Abstract
Background
There is little consensus on the most efficacious vehicle substance for vitamin D supplements. Fat malabsorption may impede the ability of patients with cystic fibrosis (CF) to absorb vitamin D in an oil vehicle. We hypothesized that vitamin D contained in a powder vehicle would be absorbed more efficiently than vitamin D contained in an oil vehicle in patients with CF.
Methods
In this double-blind, randomized controlled trial, hospitalized adults with CF were given a one-time bolus dose of 100,000 IU of cholecalciferol (D3) in a powder-based or oil-based vehicle. Serum D3, 25-hydroxyvitamin D, and parathyroid hormone concentrations were analyzed at 0, 12, 24, and 48 hours posttreatment. The area under the curve for serum D3 and the 12-hour time point were also assessed as indicators of D3 absorption.
Results
This trial was completed by 15 patients with CF. The median (interquartile range) age, body mass index, and forced expiratory volume in 1 second were 23.7 (19.9–33.2) years, 19.9 (18.6–22.6) kg/m2, and 63% (37%–80%), respectively. The increase in serum D3 and the area under the curve was greater in the powder group (P = .002 and P = .036, respectively). Serum D3 was higher at 12 hours in the powder group compared with the oil group (P = .002), although levels were similar between groups by 48 hours.
Conclusions
In adults with CF, cholecalciferol is more efficiently absorbed in a powder compared with an oil vehicle. Physicians should consider prescribing vitamin D in a powder vehicle in patients with CF to improve the absorption of vitamin D from supplements.
Keywords: cholecalciferol, ergocalciferol, vitamin D, cystic fibrosis, vitamin D3, vehicle, pancreatic insufficiency, malabsorption
Introduction
Cystic fibrosis (CF) is a genetic disorder that prevents proper epithelial surface hydration in the lungs, pancreas, and other organs. Thickened mucus in the pancreas, in particular, induces acinar scarring and defective secretion of bicarbonate, which ultimately leads to exocrine pancreatic insufficiency. This, in turn, causes intestinal malabsorption of fat-soluble vitamins and chronic nutrient deficiencies of vitamins A, D, E, and K.1 People with pancreatic insufficiency replace insufficient bicarbonate and enzymes with routine use of pancreatic enzyme replacement therapy (PERT), the dose of which is individual to the patient’s weight or per gram of fat intake in the diet. Despite the regular use of enzymes, fat absorption in CF often remains suboptimal.2,3 Vitamin D deficiency, in particular, is highly prevalent in patients with CF.4–8 In addition to its most well-defined roles in calcium homeostasis and bone maintenance, new studies in patients with CF present evidence that vitamin D plays a role in immunity and lung health.9,10 Achieving optimal vitamin D status is, therefore, an important component of CF care.
In 2012, the Cystic Fibrosis Foundation updated recommendations for vitamin D therapy in patients with CF, calling for higher doses of vitamin D to maintain optimal vitamin D status in adults and children with CF.7 However, there remains some question as to the most efficient vehicle compound for the vitamin D supplement for maximal absorption. A vehicle is a substance that may bind or carry the vitamin from one place to another and can include oils, water, or acidic-based substances. Vitamin D is a fat-soluble vitamin and is more efficiently absorbed when combined with fats/oils as the vehicle compound in healthy individuals.11 Thus, most vitamin D pills marketed in the United States are formulated with a fat-soluble vehicle.11,12 Vitamin D deficiency is often incipient even in the face of vitamin D supplementation, and it is not known whether the oil-based vehicle substances of standard vitamin D supplements are poorly absorbed by patients with CF.11–13 There have been no clinical trials conducted in the CF population to evaluate the impact of the vehicle substance on absorption of vitamin D. One 2007 pharmacokinetic trial in CF illustrated superior absorption of a water-soluble vitamin E supplement compared with oil.14 Since vitamin D supplements are produced in both water-miscible and oil-miscible forms, it is possible the vehicle may play a role in the success of vitamin D repletion in CF. The objective of this study is to determine whether oil or powder is a more effective vehicle of vitamin D3 for patients with CF. Effectiveness was evaluated with serum cholecalciferol at 12 hours and the area under the curve (AUC). Based on the current understanding that fat is malabsorbed in CF, we hypothesized that a powder vehicle would support better overall absorption of vitamin D3 in this population.
Materials and Methods
Patients
Study patients were at least 18 years old with a history of CF and were recruited from Emory University Hospital during an inpatient hospital admission for pulmonary exacerbation. Screening of new inpatients was completed daily by a research coordinator. Exclusion criteria included a history of disorders associated with hypercalcemia and/or current hypercalcemia (serum albumin corrected, serum calcium >10.8 mg/dL, or ionized calcium >5.2 mg/dL), chronic kidney disease worse than stage III (<60 mL/min), forced expiratory volume in 1 second (FEV1) predicted <20%, current significant hepatic dysfunction total bilirubin >2.5 mg/dL with direct bilirubin >1.0 mg/dL, inability to tolerate oral food or medications, current use of cytotoxic or immunosuppressive drugs, history of AIDS or illicit drug abuse, current or planned pregnancy within the following year, or current enrollment in another intervention trial. Clinical data were extracted from electronic medical health records and included age, sex, ethnicity, body mass index (BMI), comorbidities, antibiotic use, diabetes status, most recent serum 25-hydroxyvitamin D (25(OH)D), and FEV1 as a measure of lung function. Questionnaires were designed by the study team and used to assess current vitamin supplementation and enzyme use.
This study was approved by the Emory Institutional Review Board, and all patients provided written informed consent prior to study procedures. The trial was registered at clinicaltrial.gov as NCT01880346.
Intervention
Patients were randomized in blocks of 4 to powder-based or oil-based supplements containing a total amount of 100,000 IU of vitamin D3 (cholecalciferol). The powder bolus, Vitamin D3-50 Cholecalciferol, was obtained from BioTech Pharmacal (Fayetteville, AR) (50,000 IU of cholecalciferol/tablet; inactive ingredients: gelatin, lactose, cellulose, and magnesium stearate); patients received 2 tablets totaling 100,000 IU of cholecalciferol. The oil-based vitamin D3 supplements were obtained from BTR Group (Pittsfield, IL) (Maximum D3; 10,000 IU of cholecalciferol/tablet, refined soybean oil, and glycerin); patients received 10 tablets totaling 100,000 IU of cholecalciferol. A Certificate of Analysis was obtained from Analytical Research Laboratories (Oklahoma City, OK) to validate capsule content potency of both the powder and the oil cholecalciferol capsules. The powder capsule was found to contain 91.3% of the stated amount of vitamin D3, and the oil capsule was found to contain 95% of the stated amount of vitamin D3. Blood was drawn by intravenous (IV) catheter at baseline (time 0) and 12, 24, and 48 hours after cholecalciferol dosing.
Patients were instructed to take their normal or current prescription dose of pancreatic enzymes as well as a meal or nutrition supplement just prior to dosing. Not all time points were available on all patients; results of the study are reported only for participants who completed at least 0-, 12-, and 24-hour time points. A floor nurse, independent of the study investigators, directly observed the patients taking the study drug and enzymes to confirm adherence with the study protocol.
Analysis of Serum Vitamin D3, Plasma 25(OH)D, and Parathyroid Hormones
Serum vitamin D3 (serum D3) was measured with liquid chromatography– mass spectrometry (Heartland Assays, Ames, IA). Peak absorption of serum D3 was assessed using the 12-hour time point based on 2 preceding published recommendations by Lo et al6 and Farraye et al.15 The AUC for serum D3 over all time points, calculated using the trapezoidal method,16 was also assessed as an indicator of absorption, as precedented by Lark et al.4 A higher 12-hour serum D3 or higher AUC reflects greater vitamin D3 absorption.6,15 Plasma 25(OH)D and plasma parathyroid hormone (PTH) were measured with an automated chemiluminescence assay (iSYS System; Immunodiagnostic Systems, Gaithersburg, MD). Quality assurance of the plasma 25(OH)D measurements was monitored by participation in the vitamin D external quality assessment scheme (DEQAS) program. To minimize interassay variability, all samples were analyzed in 1 batch and run with controls of known concentrations.
Statistical Analysis and Power
Descriptive statistics were performed and reported as a percentage or median (interquartile range [IQR]). The Shapiro-Wilk test was conducted to test for sample normality, and those deviating from a normal distribution (baseline serum D3) were log10-transformed for statistical analyses. Vehicle (oil vs powder) group differences in baseline demographics were analyzed using Fisher exact tests for categorical variables and Student t tests for continuous variables. Student t tests were used to examine vehicle group differences in the AUC for serum D3 over 48 hours and at the individual 12-hour time point. Two-way mixed-model repeated-measures analysis of variance (ANOVA) was used to test for differences in serum D3 and plasma 25(OH)D by group, over time, and in the group-by-time interactions. Post hoc treatment and individual time responses were analyzed using Tukey’s t tests. As this is a novel pilot study and since there were no previously published trials from which to draw power calculations, a formal power analysis was not conducted. We aimed to recruit 12–24 patients for this pilot study to establish feasibility and to produce needed preliminary data for a future, adequately powered, robust clinical trial in the event we were unable to reject the null hypothesis. Statistical analyses were performed with JMP Pro 10.0.0 (SAS Institute, Cary, NC) using 2-sided tests with a 5% significance level.
Results
Patient Demographics
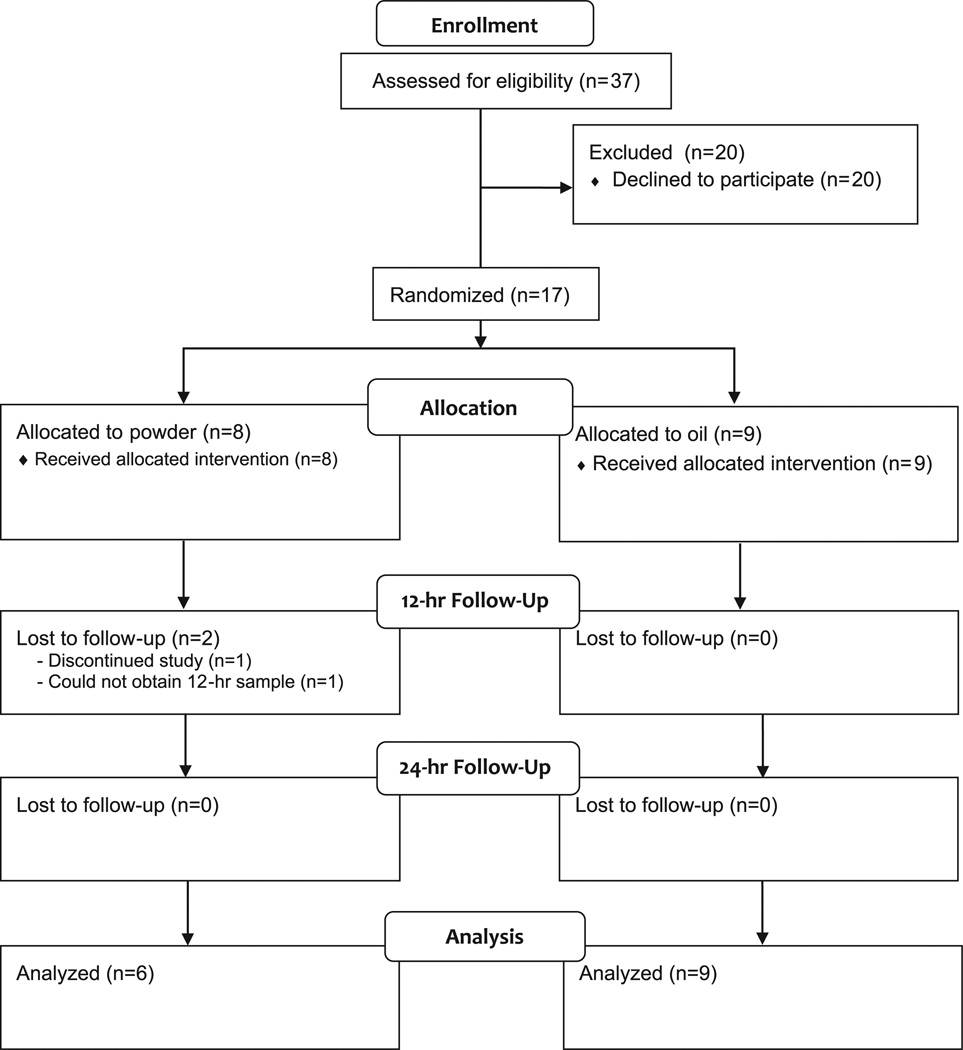
A CONSORT (Consolidated Standards of Reporting Trials) diagram of study enrollment is provided in Figure 1. A total of 17 participants were randomized to receive 100,000 IU of cholecalciferol in either an oil or a powder form. One participant withdrew from the study prior to completing all crucial time points and was therefore excluded from the final analysis. A second participant was excluded from the final analysis due to an inability to acquire an adequate blood sample at the 12-hour time point, and thus 15 patients were included in the final analysis (n = 6 in powder group, n = 9 in oil group).
Figure 1.
Flow diagram of participant enrollment. Patients with cystic fibrosis (n = 37) were screened for participation in the study. Recruitment took place over 1.25 years (January 2013–March 2014). Seventeen patients were enrolled and randomly assigned to receive either a powder or an oil vehicle of vitamin D3. One patient did not complete the intervention, and 1 patient was excluded due to inadequate sample collection.
Baseline demographic and clinical characteristics are presented in Table 1. The demographics for each group are approximately evenly distributed with no significant differences between oil or powder vehicle groups. The median (IQR) age of the group was 23.7 (19.9–33.2) years, approximately 50% were female, and 88% were of white ethnicity. One participant was of African American descent and 1 of East Asian descent. The median (IQR) BMI was 19.9 (18.6–22.6) kg/m2. Only 2 participants were not on a pancreatic enzyme regimen, and 29% were currently prescribed a vitamin D supplement. Three patients had CF-related diabetes. Finally, most participants (75%, n = 12) had baseline plasma 25(OH)D in the vitamin D insufficient range (<30 ng/mL), and 8 patients (66%) had 25(OH)D in the vitamin D deficient range (<20 ng/mL). The median (IQR) FEV1 was 63% (37%–80%) of predicted.
Table 1.
Participant Demographics.a
| Demographic | Powder (n = 6) | Oil (n = 9) | P Value |
|---|---|---|---|
| Age, y | 24.9 (20.0–33.2) | 23.1 (19.5–33.2) | .88 |
| Female sex | 83.3 | 33.3 | .11 |
| White ethnicity | 66.7 | 100.0 | .14 |
| Smoking status: never smoked | 100 | 88.9 | 1.00 |
| BMI, kg/m2 | 19.5 (17.8–21.3) | 19.9 (18.7–25.5) | .35 |
| Supplemented enzymes | 83.3 | 88.9 | 1.00 |
| Vitamin D supplements | 0 | 44.4 | .10 |
| HbA1C | 5.9 (5.2–6.3) | 5.9 (5.6–6.4) | .53 |
| Plasma total 25(OH)D, ng/mL | 25.0 (11.2–36.8) | 17.2 (9.8–26.9) | .38 |
| Serum cholecalciferol, ng/mLb | 3.9 (2.1–7.6) | 4.2 (1–7.5) | .98 |
| Serum parathyroid hormone, pg/mL | 27.4 (19.6–39.7) | 34.8 (22.3–55.6) | .43 |
| FEV1 | 64.5 (47.0–79.8) | 54.0 (25.0–82.5) | .58 |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; HbA1c, glycated hemoglobin; 25(OH)D, 25-hydroxyvitamin D.
Values reported as percentage or median (interquartile range). Statistical differences were determined with Fisher exact tests for categorical variables and t tests for continuous variables.
Log10-transformed for statistical analysis.
Serum Cholecalciferol (D3) Absorption by Group
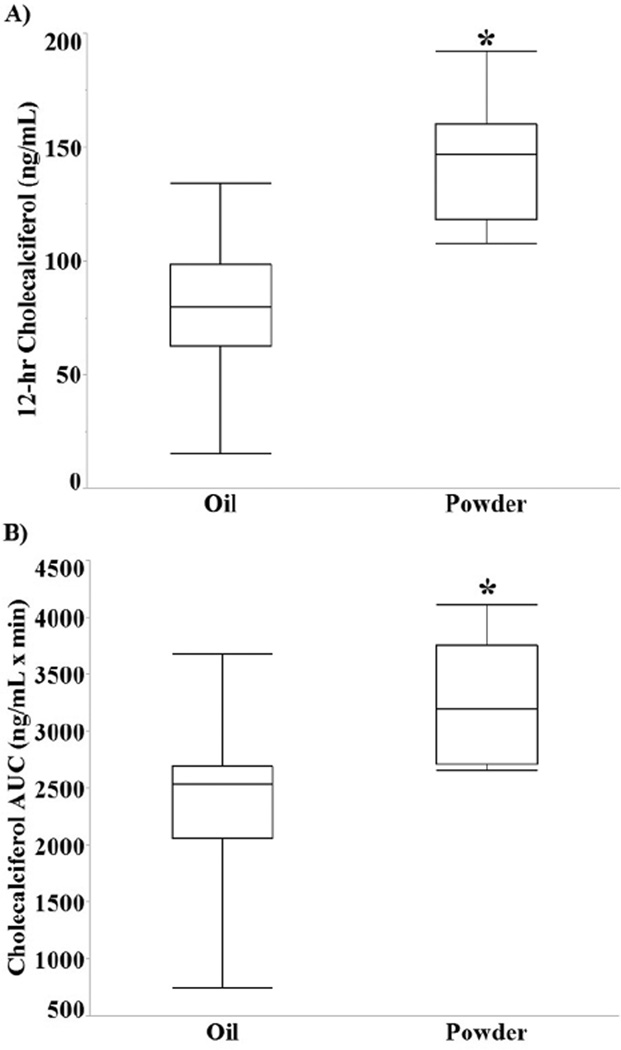
The 12-hour serum D3 concentration was significantly higher in the powder group compared with the oil group (144.2 ± 29.1 ng/mL vs 79.9 ± 33.1 ng/mL, P = .002; Figure 2A). The AUC (Figure 2B) for serum D3 was also higher in the powder group compared with the oil group (3256.5 ± 285.5 ng·h/mL vs 2393.73 ± 233.1 ng·h/mL, P = .036). Mixed-model repeatedmeasures ANOVA indicated a significant time effect (Ptime < .001), but the group and group-by-time interaction effects were not statistically significant (Pgroup = .38, Pgroup*time = .21).
Figure 2.
The serum cholecalciferol response to a bolus dose of vitamin D3. Box and whisker plots for serum levels of (A) cholecalciferol at 12 hours (*P = .002). (B) The area under the curve (AUC) for serum cholecalciferol (3256.5 ± 285.5 ng·h/mL vs 2393.73 ± 233.1 ng·h/mL, *P = .036).
Plasma 25(OH)D and PTH by Time and Group
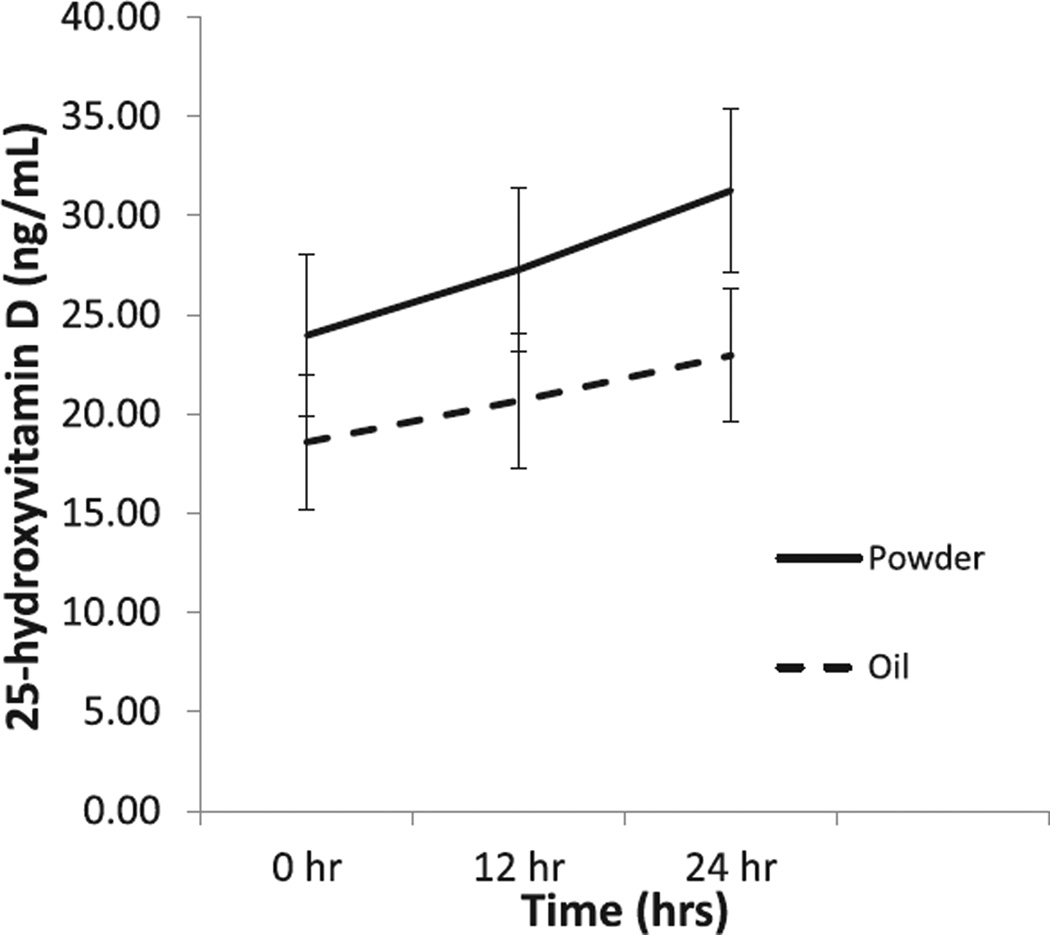
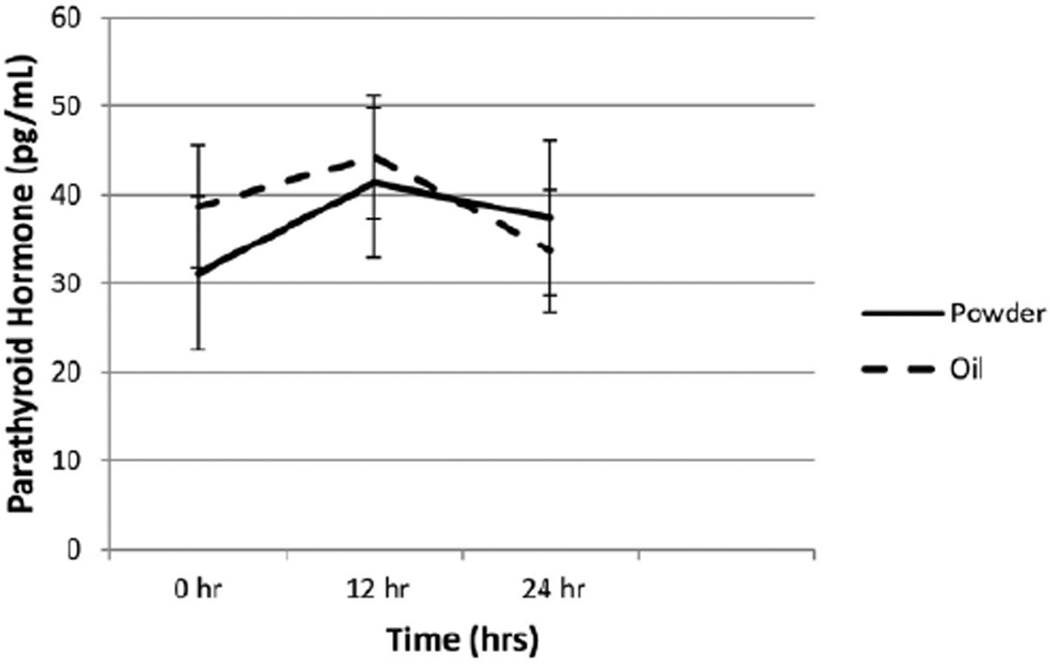
Two-way repeated-measures ANOVA analyses (Figure 3) indicated that plasma 25(OH)D increased significantly by time (Ptime = .006), although the group effect and the group-by-time interaction were not different (Pgroup = .35, Pgroup*time = .30). There were no significant changes in PTH measurements (Figure 4) between baseline and 24 hours for either the powder or the oil group (P = .19).
Figure 3.
Plasma 25-hydroxyvitamin D (25(OH)D) concentrations comparing an oil vs powder vehicle in adults with cystic fibrosis. The plasma 25(OH)D concentrations rose in both groups over time compared with baseline. There was a significant time effect from baseline to 24 hours postdose (*P = .0062) with no difference between groups.
Figure 4.
Changes in parathyroid hormone over time. There was no significant change in parathyroid hormone for group, time, or group*time (P = .190). Time effect measures from baseline to 24 hours.
Discussion
This pilot study examined the pharmacokinetic absorption of 2 different vehicle substances for cholecalciferol supplements in adults with CF. Pancreatic enzymes and bile are the primary conduits for the absorption of fats and fat-soluble vitamins, and it is well established that dietary fat aids the intestinal absorption of vitamin D.12 In CF, pancreatic scarring causes limited release of digestive enzymes into the small intestine, decreasing the absorption potential of all fats and fat-soluble vitamins. Although routine pancreatic enzymes replace this deficit, people with CF still exhibit malabsorption compared with their non-CF counterparts when consuming vitamin D with oils.17 In our study, we found that a cholecalciferol supplement prepared in a water-soluble powder vehicle was more efficiently absorbed than an oil preparation in patients with CF.
Patients of the study were largely vitamin D insufficient and were enrolled in the study during a hospital admission. Reason for hospital admission in the study was primarily to initiate antibiotic treatment for lung infections. Study exclusions were set to ensure that the patient did not currently have symptoms that might result in excretion of vitamin D3 or inhibit hepatic hydroxylation to 25(OH)D beyond expected CF malabsorption. Both groups likely had vitamin D insufficiency due to decreased sun exposure and poor nutrition intake of vitamin D prior to hospitalization. Indeed, patients with CF who are admitted with pulmonary exacerbation have lower vitamin D status than those without pulmonary exacerbation.5
This is the first trial to directly compare the differences in vitamin D absorption between a powder-based and an oil-based vehicle in patients with CF. There are few prior studies comparing vitamin D3 absorption from the gut based on vehicle alone.7 A systematic review of clinical trials evaluating different vitamin D vehicles identified 4 studies comparing oil, cellulose/lactose, and ethanol and evaluated their efficiency to improve vitamin D status in healthy participants.11 In their literature review, the authors concluded that oil was the most efficacious vehicle substance for healthy individuals, although the methods used in the studies using oil were inconsistent and were studied in patients without fat malabsorption.11 Our study showed a significant rise at 12 hours in serum D3 in response to a cholecalciferol supplement contained in a powder vehicle compared with a cholecalciferol supplement contained in an oil vehicle in patients with CF. Thus, in contrast to previous suggestions in healthy individuals, our data suggest that patients with CF may have better absorption of vitamin D contained in a powder-based vehicle, as opposed to oil.
Previous studies have examined vitamin D absorption in patients with intestinal malabsorption and demonstrated that peak vitamin D absorption occurs at approximately 12 hours following supplementation.6,15 Lo et al6 developed an oral challenge test quantifying the difference in vitamin D bioavailability between healthy controls and patients with fat malabsorption syndromes using a 50,000-IU bolus dose of D2 and found that serum D2 began to rise 4 hours after ingestion and reached a peak at 12 hours after ingestion. Farraye et al15 confirmed a 12-hour peak in serum D2 with vitamin D supplementation in patients with Crohn’s disease.15 This 12-hour time point, in addition to total AUC for serum D3, was the primary end point for this study and was used as an indicator of vitamin absorption to show superiority of a powder-based vs oil-based vehicle in patients with CF.14 Notably, serum D3 concentrations did not differ between groups after 48 hours, and levels returned to baseline values. Cholecalciferol has a half-life of approximately 24 hours, explaining the decrease in serum D3 by 48 hours.4 The clinical implications for greater cholecalciferol absorption by 12 hours are yet to be determined with longer follow-up.
In our study, there was a similar rise of serum 25(OH)D in both groups from baseline to 24 hours. Interestingly, and although not statistically significant and not the primary end point for this study, the powder group had higher plasma 25(OH)D levels toward the sufficiency range than the oil group (31.3 ± 4.2 ng/mL and 22.9 ± 3.4 ng/mL, respectively). Peak plasma 25(OH)D concentrations, however, are usually not reached until 48–72 hours following supplementation, and thus we cannot fully determine the impact of the vehicle on serum 25(OH)D concentrations.4 Future head-to-head studies of cholecalciferol supplementation in a powder vs oil form with a longer period of follow-up are needed to determine the impact of vehicle on vitamin D status.
Our findings may explain why there have been differences reported in the literature regarding the bioavailability of vitamin D2 (ergocalciferol) and D3 in CF.18–21 Several studies have compared differences in the serum 25(OH)D response to oral vitamin D2 and D3 supplementation; however, the vehicles used in these studies were either primarily oil based or did not control for the type of vehicle when comparing vitamin D2 and D3.18,19,17,22 Conversely, several clinical trials supplementing vitamin D3 have mainly employed powder vehicles (comprising lactose, cellulose, and magnesium stearate).11 Khazai et al17 found that serum 25(OH)D increased significantly via a powder- based bolus of cholecalciferol compared with an oil-based bolus of ergocalciferol. The vehicles used, therefore, may have presented as possible confounders in previous pharmacokinetic studies. The Cystic Fibrosis Foundation recommends the use of vitamin D3 for supplement use in CF due to its greater pharmacokinetic potency; however, the more efficient absorption of vitamin D3 (as opposed to vitamin D2) may have been due to the differences in the vehicle substance.7,10
Strengths and Limitations
Strengths of this study include the randomized controlled design and direct observation of the patient during ingestion of the study drug. To our knowledge, this is the first trial to measure a head-to-head comparison of 2 vehicles of cholecalciferol in an adult CF population. The study was limited by its small sample size and in its ability to assess long-term effectiveness of each vehicle due to a short collection period of 48 hours; however, our primary objective in this pilot study was to measure short-term absorption of cholecalciferol to determine vehicle efficacy. Finally, this study population was recruited during an inpatient hospital admission, and the overall health status of study patients was considered “poor.” It is unknown the extent to which CF acute illness affects metabolic processes such as nutrient absorption.
Conclusions
In this randomized, double-blinded, controlled pilot trial, a vitamin D3 supplement had better absorption contained in a powder vehicle compared with supplements contained in an oil vehicle. This is the first clinical trial to evaluate such a direct vehicle substance of vitamin D3 absorption. These findings may also explain some of the differences in efficacy of different vitamin D supplements in the CF population. The new information should provide additional guidance for clinicians when deciding on the vitamin D supplement to use in patients with CF. Additional research is still needed to determine if improved efficacy of vitamin D absorption leads to better long-term vitamin D status in the CF population.
Clinical Relevancy Statement.
Patients with cystic fibrosis have fat malabsorption, increasing the risk of vitamin D deficiency, which is commonly treated with oral supplementation with vitamin D. The supplements of vitamin D contain either oil-based or powder-based vehicles. This study demonstrates that an oil-based vitamin D supplement is not as well absorbed as a powder-based supplement. These findings are clinically relevant to physicians, pharmacists, nurses, and dietitians caring for patients with cystic fibrosis.
Acknowledgments
Financial disclosure: This work was supported by grants from the Cystic Fibrosis Foundation (HERMES14H0 and TANGPR11A0) and the National Center for Advancing Translational Sciences of the and National Institutes of Health (K01 DK102851, UL1 TR000454).
Footnotes
Statement of Authorship
W. A. Hermes, J. A. Alvarez, and V. Tangpricha contributed to conception/design of the research; W. A. Hermes, J. A. Alvarez, V. Tangpricha, M. J. Lee, S. Chesdachai, D. Lodin, and R. Horst contributed to acquisition, analysis, or interpretation of the data; W. A. Hermes, J. A. Alvarez, and V. Tangpricha drafted the manuscript; and W. A. Hermes, J. A. Alvarez, V. Tangpricha, M. J. Lee, S. Chesdachai, D. Lodin, and R. Horst critically revised the manuscript. All authors read and approved the final manuscript and agree to be fully accountable for ensuring the integrity and accuracy of the work.
References
- 1.Gibson-Corley KN, Meyerholz DK, Engelhardt JF. Pancreatic pathophysiology in cystic fibrosis. J Pathol. 2016;238(2):311–320. doi: 10.1002/path.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle MP, Noschese ML, Watts SL, Davis ME, Stenner SE, Lechtzin N. Failure of high-dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(2):212–217. doi: 10.1164/rccm.200403-387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr. 2007;86(6):1694–1699. doi: 10.1093/ajcn/86.5.1694. [DOI] [PubMed] [Google Scholar]
- 4.Lark RK, Lester GE, Ontjes DA, et al. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr. 2001;73(3):602–606. doi: 10.1093/ajcn/73.3.602. [DOI] [PubMed] [Google Scholar]
- 5.Lee MJ, Alvarez JA, Smith EM, et al. Changes in mineral micronutrient status during and after pulmonary exacerbation in adults with cystic fibrosis. Nutr Clin Pract. 2015;30(6):838–843. doi: 10.1177/0884533615589991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644–649. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 7.Tangpricha V, Kelly A, Stephenson A, et al. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97(4):1082–1093. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 8.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69(3):374–381. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol. doi: 10.1016/j.jsbmb.2015.09.013. [published online September 20, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 11.Grossmann RE, Tangpricha V. Evaluation of vehicle substances on vitamin D bioavailability: a systematic review. Mol Nutr Food Res. 2010;54(8):1055–1061. doi: 10.1002/mnfr.200900578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson-Hughes B, Harris SS, Lichtenstein AH, Dolnikowski G, Palermo NJ, Rasmussen H. Dietary fat increases vitamin d-3 absorption. J Acad Nutr Diet. 2015;115(2):225–230. doi: 10.1016/j.jand.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Kalivianakis M, Minich DM, Bijleveld CM, et al. Fat malabsorption in cystic fibrosis patients receiving enzyme replacement therapy is due to impaired intestinal uptake of long-chain fatty acids. Am J Clin Nutr. 1999;69(1):127–134. doi: 10.1093/ajcn/69.1.127. [DOI] [PubMed] [Google Scholar]
- 14.Papas K, Kalbfleisch J, Mohon R. Bioavailability of a novel, water-soluble vitamin E formulation in malabsorbing patients. Dig Dis Sci. 2007;52(2):347–352. doi: 10.1007/s10620-006-9489-2. [DOI] [PubMed] [Google Scholar]
- 15.Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17(10):2116–2121. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 16.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khazai NB, Judd SE, Jeng L, et al. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94(6):2037–2043. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66(9):1072–1074. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossmann RE, Zughaier SM, Kumari M, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol. 2012;4(2):191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr. 2003;77(6):1478–1483. doi: 10.1093/ajcn/77.6.1478. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd D, Belessis Y, Katz T, Morton J, Field P, Jaffe A. Single high-dose oral vitamin D(3) (stoss) therapy—a solution to vitamin D deficiency in children with cystic fibrosis? J Cyst Fibros. 2013;12(2):177–182. doi: 10.1016/j.jcf.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Simoneau T, Sawicki GS, Milliren CE, Feldman HA, Gordon CM. A randomized controlled trial of vitamin D replacement strategies in pediatric CF patients. J Cyst Fibros. doi: 10.1016/j.jcf.2015.07.004. [published online July 23, 2015] [DOI] [PubMed] [Google Scholar]