Scholar]
- 30.Evans D, Menezes C, Mahomed K, et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29:892–900. doi: 10.1089/aid.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofo-vir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. 2016;11:18–26. doi: 10.1097/COH.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–F19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 33.Stein ZA. HIV prevention: the need for methods women can use. Am J Public Health. 1990;80:460–462. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.