Summary
The assessment of early pulmonary disease and its severity can be difficult in children as they often cannot perform procedures such as spirometry. Computed tomography provides detailed structural images of the pulmonary parenchyma but its major drawback is that the patient is exposed to ionizing radiation. In this context, MRI is a promising technique for the evaluation of pediatric lung disease, especially when serial imaging is needed. Traditionally, MRI played a small role in evaluating the pulmonary parenchyma. Because of its low proton density, the lungs display low signal intensity on conventional proton-based MRI. Hyperpolarized (HP) gases are inhaled contrast agents with an excellent safety profile and provide high signal within the lung allowing for high temporal and spatial resolution imaging of the lung airspaces. Besides morphological information, HP MR images also offer valuable information about pulmonary physiology. HP gas MRI has already made new contributions to the understanding of pediatric lung diseases and may become a clinically useful tool. In this article, we discuss the HP gas MRI technique, special considerations that need to be considered when imaging children, and the role of MRI in two of the most common chronic pediatric lung diseases, asthma and cystic fibrosis. We also will discuss how HP gas MRI may be used to evaluate normal lung growth and development, and the alterations occurring in chronic lung disease of prematurity and in patients with a congenital diaphragmatic hernia.
Keywords: Hyperpolarized Gas MRI, Cystic fibrosis, Asthma
Introduction
Pulmonary function tests (PFTs) are the gold standard for the monitoring of diffuse lung disease in children aged ≥ 6 years; in younger children PFTs can usually not be obtained because they cannot perform the required forced breathing maneuvers. In a few clinical centers passive PFTs is performed in sedated children but the requirement for sedation limits the utility of this technique. Furthermore, since PFTs provide only global information of the lung function, they are relatively insensitive to detecting early lung disease. The ongoing development of new therapies to modify the course of diffuse pediatric lung diseases, such as cystic fibrosis (CF), highlights the need for sensitive outcome measures that can make global and regional assessments of disease for clinical care and interventional trials.
Modern multidetector computed tomography (CT) scanners permit submillimeter imaging of the entire chest in a single breath hold. The excellent spatial resolution images provided by CT makes this modality ideal for evaluating the pulmonary parenchyma. CT provides regional information and is more sensitive than PFTs and chest x-ray to detect early lung parenchymal abnormalities. The major disadvantage of CT is that it involves ionizing radiation. In recent years, there has been a decline in CT-related radiation exposure thanks to dose-saving strategies and improvements in scanner technology. Despite these efforts, radiation remains a very important concern in the pediatric population, because children are considerably more sensitive to the carcinogenic effects of ionizing radiation than adults and have a longer life expectancy in which to express risk (1).
In this context, the lack of ionizing radiation in Magnetic Resonance Imaging (MRI) makes the technique ideal for use in the pediatric population, especially when repeated or serial studies are required. On global (whole-lung) and regional scales, MRI can also provide functional and morphological evaluations of the pulmonary parenchyma. Traditionally, proton MR has had a small role in evaluating lung parenchyma. Because of its low proton density, the normal pulmonary parenchyma displays with low signal intensity on MR images. In addition, the signal is further degraded by the susceptibility effects induced by its many air-tissue interfaces and by cardiac and respiratory movement. MR imaging in young children is especially challenging because of motion and their inability to follow breath hold instructions. Hyperpolarized (HP) gases are inhaled contrast agents that permit imaging of the lung airspaces. HP gas MRI has become an important research tool not only for morphological and functional evaluation of normal pulmonary physiology, but also for regional quantification of pathologic changes occurring in diffuse lung diseases (2–7).
In this article, we discuss the HP gas MRI technique and special considerations that need to be taken into account when imaging the pediatric population. We review the role in the technique in two of the most common chronic pediatric lung diseases, asthma and cystic fibrosis. Further, we will discuss its role in the evaluation of normal lung growth, and in assessing the development of the structural alterations that occur in children with chronic lung disease of prematurity and in patients with congenital diaphragmatic hernia.
HP Gas MRI Technique
HP gases are prepared using a laser-based system called a polarizer, separate from the MR scanner, wherein the nuclear spins of the gas atoms are brought into preferential alignment with a weak magnetic field. This achieves a signal strength many times higher than that which would be obtained if the subject were to inhale the gas without this preparation. After polarization, the gas is transferred to a plastic bag, transported to the MR scanner, and inhaled by the subject through a small plastic tube or using a gas-administration apparatus.
HP Gases: Helium-3 and Xenon-129
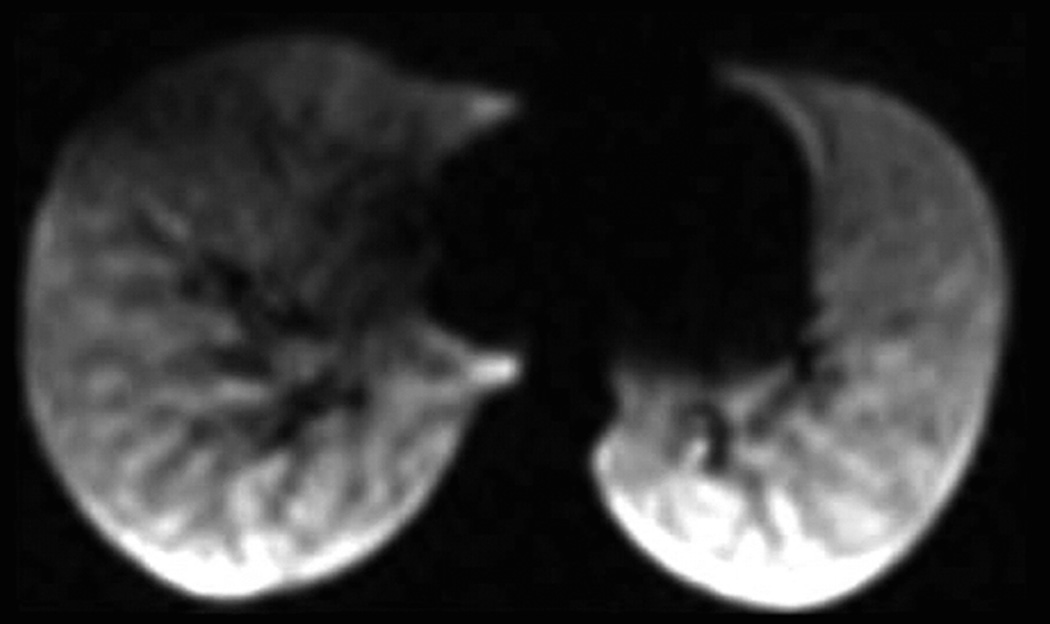
Helium-3 and xenon-129 are two non-radioactive isotopes of noble gases that can be hyperpolarized and have different physiologic properties (Table 1). They are classified as investigational drugs by the US Food and Drug Administration (FDA). Owing to its very low solubility in blood and tissue, helium is relatively biologically inert, and remains almost entirely within the airspaces when inhaled. Conversely, xenon is weakly soluble in lipids and in blood and tissue. This weak solubility makes xenon biologically active and gives it anesthetic properties at high doses. For imaging, the solubility of xenon in tissues allows detection of the regional gas transfer between the alveoli and the tissue in the lung (8,9). Although most research in humans has used HP helium-3, a reduction in the world supply of helium-3 and consequent increases in price turned attention to HP xenon-129. Compared to helium-3, xenon-129 provides intrinsically lower signal due to its smaller magnetic moment. Recent advances in gas-polarization technology for xenon-129 have enabled studies that found ventilation, diffusion, and partial pressure of oxygen imaging with HP xenon-129 is comparable to HP helium-3 (10–13)(Fig.1).
Table 1. Hyperpolarized Gases.
Comparison of physical and physiologic properties of hyperpolized Helium-3 and Xenon-129.
| Helium-3 | Xenon-129 | |
|---|---|---|
|
Gyromagnetic ratio [MHz/T] |
32.4 | 11.8 |
| Blood Solubility (%) | 1 | 17 |
| Side effects | No known side effects | CNS (euphoric and anesthetic) side effects |
| Atomic mass (u) | 3.016 | 128.904 |
|
Free diffusion coefficient in air (cm2/s) |
0.86 | 0.06 |
| Supply | Tritium decay (1.3×10–4% on Earth) Shortage of supply |
Earth´s atmosphere (26.44% on Earth) Readily available |
| Price (approx. $/L) | 900 | 200 |
CNS: Central Nervous System
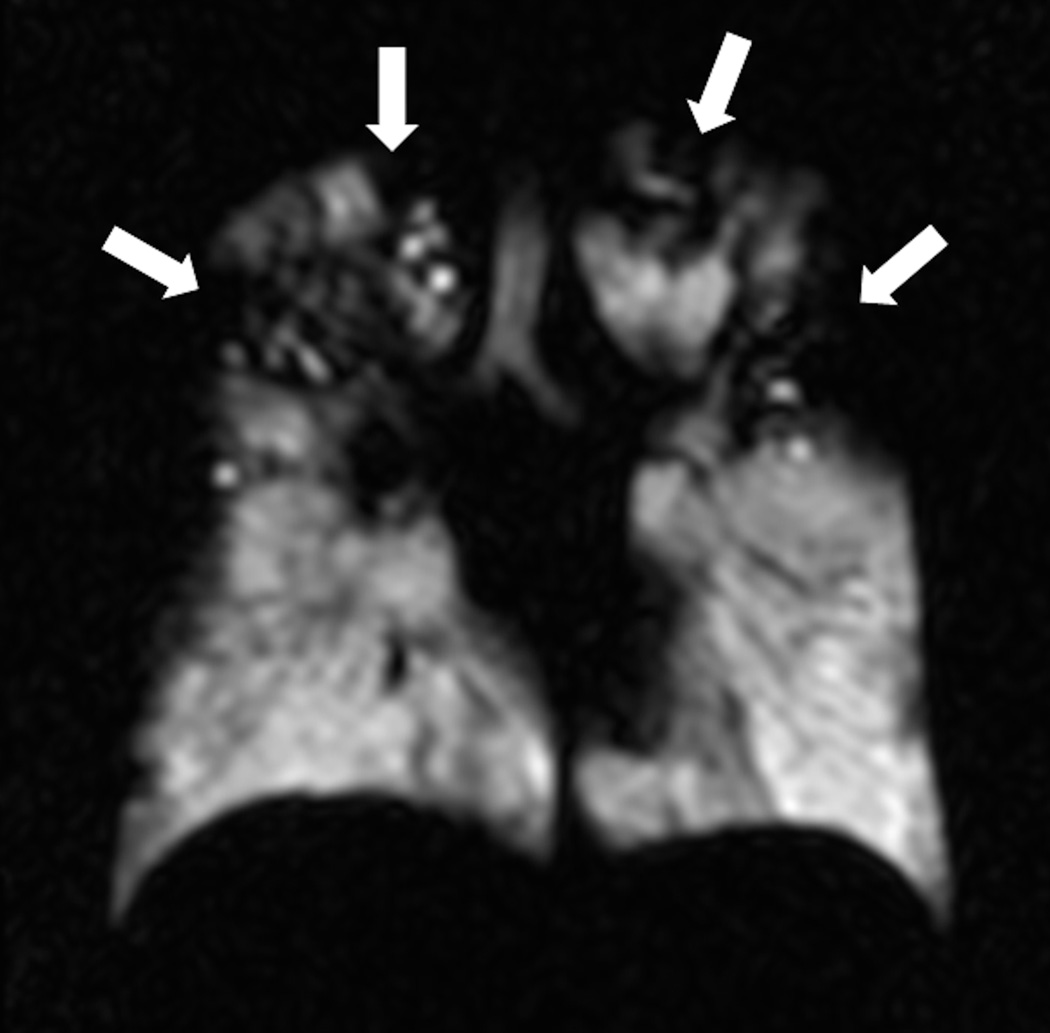
Fig. 1. Ventilation imaging: comparison of Helium-3 and Xenon-129 imaging.
Twenty-eight year-old male with CF (FEV1 57%). Coronal Helium-3 (a) and Xenon-129 (b) MR ventilation images performed on the same day show similar appearing upper lobe predominant ventilation defects (arrows).
MR Sequences
1. Ventilation Imaging
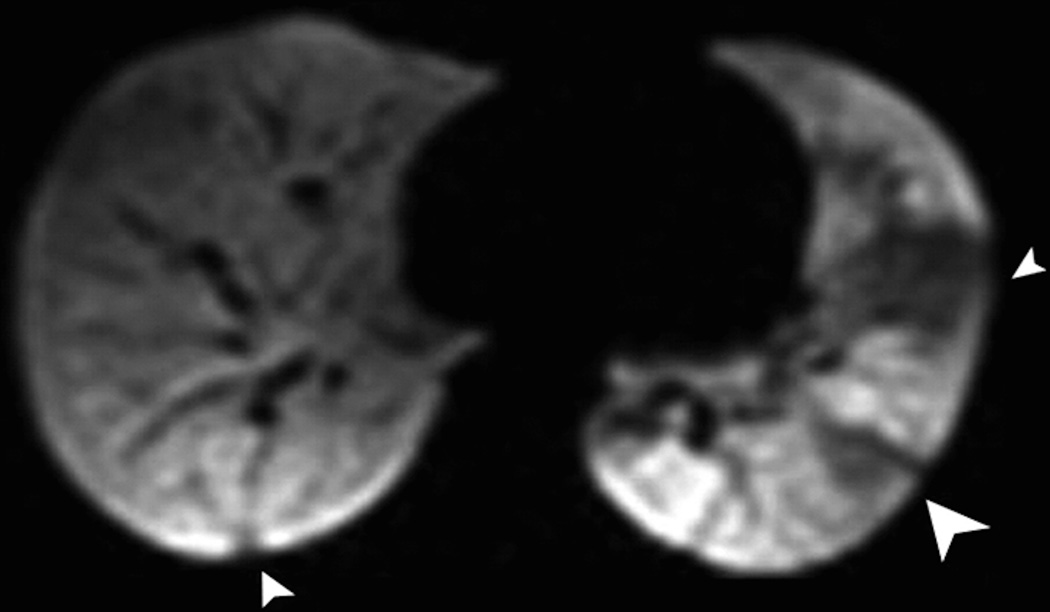
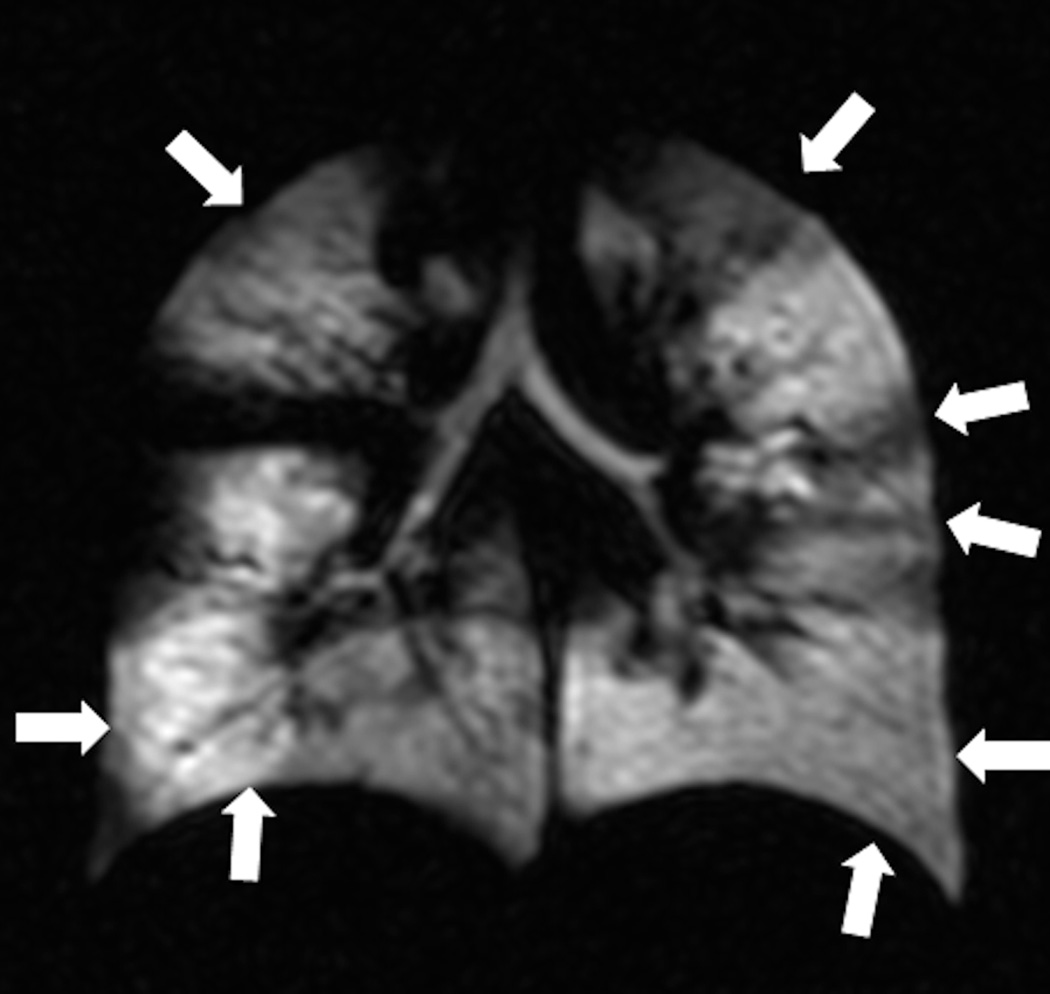
Images of lung ventilation are obtained using spin density imaging during a breath hold following the inhalation of HP gas. When the HP gas is inhaled the airspaces are filled, and when subsequently MR images are obtained, the changes in the regional ventilation resulting from airflow obstruction are depicted. In healthy subjects the gas distributes uniformly throughout the airspaces producing uniformly high signal throughout the pulmonary parenchyma. When there is disease that leads to reduction of local airflow, the airspaces distal to that area are poorly ventilated and appear dark on ventilation images, resulting in so-called ventilation defects (Fig.2). This is conceptually similar to nuclear medicine lung ventilation imaging, albeit with better temporal and spatial resolution. Modern computational processing methods permit automated quantification of ventilation defects on HP gas MRI (Fig. 3) (14,15).
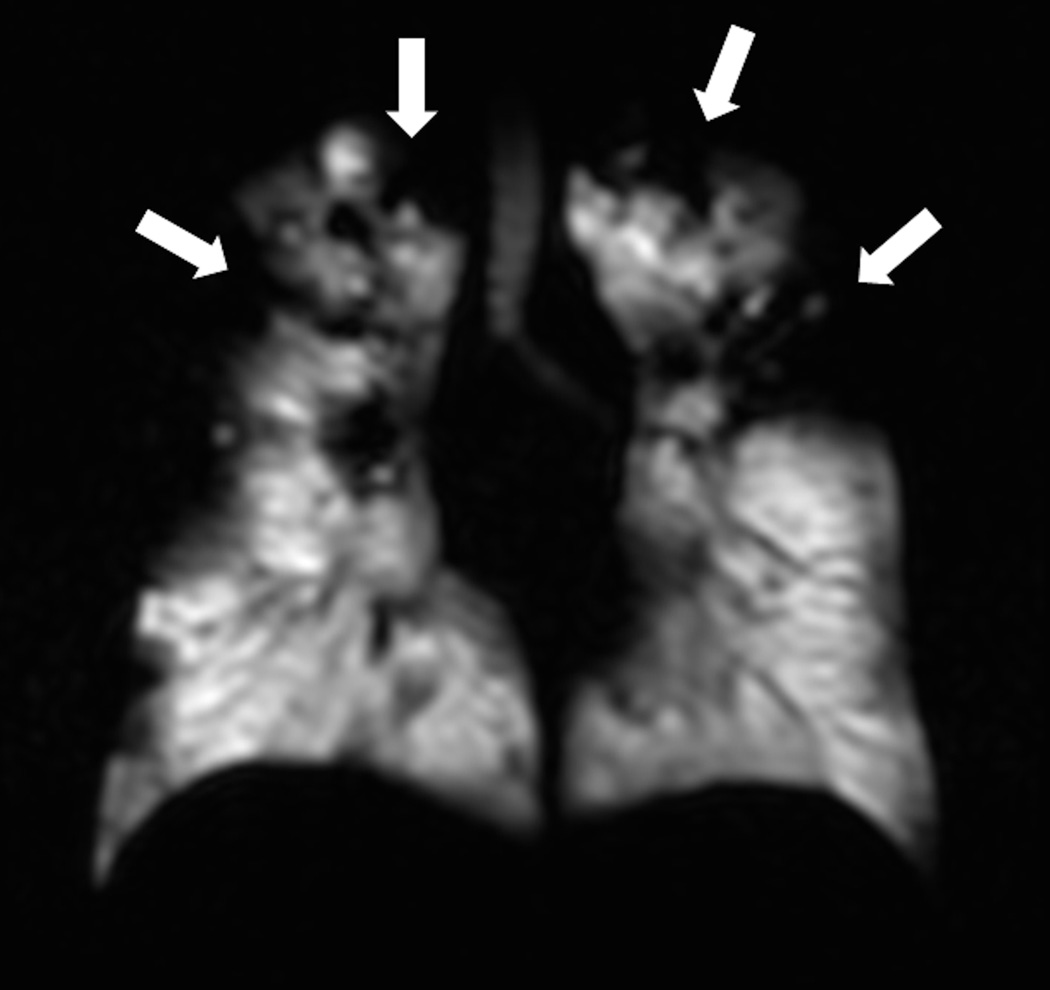
Fig. 2. Helium-3 MR ventilation images.
Coronal images show homogenous distribution of signal throughout the lung parenchyma in a subject without lung disease (a) and multiple ventilation defects in a patient with cystic fibrosis (b).
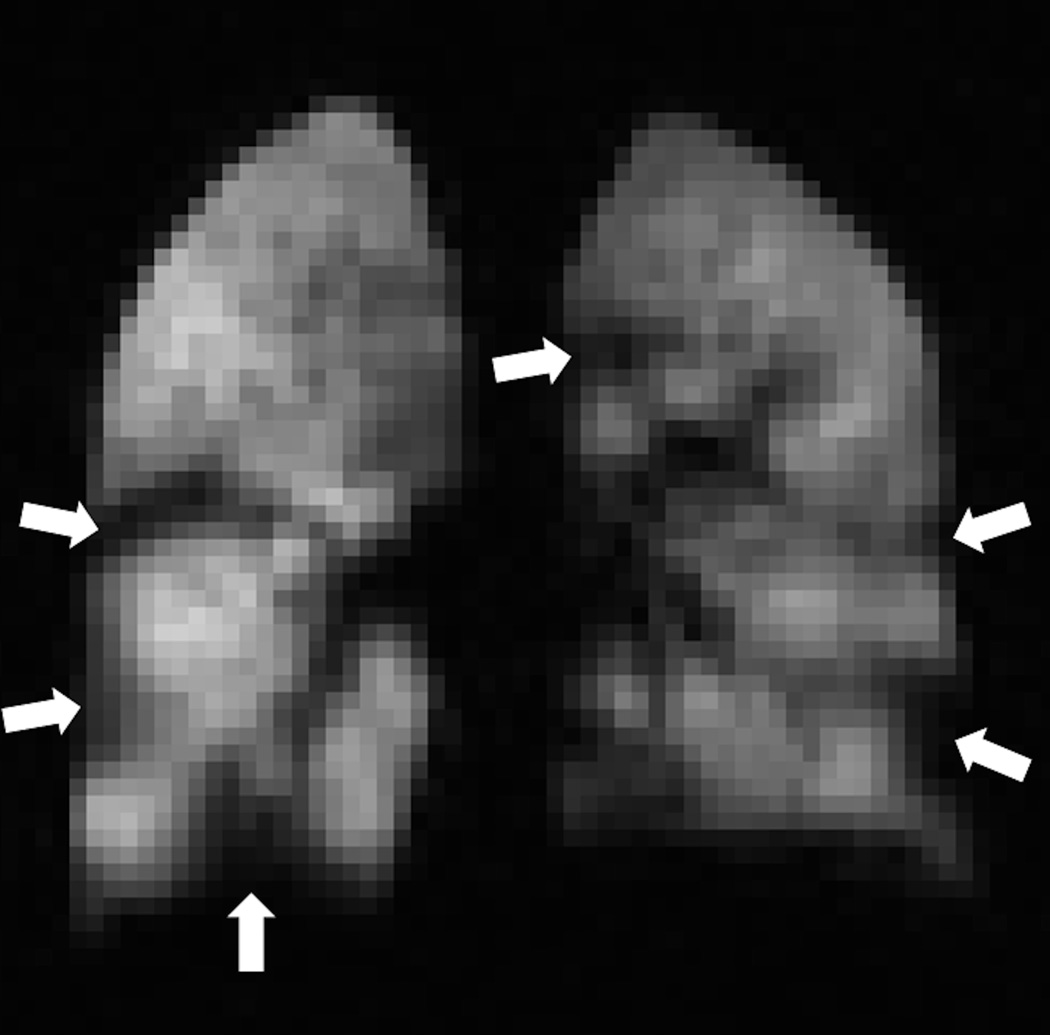
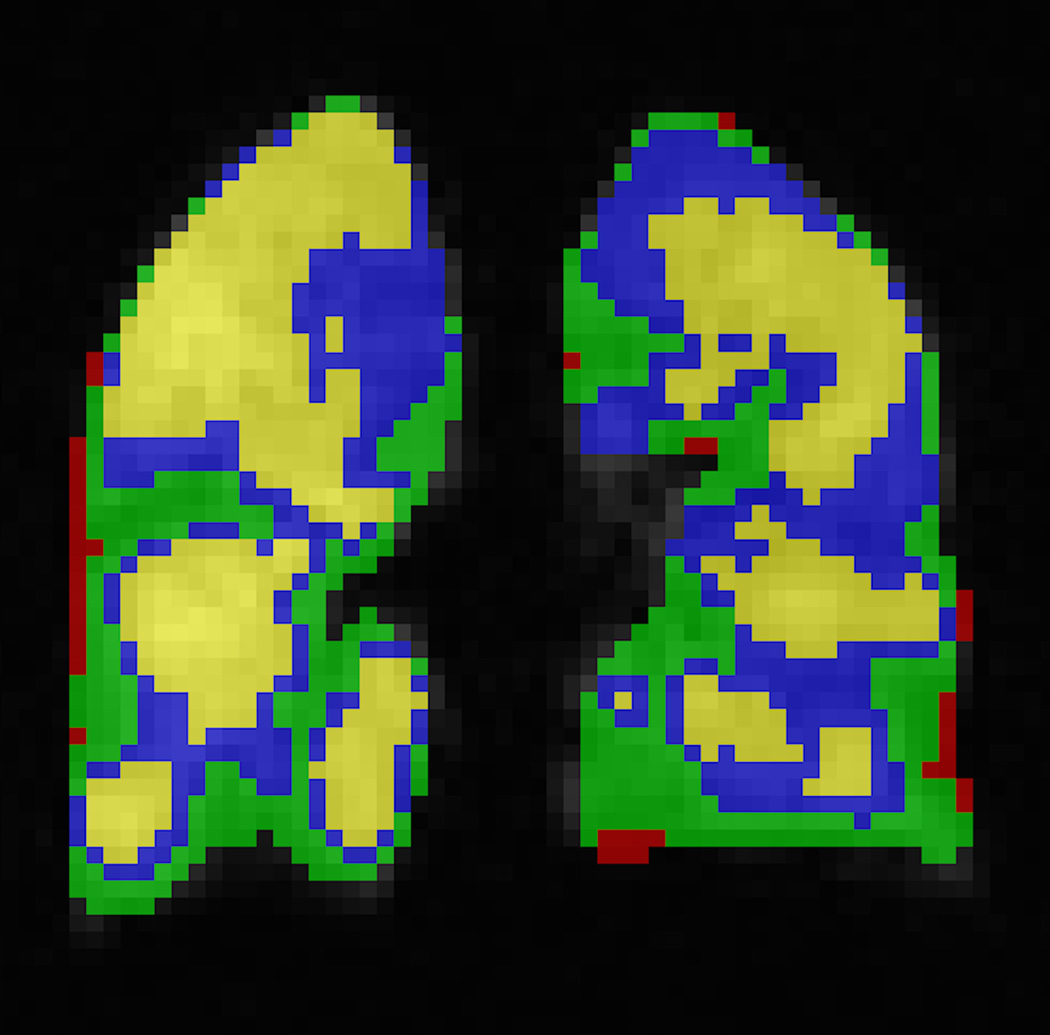
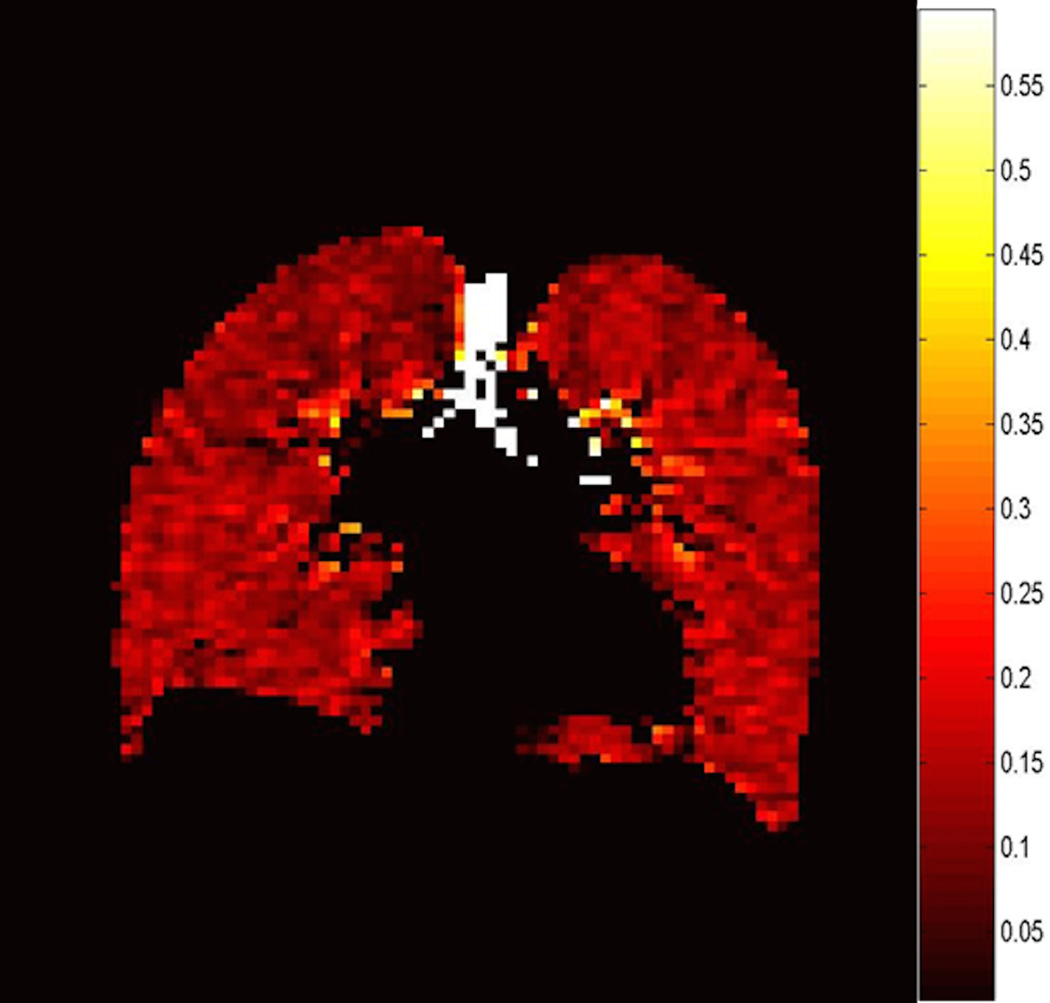
Fig. 3. Automatic segmentation of ventilated lung volume.
HP helium-3 ventilation MRI (left) in a pediatric patient shows multiple bilateral ventilation defects (arrows). Segmentation using an automatic ventilation-based segmentation algorithm (14) (b) allows precise quantification of the ventilated lung volume. The color legend in b is as follows: red-ventilation defect, green-hypoventilation, blue/yellow-normal ventilation.
Dynamic ventilation MRI is performed using ultrafast spin density imaging while the subject inhales or exhales the gas. The technique is used to show the distribution of the gas in the lung parenchyma over time with sub-second time resolution.
2. Diffusion Imaging
Proton-based diffusion-weighted (DW) MR imaging studies the random Brownian motion of water molecules within a voxel of tissue. A similar principle can be applied to HP gas MR to assess the motion of HP gas atoms within the alveolar spaces and the degree of restriction by alveolar walls as measured by an apparent diffusion coefficient (ADC) (16). The ADC provides a noninvasive quantitative measure that correlates with alveolar dimensions (17–22). Precise correlations between the global and regional ADC measured by HP gas MRI and alveolar size as measured histologically have been reported (17,23–25).
DW HP gas MRI is one of the most extensively developed HP-gas methods for studying emphysema (21,24,26–28). Enlarged airspaces have been detected using DW HP helium-3 and xenon-129 MR imaging in animal models and humans with emphysema (21–24,26,27,29). The technique has also applications in pediatric lung diseases as we will further discuss below.
3. Oxygen Sensitive Imaging
The rate of loss of the polarization of the gas is strongly influenced by the local environment. In the presence of oxygen, due to its paramagnetic effect, the longitudinal relaxation of HP gas is accelerated. By assessing the rate of decay of polarization (measuring the T1) of HP helium-3 or xenon-129 within the lung, it is possible to obtain an indirect measurement of the alveolar partial pressure of oxygen (30,31).
4. Alveolar-Capillary Gas Transfer
Upon inhalation, HP xenon-129 follows the functional pathway of gas exchange in the lung by diffusing from the alveolar airspaces into the alveolar septa (blood and tissue) (32). Since the spectral peaks associated with xenon-129 magnetization in the different pulmonary compartments are at different chemically shifted frequencies, it is possible to monitor the kinetics of xenon-129 magnetization diffusion from one compartment to another and to distinguish the xenon gas- and dissolved-phases.
Several image acquisition methods have been developed to obtain information of gas exchange and alveolar-capillary gas uptake. In chemical shift saturation recovery (CSSR), a frequency-selective radio frequency (RF) pulse is initially employed to destroy the xenon-129 magnetization in the dissolved phase. Recovery of the dissolved phase xenon-129 magnetization, by diffusion of HP xenon-129 from the alveolar gas space to septal tissue, is then observed (33). Compared to CSSR, which directly measures the dissolved-phase xenon magnetization, the xenon polarization transfer contrast (XTC) method measures the diffusion into the dissolved phase indirectly by looking at the attenuation in the gas phase signal after multiple opportunities for interphase diffusion (8,33). Parenchymal tissue to alveolar-volume ratio, surface-to-volume ratio and blood–gas barrier thickness can be determined (32).
Since little work has been done with oxygen sensitive and alveolar gas transfer imaging in children, we will not further discussed it and we will focus on the applications of ventilation and diffusion imaging in the pediatric population.
Considerations for MR Imaging in Infants and Young Children
Children are difficult to evaluate with MR imaging because they often cannot remain motionless or comply with breathing instructions. Clear explanation and coaching on breathing maneuvers are essential. In infants, it is necessary to restrain motion similarly to other clinical imaging procedures. Parents are also usually asked not to feed the infant for 2 hours before the MR appointment. Children may be fed immediately prior to being placed in the MR scanner, and parents are encouraged to be in the scanner room with their child during the MR scanning. If tolerated, skull-caps with noise attenuating material over the ears may prevent the child from being startled by the noise of brief MRI acquisitions. Helium-3 gas delivery methods have been developed based on clinical methods for delivering oxygen and inhaled medications to infants and young children. Stuffed animals and other toys are usually used to distract infants during the short MRI acquisition times.
In adults, HP gas MR imaging of the entire lung volume requires a 4- to 20-second breath-hold. Because children have smaller lungs, the duration of the breath-hold is usually shorter, but still beyond the ability of infants and young children. The acquisition time can be reduced by a factor of ten with spiral-sampling-based strategies, thus making possible imaging of free breathing infants and young children (Fig.4). The helium-3 MR acquisition time for a set of images covering the whole lung is less than 1 second, and the total time the subject is in the MR scanner less than 10 minutes. The acquisition time is comparable to or shorter than that of a CT scan. The extremely short acquisition time allows helium-3 MRI in non-sedated infants. Helium-3 MRI has been successfully performed in children as young as 10 months. Specially designed infant-sized helium-3 MRI RF coils must be used to obtain high spatial resolution imaging in small children (Fig.5). Since the gas is polarized outside the scanner, it is possible to image HP gases with a very low magnetic field strength scanner that could easily be located in an NICU.
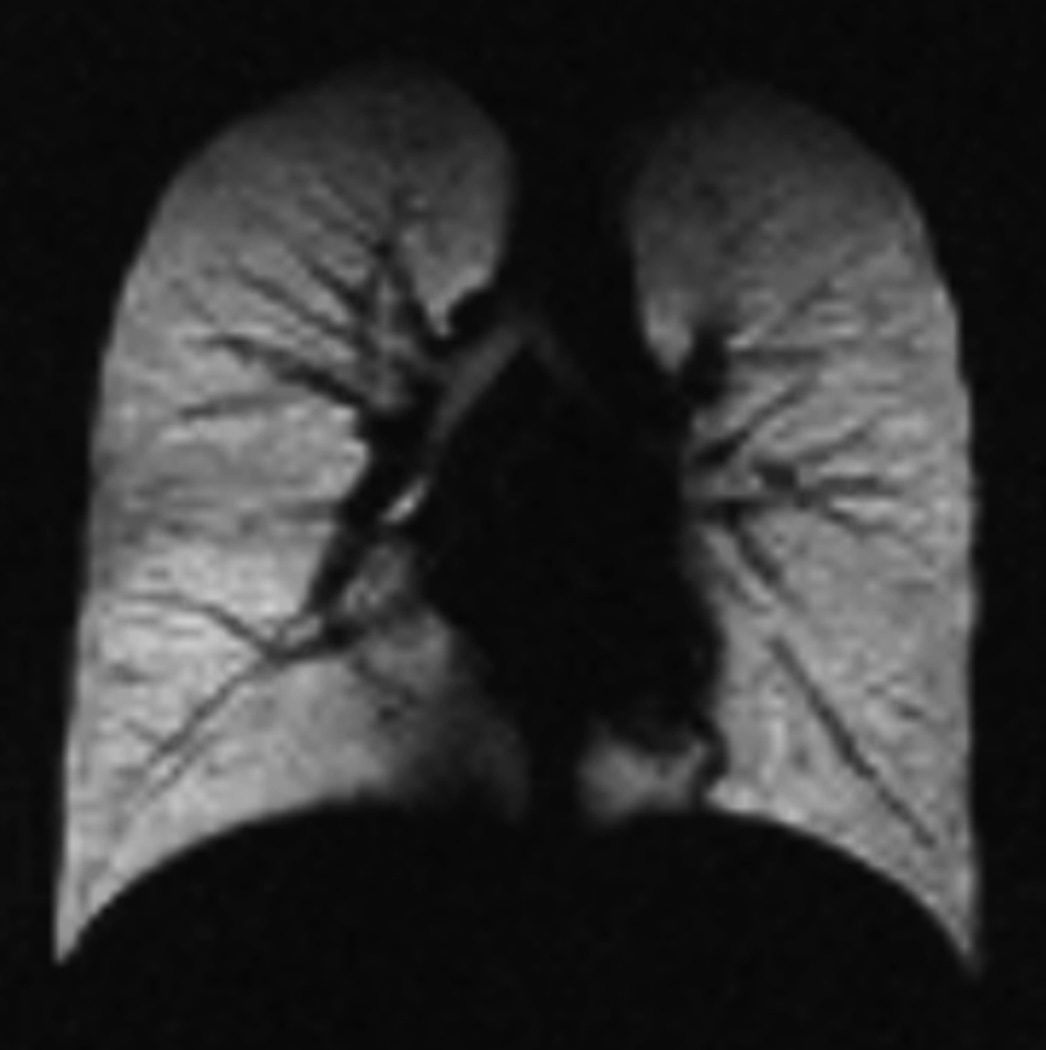
Fig. 4. Free-breathing ventilation helium-3 MRI.
MR images obtain during free breathing show homogeneous distribution of signal throughout the lung parenchyma in a 4-year-old healthy child (a) and several ventilation defects in a 5-year-old child with cystic fibrosis (arrows) (b).
Fig. 5.
Infant sized coil tuned to the RF frequency to the HP gas enables fast, high-resolution head and smaller field-of-view imaging.
The primary safety advantage of HP gas MRI is the absence of ionizing radiation, and helium-3 itself is not radioactive. Its chemical properties are not altered by polarization such that it retains low solubility in blood or tissue and is thus biologically inert. Inhaled helium-3 remains within the airspaces and is exhaled in subsequent breaths. To date, HP gas MRI has been well tolerated and used safely (34,35). In a review of the safety data of HP helium-3 MRI performed at our institution during three consecutive years in 313 subjects, we found mild respiratory adverse events in 7% of the patients with spontaneous resolution in all cases (36). Children were not more likely to have an adverse event than adults, and patients with lung disease were not more likely to have an adverse event than healthy volunteers. Thus inhaled helium-3 has the outstanding safety profile required for use in infants.
Although there are concerns about the anesthetic properties of xenon-129 and experience with this gas is more limited, its safety and tolerability have also been recently demonstrated in adult healthy subjects and patients with pulmonary disease (11,37). In our institution, we have safely performed HP xenon-129 MR imaging in healthy children and children with pulmonary diseases.
Use of HP Gas MRI in Children
Asthma
Asthma is the most common chronic disorder of childhood. It is a chronic inflammatory disorder of the lung that predominantly involves the small and medium-sized airways. Symptoms are usually associated with variable airway obstruction and impaired ventilation caused by such changes as airway hyper-responsiveness and bronchoconstriction, airway wall edema, mucus hypersecretion, and airway remodeling with smooth muscle hypertrophy and subepithelial fibrosis.
Spirometry measurements of airflow obstruction tend to overestimate large airway constriction and underestimate small airways disease and, moreover, cannot identify specific airways responsible for regional airflow obstruction (38). Current imaging techniques are of limited value in asthma. CT provides detailed images of the bronchial walls and air trapping, but it is not clear whether these indirect assessments of aeration correlate with asthma severity. CT provides only indirect functional information about lung ventilation. HP gas MRI can evaluate functional alterations of airflow within the lung caused by structural changes of the airways. Because no radiation is involved, serial imaging permits the safe assessment of temporal changes within the lungs of asthmatics.
Most studies with HP gas MRI have been performed on adult asthmatic patients. These studies have found ventilation defects in asthmatics, even when asymptomatic (39). There is correlation between the number and size of ventilation defects detected by HP helium-3 MRI and disease severity as measured by spirometry or the frequency of symptoms (4).
Recently, lung defect volume percentage was found to correlate with several clinical features of asthma such as severity, symptom score, medication requirement, airway physiology, and atopic markers in children with mild and severe asthma (40). Another recent study (41) using helium-3 MR in a subset of 44 children participating in the Childhood Origins of Asthma (COAST) study (42)found that children with asthma had more and larger ventilation defects and a greater degree of restricted gas diffusion (lower average root-mean-square diffusion length) than non-asthmatic children in this cohort, without correlation between the two measures. The authors hypothesized that the dimensions of the small airways and alveolar spaces are smaller in children with asthma. Results in adults contradict those in children (43) and suggest that there may be age-related or duration of disease related differences in structural features of asthma. Thus, further investigation is needed.
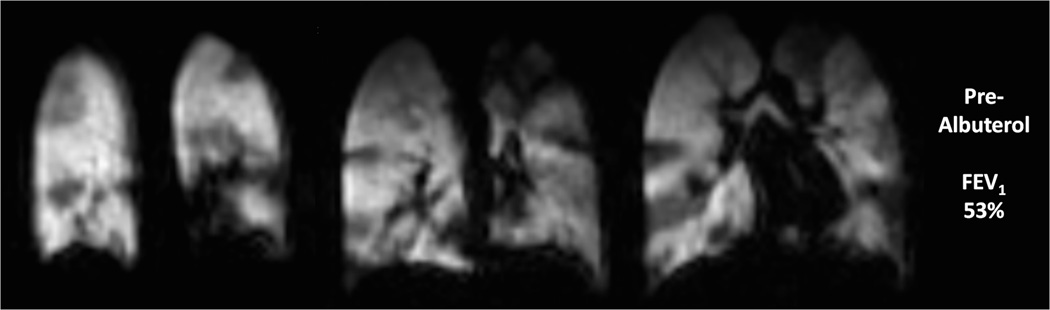
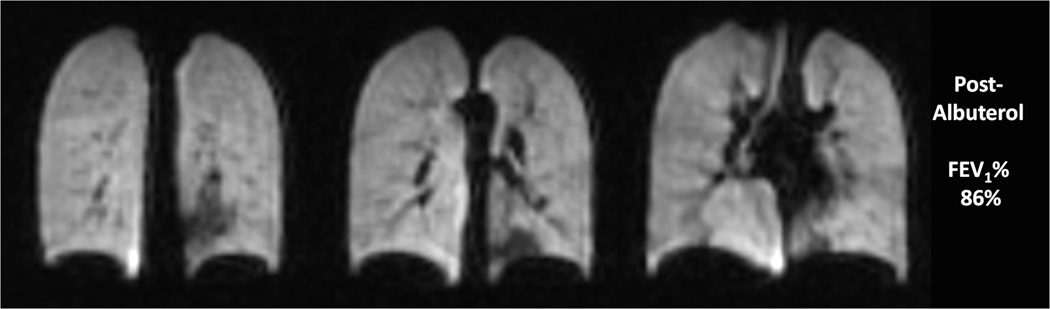
Ventilation defects can be induced medically with methacholine (an inhaled direct bronchoconstrictor) (44,45) and with exercise, and can be reversed by albuterol (an inhaled bronchodilator) (Fig.6). The percent increase in defects from premethacholine to postmethacoline administration was much greater than the percent decrease in spirometric values (45)
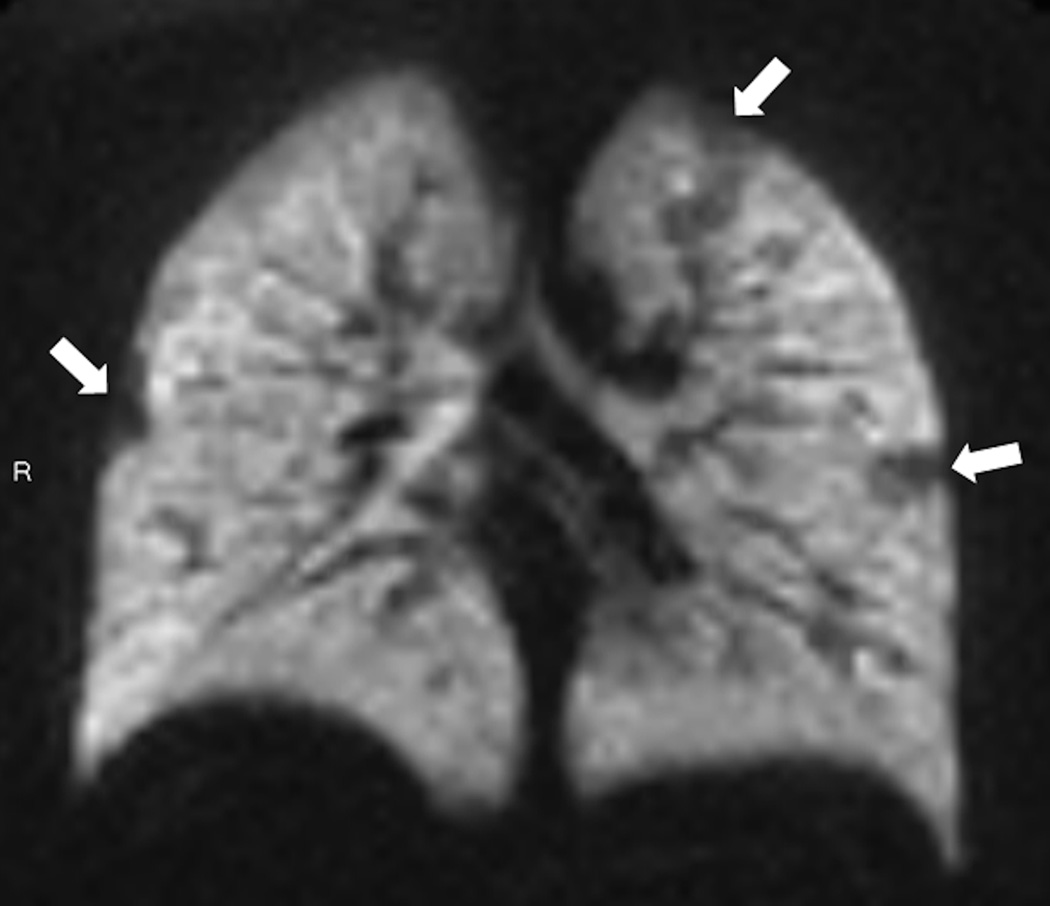
Fig. 6. Ventilation changes after inhalation of albuterol in 10 year old male with asthma.
Coronal helium-3 ventilation images at baseline (a) (FEV1 53%) show extensive bilateral ventilation defects which decreased after treatment with albuterol (b) (FEV1 86%)
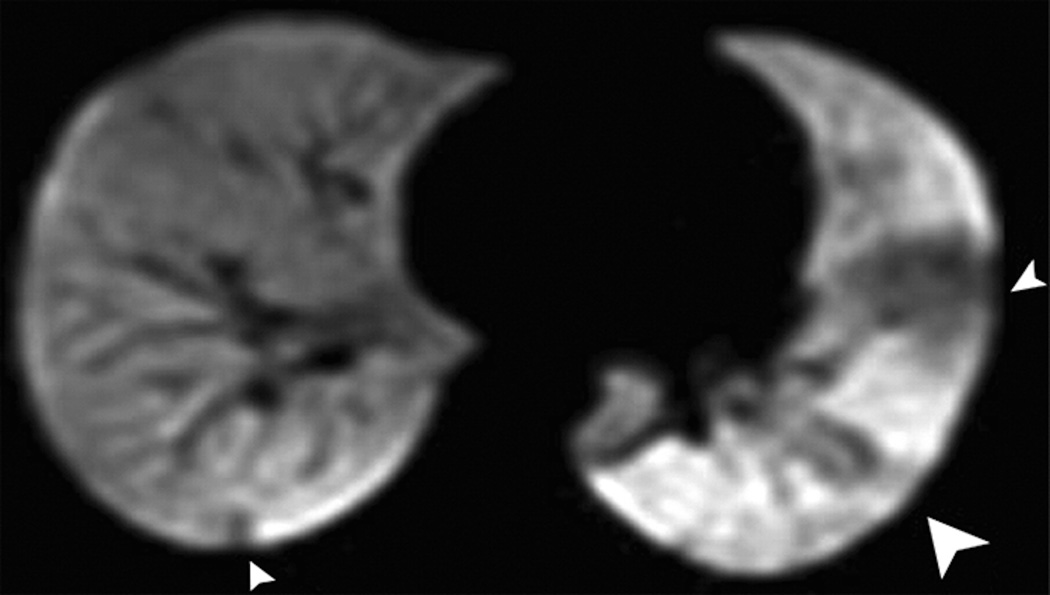
HP gas MRI has given new insight in the of regional airflow variability within the lungs of patients with asthma in that ventilation can defects persist or recur in the same location with time (45) regardless of disease severity or treatment (46) (Fig.7). This suggests that the severity of the disease within the lung varies regionally. The use of HP gas MRI may have important implications for regional treatments such as local aerosolized anti-inflammatory agents or bronchial thermoplasty, where the airway wall smooth muscle is RF ablated during bronchoscopy. In fact, in a study of 10 patients with severe asthma who received treatment with bronchial thermoplasty (47), quantification of regional ventilation changes indicated that ventilation defects decrease as a function of time after treatment. In particular, ventilation defects first increased early post-treatment but then decreased with time.
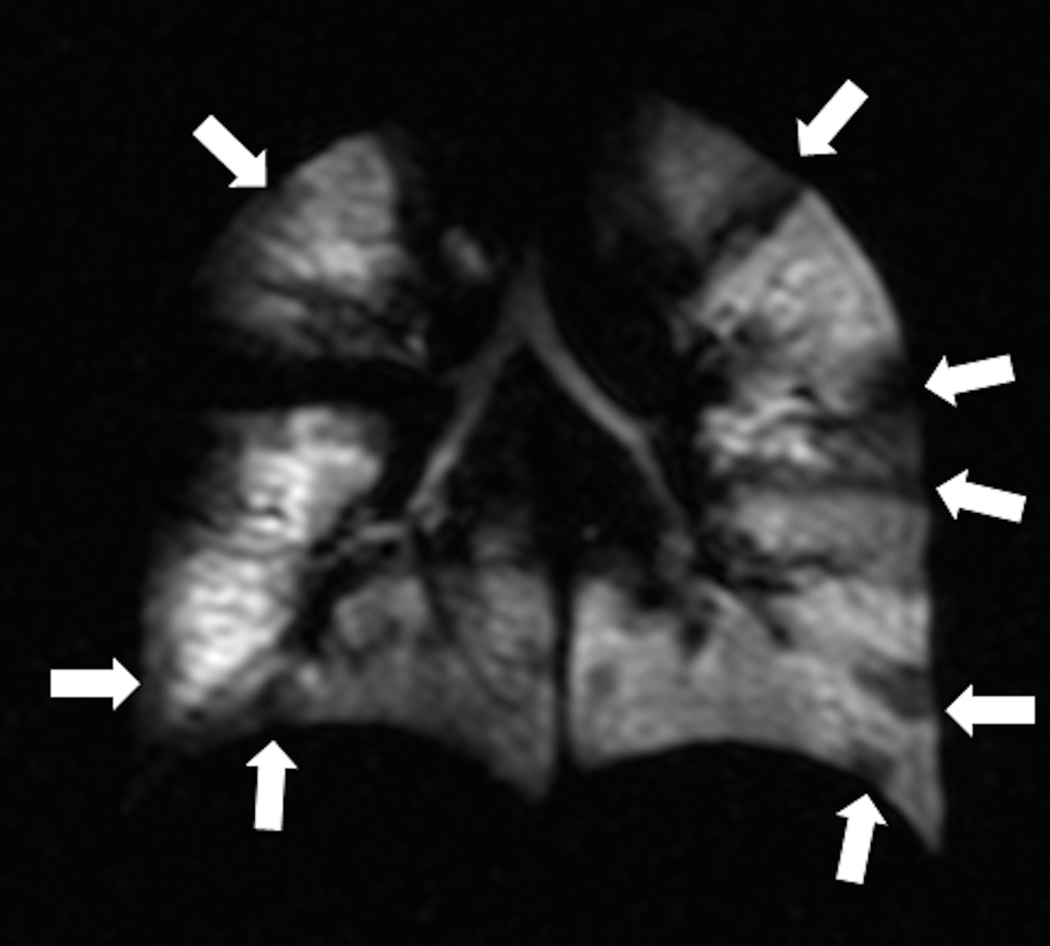
Fig. 7. Recurrence of ventilation defects in 19 year old female with asthma.
HP Helium-3 MR images performed at 3 different time points (a: baseline, b: 55 days later, c: 93 days after a) show three ventilation defects at baseline (arrowheads) (a) that resolved at 55 days (b) and recurred at 93 days with exam location and size as at baseline (arrowheads) (c) (Adapted with permission from: de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP 3rd, Platts-Mills TA. Radiology 2009, 250, 567–575. DOI: 10.1148/radiol.2502080188).
Dynamic ventilation images permit analysis of the kinetics of air trapping in asthmatic patients with good concordance between the areas of air trapping depicted by HP gas MRI and CT (48).
Cystic Fibrosis
Cystic fibrosis (CF), is an autosomal recessive disorder caused by mutations on the CF transmembrane conductance regulator (CFTR) gene. Impaired function of the CFTR protein causes abnormal chloride transport and increased viscosity of exocrine secretions in the pulmonary airways and GI tract. Abnormal airways in the lung are prone to infection and exhibit a marked and prolonged inflammatory response. The cycle of infection and inflammation along with obstruction due to abnormal mucous produces progressive structural damage causing bronchiectasis and fibrosis. Progressive lung disease remains the primary cause of death in CF (49) and imaging monitoring is usually necessary. With an estimated incidence of one in 2500 live births (50), CF is the most common fatal genetic disease in Caucasians.
Proton-based or conventional MRI may be an imaging alternative to study structural abnormalities in patients with CF. Spatial resolution of MRI is lower than that of CT, however MRI can detect bronchial wall thickening, mucus plugging, bronchiectasis, air-fluid level, and airspace consolidations (51,52). New ultrashort TE techniques hold promise for greatly improving image quality in MRI of the pulmonary parenchyma (53). A detailed description of proton-based MR in CF patients is beyond the scope of this article.
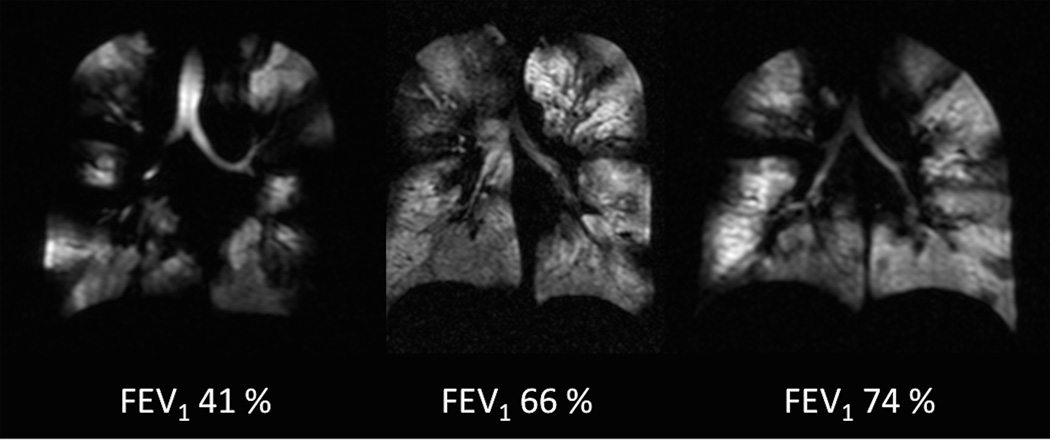
Functional information can be obtained by using HP gases. Studies investigating HP gas in CF patients were mostly performed using ventilation imaging. HP helium-3 MR uncovered ventilation defects in young adults with CF (6), and children with CF (54). CF patients had more defects than age-matched healthy subjects (50). Defects were also present in CF patients with normal pulmonary function tests (50,55) (Fig.8).
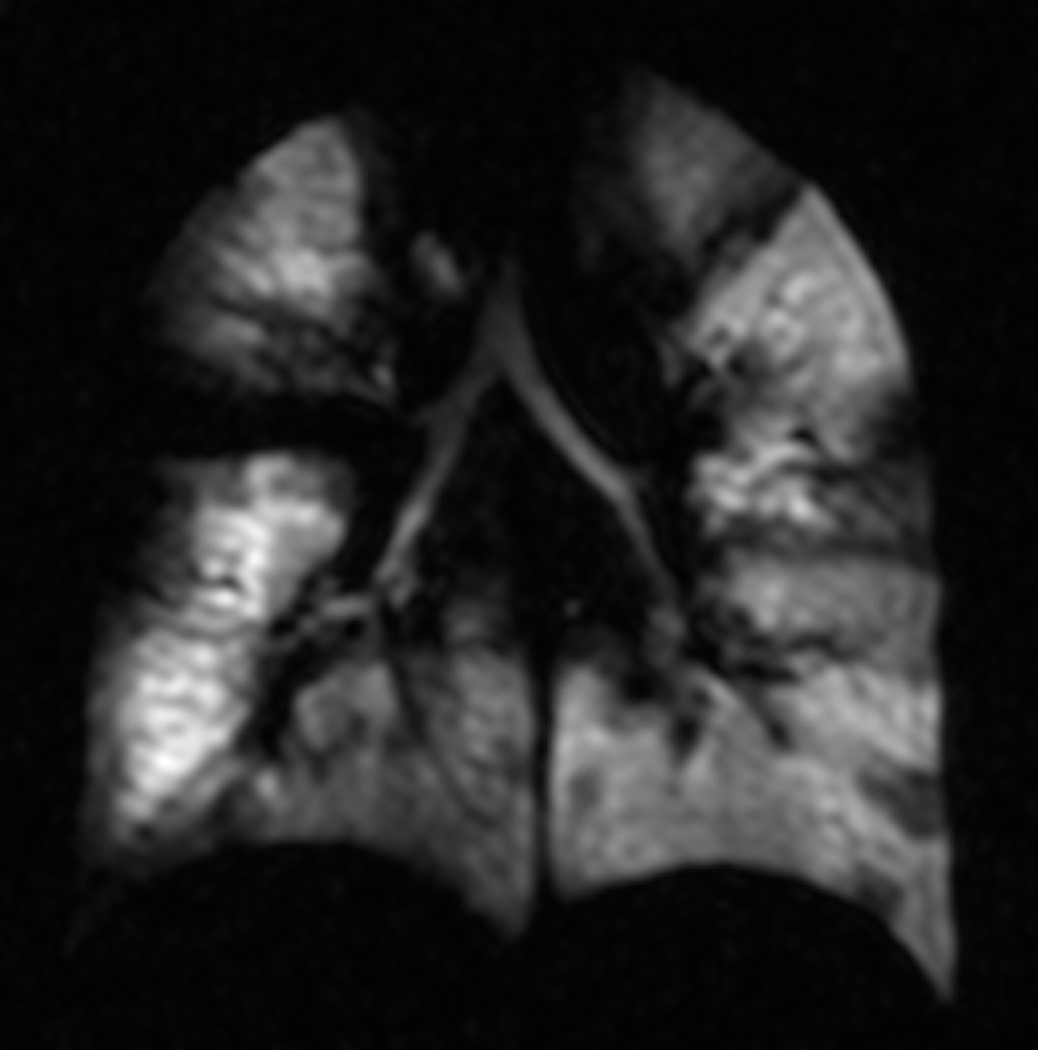
Fig. 8. Ventilation defects in cystic fibrosis.
Coronal helium-3 MR ventilation images in three patients with cystic fibrosis reveal multiple bilateral ventilation defects regardless the spirometry results.
HP gas ventilation MRI correlates strongly with structural CT abnormalities (Bhalla score) (56) and spirometry (50,56), but correlation between the MRI findings and those of chest radiograph (Swachman and Chrispin-Norman scores) is poor (54). Conversely, the correlation between HP gas MRI and spirometry is stronger than that of CT (56). The abnormalities depicted with HP helium-3 MRI are more extensive than those seen with conventional proton MRI (6).
Ventilation defects in CF patients change with treatment including bronchodilator (Fig.9 ) and mechanical airway clearance (50,57,55). HP helium-3 MRI findings are reproducible over time (58). Hence, HP gas MRI can be a sensitive test for depicting early disease and has the potential to become a biomarker of pulmonary CF.
Fig. 9. Ventilation changes after inhalation of albuterol in 16-year old female with cystic fibrosis.
Coronal helium-3 ventilation images show extensive bilateral ventilation defects before treatment (arrows) (a) which decreased after treatment with albuterol (b). FEV 1 increased from 74% to 75% with treatment.
The life expectancy of CF patients increased substantially from 14 to 40 years during 1969 to 2013 (49) and is expected to continue to improve. Many different mutations have been identified in the CFTR gene, prompting development of drugs specific to a mutated gene product. In this context, sensitive outcome measurements are needed. Ivacaftor (Kalydeco®, Vertex Pharmaceuticals) is the first of these drugs and targets patients with the G551D (glycine to aspartate change in nucleotide 1784 in exon 11) mutation. The drug is a CFTR potentiator since it is responsible for improved chloride transport via the G551D-CFTR protein (59). Ivacaftor is approved for the treatment of CF in patients aged ≥ 6 years with G551D mutation of CFTR. HP gas MRI recently detected changes in ventilation in CF patients after Ivacaftor therapy (60). Thirty days of treatment with Ivacaftor led to a substantial reduction in total ventilation defects and total defect volume as assessed by HP gas MRI.
Lung growth
Knowledge of human lung development is based upon a few autopsy studies with a small number of subjects (61–63). Alveolar development starts 25 to 40 weeks post-conception and continues during the first few years of life (64,65). Approximately 20 million alveoli are thought to be present at birth and progressively increase to 200 million by the age of three years (61–63). Beyond approximately 8 years of age, most lung growth has been thought to be secondary to enlargement of preexisting alveoli (66,67). Interestingly, recent data in mammals (68–70) from studies using modern morphometric techniques suggest that alveolarization continues throughout lung growth. Since DW HP helium-3 MRI has demonstrated that the ADC provides information about the alveolar size and morphology (20–22) that correlates with histology (17,23–25), HP gas MRI may be able to non-invasively evaluate normal lung growth and development.
In a group of 29 normal subjects, DW HP helium-3 MRI demonstrated an increase in ADC with increasing subject age, with a 55% increase in mean ADC from the youngest (4 years of age) to the oldest (30 years of age) subject. This ADC pattern likely reflects increasing alveolar size as the lung grows during childhood (71). Another study using DW HP helium-3 MRI in 109 subjects with an age 7–21 years (median 12.8 yr) suggested that alveolarization also continues during childhood and adolescence (72). The alveolar dimensions increased with age and lung size, at a rate much less than would be expected if lung growth occurred only by expansion of the existing airspaces. The explanation for these results was that the lung grows largely by neoalveolarization through childhood and adolescence.
Chronic lung disease of prematurity
As noted above, formation of alveoli occurs late in gestation and during the first years of life (64,65). At 24–26 weeks’ gestation infant lungs are in the canalicular phase of development, a time when alveolar and distal vascular development commences (64). Chronic lung disease of prematurity, also known bronchopulmonary dysplasia (BPD), is thought to be at least partially due to an alteration in normal lung development. When an infant is born prematurely, its lungs must act as a gas exchange organ despite being at a time when alveoli are still forming (65). At least in some premature infants this appears to result in alveoli that are abnormally large and fewer in number (64,65,73,74). Surfactant deficiency, perinatal insults such infection and damage from the ventilation supportive care, and alterations in lung development interact to impair lung function (65). Unfortunately, morphological alterations of lung structure in children with BPD are not well characterized, since lung tissue from living children is not readily available and lung autopsy specimens have rarely been studied (75). Alveolar size and data on normal lung growth and development may be gleaned from DW HP gas MRI. Alterations in the normal lung growth and development caused by prematurity and childhood lung diseases may therefore be amenable to study with MR imaging.
A study of children with history of preterm birth and BPD and of corresponding normal children detected alterations of lung structure in children with BPD as shown by DW HP helium-3 MR (76). Elevated ADC values (Fig.10), suggestive of enlarged alveoli, occurred in children with a history of BPD who had the same lung volumes as age-matched healthy control children. Although limited, histological data support the observation that children with BPD have larger but fewer alveoli than healthy children. The elucidation of the disease process of BPD and its pulmonary consequences require more study for optimal treatment (65), and HP gas MRI may be a critical tool.
Fig. 10. Diffusion weighted HP helium-3 MRI: Detection of alveolar enlargement in Bronchopulmonary Displasia (BPD).
Coronal HP helium-3 ADC map in 10-year-old healthy child (a) and 12-old patient with BPD (c). ADC maps show diffusely elevated values in the BPD patient (b) compared to the healthy child (a) (0.33 cm2/s vs 0.15 cm2/s respectively).
Congenital Diaphragmatic Hernia
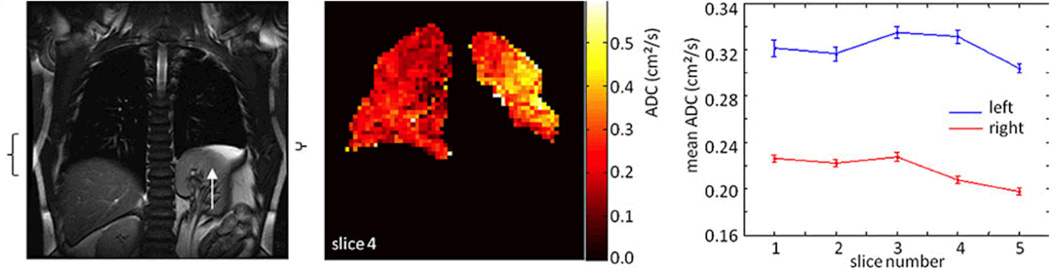
Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm with associated pulmonary hypoplasia and abnormal pulmonary vasculature. A recent study used HP helium-3 DW MRI to evaluate 9 young adults with a history of repaired congenital diaphragmatic hernia (CDH) and noted higher ADC values for the ipsilateral lung than the contralateral lung in 8 of 9 subjects (Fig. 11) (77). Ventilation defects and smaller ventilation volume was also found in the ipsilateral lung in six patients. Enlarged alveolar dimension and decreased alveolar number in the ipsilateral lung support post-mortem findings of larger and fewer alveoli in both lungs with the ipsilateral side affected more than the contralateral in young children after repair of CDH (78,89), suggesting an arrest in normal alveolar development.
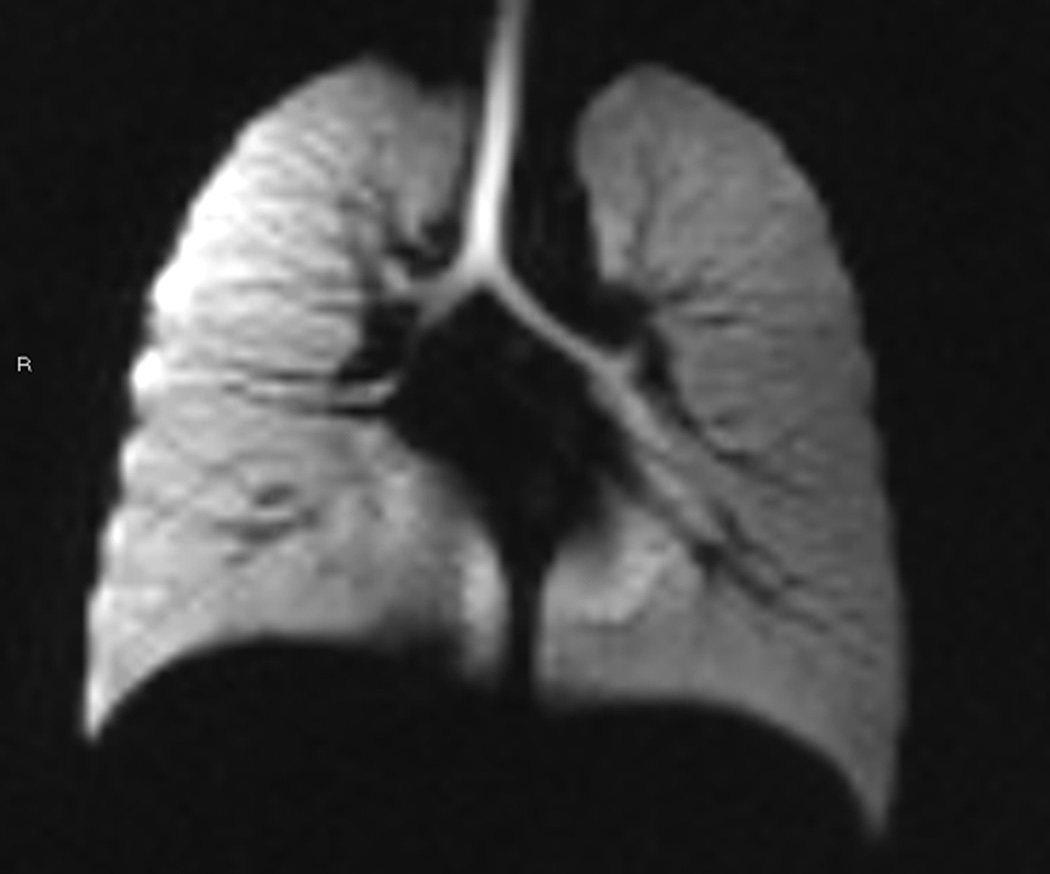
Fig. 11. Diffusion weighted HP helium-3 MRI in congenital diaphragmatic hernia.
Coronal proton MR image (a) and HP helium-3 ADC map (b) in a patient with left-sided congenital diaphragmatic hernia. Left diaphragmatic elevation (white arrow) is seen on the proton images. Black brackets on the sides of the proton image (a) show the extent of diaphragm movement ipsilateral and contralateral measured from the proton dynamic images(not shown). HP helium-3 ADC map (b) shows elevated values in the left lung when compared to the right lung. In the graph, ADC values of the ipsilateral lung (blue) and contralateral lung (red) are plotted for all slices, error bars show the standard deviation of the mean. (Adapted with permission from: Spoel M, Marshall H, IJsselstijn H, et al. Pulmonary ventilation and micro-structural findings in congenital diaphragmatic hernia. Pediatr Pulmonol. 2015 Oct 9. doi: 10.1002/ppul.23325 [Epub ahead of print])
Current limitations and future directions
HP gas MRI has opened a new window into pulmonary imaging. It has demonstrated to be a valuable research tool that provides meaningful functional and morphological information of a full range of lung diseases providing a new insight in our understanding of their pathophysiology. Nonetheless, further technological development is still required to turn this promising imaging technique into an established clinical tool.
Most of the initial human research with HP gas MRI has been performed using helium-3. Xenon-129 could become widely used due ongoing advances in xenon polarization which permit higher polarization rates and recent contraction in helium-3 supply leading to increasing costs of the gas. The excellent safety and tolerability of xenon-129 has been proven in adult subjects (10).
Continuous research and development of novel therapies with the potential of cure or delay the progression of pediatric respiratory diseases make obvious the need for a non-invasive method to monitor the start and the progression of early lung damage in infants and young children as well as to closely track and quantify early changes after treatment to assess the effectiveness. The lack of ionizing radiation and the capability to perform free-breathing imaging make HP Gas MRI the ideal imaging method for these purposes. HP gas MRI-based endpoints or scores have the potential to become surrogate markers of disease, ideally using quantitative segmentation methods but further validation is needed.
Conclusion
HP gas MRI has two main advantages over CT: lack of ionizing radiation and the ability to directly provide functional information of the lung. Its imaging capabilities have already uncovered new information about pediatric lung disease. HP gas MR found ventilation defects in patients with asthma and CF even when patients had normal PFTs. A good correlation exists between HP gas MRI findings, and structural CT abnormalities and spirometry, and HP gas MR has detected changes after treatment and persistence over time. The technique also demonstrated the heterogeneity of asthma which may have important implications for regional treatments. Early results in BPD and congenital diaphragmatic hernia suggest that HP DW MRI can detect abnormal alveolar development. For all these reasons, HP gas MRI can be a sensitive test for assessing early pulmonary disease and may become an excellent biomarker. Despite the current limited availability of the technique, if clinical applications for HP gas MR increase, regulatory and adoptive barriers will likely decline.
Acknowledgments
HP Gas MRI in Pediatric Respiratory Diseases
Funding for this work was provided by NIH (R01-HL66479, 1R43 HL117337-01, 1R43 HL108452-01A1), The Hartwell Foundation, and Vertex Pharmaceuticals Incorporated. General research support was provided by Siemens Healthcare.
References
- 1.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(2):121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flors L, Altes TA, Mugler JP3, et al. New insights into lung diseases using hyperpolarized gas MRI. Radiologia. 2015;57(4):303–313. doi: 10.1016/j.rx.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Altes TA, Powers PL, Knight-Scott J, et al. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging. 2001;13:378–384. doi: 10.1002/jmri.1054. [DOI] [PubMed] [Google Scholar]
- 4.de Lange EE, Altes TA, Patrie JT, et al. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest. 2006;130:1055–1062. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 5.Gast KK, Viallon M, Eberle B, et al. MRI in lung transplant recipients using hyperpolarized 3He: comparison with CT. J Magn Reson Imaging. 2002;15:268–274. doi: 10.1002/jmri.10070. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly LF, MacFall JR, McAdams HP, et al. Cystic fibrosis: combined hyperpolarized 3He-enhanced and conventional proton MR imaging in the lung--preliminary observations. Radiology. 1999;212:885–889. doi: 10.1148/radiology.212.3.r99se20885. [DOI] [PubMed] [Google Scholar]
- 7.Fichele S, Woodhouse N, Swift AJ, et al. MRI of helium-3 gas in healthy lungs: posture related variations of alveolar size. J Magn Reson Imaging. 2004;20:331–335. doi: 10.1002/jmri.20104. [DOI] [PubMed] [Google Scholar]
- 8.Ruppert K, Brookeman JR, Hagspiel KD, Mugler JP., 3rd Probing lung physiology with xenon polarization transfer contrast (XTC) Magn Reson Med. 2000;44:349–357. doi: 10.1002/1522-2594(200009)44:3<349::aid-mrm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A. 2006;103:18278–18283. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patz S, Muradian I, Hrovat MI, et al. Human pulmonary imaging and spectroscopy with hyperpolarized 129Xe at 0.2T. Acad Radiol. 2008;15:713–727. doi: 10.1016/j.acra.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driehuys B, Martinez-Jimenez S, Cleveland ZI, et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology. 2012;262:279–289. doi: 10.1148/radiol.11102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushik SS, Cleveland ZI, Cofer GP, et al. Diffusion-weighted hyperpolarized (129)Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med. 2010 doi: 10.1002/mrm.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleveland ZI, Cofer GP, Metz G, et al. Hyperpolarized Xe MR imaging of alveolar gas uptake in humans. PLoS One. 2010;5:e12192. doi: 10.1371/journal.pone.0012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tustison NJ, Avants BB, Flors L, et al. Ventilation-based segmentation of the lungs using hyperpolarized (3)He MRI. J Magn Reson Imaging. 2011;34:831–841. doi: 10.1002/jmri.22738. [DOI] [PubMed] [Google Scholar]
- 15.He M, Kaushik SS, Robertson SH, et al. Extending semiautomatic ventilation defect analysis for hyperpolarized (129)Xe ventilation MRI. Acad Radiol. 2014;21:1530–1541. doi: 10.1016/j.acra.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lange EE. Science to practice: what is new about detecting emphysema? Radiology. 2006;239:619–620. doi: 10.1148/radiol.2393060147. [DOI] [PubMed] [Google Scholar]
- 17.Mata JF, Altes TA, Cai J, et al. Evaluation of emphysema severity and progression in a rabbit model: comparison of hyperpolarized 3He and 129Xe diffusion MRI with lung morphometry. J Appl Physiol. 2007;102:1273–1280. doi: 10.1152/japplphysiol.00418.2006. [DOI] [PubMed] [Google Scholar]
- 18.Fain SB, Altes TA, Panth SR, et al. Detection of age-dependent changes in healthy adult lungs with diffusion-weighted 3He MRI. Acad Radiol. 2005;12:1385–1393. doi: 10.1016/j.acra.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Gierada DS, Woods JC, Jacob RE, et al. Emphysema quantification in inflation-fixed lungs using low-dose computed tomography and 3He magnetic resonance imaging. J Comput Assist Tomogr. 2010;34:773–779. doi: 10.1097/RCT.0b013e3181e480f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen XJ, Moller HE, Chawla MS, et al. Spatially resolved measurements of hyperpolarized gas properties in the lung in vivo. Part I: diffusion coefficient. Magn Reson Med. 1999;42:721–728. doi: 10.1002/(sici)1522-2594(199910)42:4<721::aid-mrm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Saam BT, Yablonskiy DA, Kodibagkar VD, et al. MR imaging of diffusion of (3)He gas in healthy and diseased lungs. Magn Reson Med. 2000;44:174–179. doi: 10.1002/1522-2594(200008)44:2<174::aid-mrm2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Yablonskiy DA, Sukstanskii AL, Leawoods JC, et al. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci U S A. 2002;99:3111–3116. doi: 10.1073/pnas.052594699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peces-Barba G, Ruiz-Cabello J, Cremillieux Y, et al. Helium-3 MRI diffusion coefficient: correlation to morphometry in a model of mild emphysema. Eur Respir J. 2003;22:14–19. doi: 10.1183/09031936.03.00084402. [DOI] [PubMed] [Google Scholar]
- 24.Chen XJ, Hedlund LW, Moller HE, Chawla MS, Maronpot RR, Johnson GA. Detection of emphysema in rat lungs by using magnetic resonance measurements of 3He diffusion. Proc Natl Acad Sci U S A. 2000;97:11478–11481. doi: 10.1073/pnas.97.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods JC, Choong CK, Yablonskiy DA, et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med. 2006;56:1293–1300. doi: 10.1002/mrm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., 3rd Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes--initial experience. Radiology. 2002;222:252–260. doi: 10.1148/radiol.2221001834. [DOI] [PubMed] [Google Scholar]
- 27.Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006;239:875–883. doi: 10.1148/radiol.2393050111. [DOI] [PubMed] [Google Scholar]
- 28.Mugler JP, 3rd, Wang C, Miller GW, et al. Helium-3 diffusion MR imaging of the human lung over multiple time scales. Acad Radiol. 2008;15:693–701. doi: 10.1016/j.acra.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanoli TS, Woods JC, Conradi MS, et al. In vivo lung morphometry with hyperpolarized 3He diffusion MRI in canines with induced emphysema: disease progression and comparison with computed tomography. J Appl Physiol. 2007;102:477–484. doi: 10.1152/japplphysiol.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deninger AJ, Eberle B, Ebert M, et al. (3)he-MRI-based measurements of intrapulmonary p(O2) and its time course during apnea in healthy volunteers: first results, reproducibility, and technical limitations. NMR Biomed. 2000;13:194–201. doi: 10.1002/1099-1492(200006)13:4<194::aid-nbm643>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Deninger AJ, Eberle B, Bermuth J, et al. Assessment of a single-acquisition imaging sequence for oxygen-sensitive (3)He-MRI. Magn Reson Med. 2002;47:105–114. doi: 10.1002/mrm.10032. [DOI] [PubMed] [Google Scholar]
- 32.Dregely I, Mugler JP, 3rd, Ruset IC, et al. Hyperpolarized Xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J Magn Reson Imaging. 2011;33:1052–1062. doi: 10.1002/jmri.22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patz S, Hersman FW, Muradian I, et al. Hyperpolarized (129)Xe MRI: a viable functional lung imaging modality? Eur J Radiol. 2007;64:335–344. doi: 10.1016/j.ejrad.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirby M, Parraga G. Pulmonary functional imaging using hyperpolarized noble gas MRI: six years of start-up experience at a single site. Acad Radiol. 2013;20:1344–1356. doi: 10.1016/j.acra.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Lutey BA, Lefrak SS, Woods JC, et al. Hyperpolarized 3He MR imaging: physiologic monitoring observations and safety considerations in 100 consecutive subjects. Radiology. 2008;248:655–661. doi: 10.1148/radiol.2482071838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lange E, Altes T, Harding D, Harrell F, Mata J, Wright C. Hyperpolarized gas MR imaging of the lung: safety assessment of inhaled helium-3; Chicago, IL. Presented at: Radiological Society of North America (RSNA).2003. [Google Scholar]
- 37.Shukla Y, Wheatley A, Kirby M, et al. Hyperpolarized 129Xe magnetic resonance imaging: tolerability in healthy volunteers and subjects with pulmonary disease. Acad Radiol. 2012;19:941–951. doi: 10.1016/j.acra.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Svenningsen S, Guo F, Kirby M, et al. Pulmonary functional magnetic resonance imaging: asthma temporal-spatial maps. Acad Radiol. 2014;21:1402–1410. doi: 10.1016/j.acra.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Altes TA, Powers PL, Knight-Scott J, et al. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging. 2001;13:378–384. doi: 10.1002/jmri.1054. [DOI] [PubMed] [Google Scholar]
- 40.Altes TA, Mugler JP, 3rd, Ruppert K, et al. Clinical correlates of lung ventilation defects in asthmatic children. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.08.045. S0091-6749(15)01335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cadman RV, Lemanske RF, Jr, Evans MD, et al. Pulmonary 3He magnetic resonance imaging of childhood asthma. J Allergy Clin Immunol. 2013;131:369–376. doi: 10.1016/j.jaci.2012.10.032. e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Altes TA, Mugler JP, 3rd, et al. Assessment of the lung microstructure in patients with asthma using hyperpolarized 3He diffusion MRI at two time scales: comparison with healthy subjects and patients with COPD. J Magn Reson Imaging. 2008;28:80–88. doi: 10.1002/jmri.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samee S, Altes T, Powers P, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003;111:1205–1211. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 45.de Lange EE, Altes TA, Patrie JT, et al. The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol. 2007;119:1072–1078. doi: 10.1016/j.jaci.2006.12.659. [DOI] [PubMed] [Google Scholar]
- 46.de Lange EE, Altes TA, Patrie JT, et al. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009;250:567–575. doi: 10.1148/radiol.2502080188. [DOI] [PubMed] [Google Scholar]
- 47.Thomen RP, Sheshadri A, Quirk JD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274:250–259. doi: 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes JH, O’Halloran RL, Brodsky EK, Jung Y, Block WF, Fain SB. 3D hyperpolarized He-3 MRI of ventilation using a multi-echo projection acquisition. Magn Reson Med. 2008;59:1062–1071. doi: 10.1002/mrm.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wielputz MO, Eichinger M, Puderbach M. Magnetic resonance imaging of cystic fibrosis lung disease. J Thorac Imaging. 2013;28:151–159. doi: 10.1097/RTI.0b013e31828d40d4. [DOI] [PubMed] [Google Scholar]
- 50.Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol. 2005;12:1423–1429. doi: 10.1016/j.acra.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Puderbach M, Eichinger M, Gahr J, et al. Proton MRI appearance of cystic fibrosis: comparison to CT. Eur Radiol. 2007;17:716–724. doi: 10.1007/s00330-006-0373-4. [DOI] [PubMed] [Google Scholar]
- 52.Eichinger M, Optazaite DE, Kopp-Schneider A, et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol. 2012;81:1321–1329. doi: 10.1016/j.ejrad.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 53.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70:1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Beek EJ, Hill C, Woodhouse N, et al. Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-Helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol. 2007;17:1018–1024. doi: 10.1007/s00330-006-0392-1. [DOI] [PubMed] [Google Scholar]
- 55.Bannier E, Cieslar K, Mosbah K, et al. Hyperpolarized 3He MR for sensitive imaging of ventilation function and treatment efficiency in young cystic fibrosis patients with normal lung function. Radiology. 2010;255:225–232. doi: 10.1148/radiol.09090039. [DOI] [PubMed] [Google Scholar]
- 56.McMahon CJ, Dodd JD, Hill C, et al. Hyperpolarized 3helium magnetic resonance ventilation imaging of the lung in cystic fibrosis: comparison with high resolution CT and spirometry. Eur Radiol. 2006;16:2483–2490. doi: 10.1007/s00330-006-0311-5. [DOI] [PubMed] [Google Scholar]
- 57.Woodhouse N, Wild JM, van Beek EJ, Hoggard N, Barker N, Taylor CJ. Assessment of hyperpolarized 3He lung MRI for regional evaluation of interventional therapy: a pilot study in pediatric cystic fibrosis. J Magn Reson Imaging. 2009;30:981–988. doi: 10.1002/jmri.21949. [DOI] [PubMed] [Google Scholar]
- 58.O’Sullivan B, Couch M, Roche JP, et al. Assessment of repeatability of hyperpolarized gas MR ventilation functional imaging in cystic fibrosis. Acad Radiol. 2014;21:1524–1529. doi: 10.1016/j.acra.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Davis P, Yasothan U, Kirkpatrick P. Ivacaftor. Nat Rev Drug DiscoV. 2012;11:349–50. doi: 10.1038/nrd3723. [DOI] [PubMed] [Google Scholar]
- 60.Altes T, Johnson M, Mugler JP3, et al. Hyperpolarized helium-3 MRI detects the effects of a CFTR potentiatior (Ivacaftor) therapy in subjects with cystic fibrosis and the G551D mutation; Melburne, Australia. Preceedings of the 20th Annual Meeting of the ISMRM.2012. [Google Scholar]
- 61.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thurlbeck WM, Angus GE. Growth and aging of the normal human lung. Chest. 1975;67:3S–6S. doi: 10.1378/chest.67.2_supplement.3s. [DOI] [PubMed] [Google Scholar]
- 63.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev. 1986;13:1–11. doi: 10.1016/0378-3782(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 64.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 65.Hayes D, Jr, Feola DJ, Murphy BS, Shook LA, Ballard HO. Pathogenesis of bronchopulmonary dysplasia. Respiration. 2010;79:425–436. doi: 10.1159/000242497. [DOI] [PubMed] [Google Scholar]
- 66.Hislop AA. Airway and blood vessel interaction during lung development. J Anat. 2002;201:325–334. doi: 10.1046/j.1469-7580.2002.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunnill M. Postnatal growth of the lung. Thorax. 1962;17:329–333. [Google Scholar]
- 68.Hyde DM, Blozis SA, Avdalovic MV, et al. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2007;293:L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- 69.Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest. 1994;94:405–412. doi: 10.1172/JCI117337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kovar J, Sly PD, Willet KE. Postnatal alveolar development of the rabbit. J Appl Physiol (1985) 2002;93:629–635. doi: 10.1152/japplphysiol.01044.2001. [DOI] [PubMed] [Google Scholar]
- 71.Altes TA, Mata J, de Lange EE, Brookeman JR, Mugler JP., 3rd Assessment of lung development using hyperpolarized helium-3 diffusion MR imaging. J Magn Reson Imaging. 2006;24:1277–1283. doi: 10.1002/jmri.20723. [DOI] [PubMed] [Google Scholar]
- 72.Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 74.Cheu HW, Lally KP, Clark R, Harrell S, Null D. Open lung biopsy in the critically ill newborn. Pediatrics. 1990;86:561–563. [PubMed] [Google Scholar]
- 75.Jobe AH. An unknown: lung growth and development after very preterm birth. Am J Respir Crit Care Med. 2002;166:1529–1530. doi: 10.1164/rccm.2209012. [DOI] [PubMed] [Google Scholar]
- 76.Altes T, Mata J, Froh D, Paget-Brown A, de Lange E, Mugler J. Abnormalities of lung structure in children with bronchopulmonary dysplasia as assessed by diffusion hyperpolarized helium-3 MRI; Seattle, WA. Presented at: International Society of Magnetic Resonance in Medicine (ISMRM).2006. [Google Scholar]
- 77.Spoel M, Marshall H, IJsselstijn H, et al. Pulmonary ventilation and micro-structural findings in congenital diaphragmatic hernia. Pediatr Pulmonol. 2015 doi: 10.1002/ppul.23325. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 78.Thurlbeck WM, Kida K, Langston C, et al. Postnatal lung growth after repair of diaphragmatic hernia. Thorax. 1979;34:338–343. doi: 10.1136/thx.34.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hislop A, Reid L. Persistent hypoplasia of the lung after repair of congenital diaphragmatic hernia. Thorax. 1976;31:450–455. doi: 10.1136/thx.31.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]