Abstract
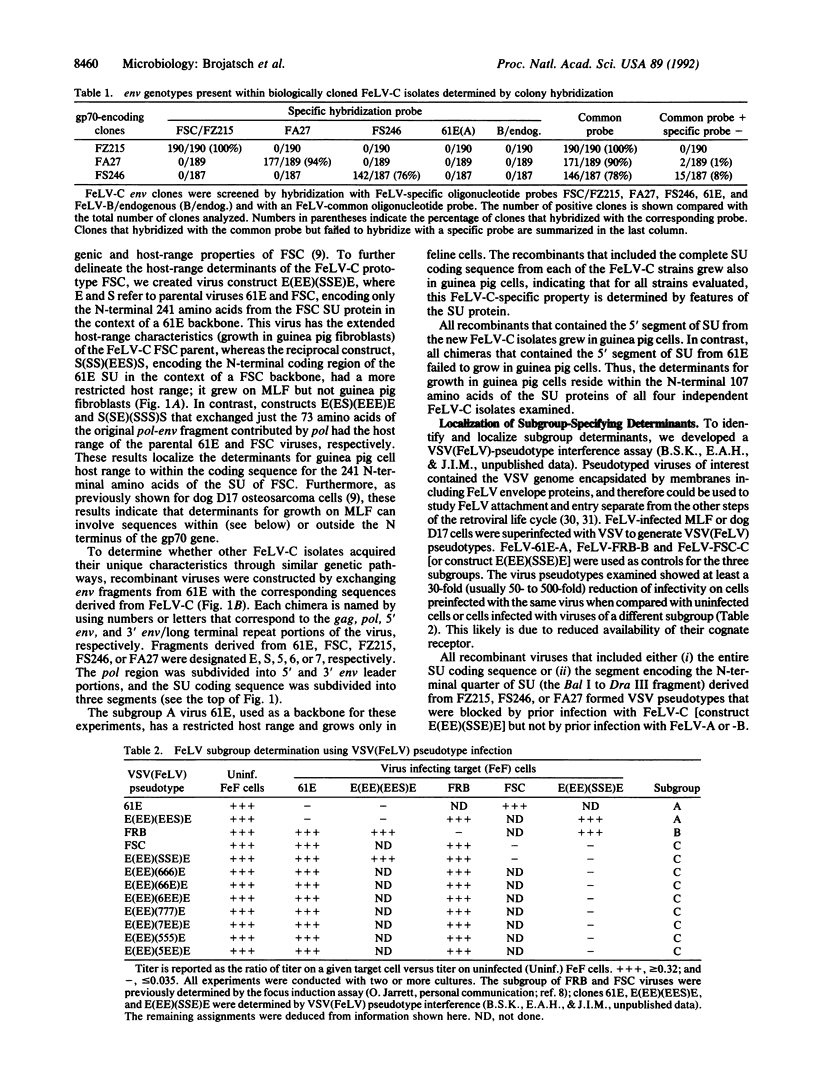
Feline leukemia viruses (FeLVs) belonging to the C subgroup induce aplastic anemia in domestic cats and have the ability, unique among FeLV strains, to proliferate in guinea pig fibroblasts in tissue culture. Previous studies have shown that the pathogenic and host range specificity of a prototype molecular clone of FeLV-C [FeLV-Sarma-C (FSC)] colocalize to a region encoding the 3' 73 amino acids of the pol gene product and the N-terminal 241 amino acids of the envelope surface glycoprotein named SU. Here, we amplified, via PCR, cloned, and sequenced the SU coding sequence from three additional anemia-inducing subgroup C FeLV isolates. Chimeric viruses were constructed by replacement of fragments of FeLV-C envelope genes into the FeLV-A prototype virus 61E. Using a modified vesicular stomatitis virus-FeLV pseudotype assay, we demonstrated that the subgroup C receptor specificity for each virus was determined by changes within the N-terminal 87-92 amino acids of SU, in which most changes occurred within the 15- to 20-amino-acid first variable region (V1). Determinants for growth in guinea pig cells colocalized to this region. Despite the consistent localization of biological determinants, the only consistent features that distinguished the deduced FeLV-A and FeLV-C proteins was one lysine-to-arginine change and a structural prediction of an alpha-helix in FeLV-A proteins versus random coil in FeLV-C proteins within V1. However, arginine in equilibrium with lysine substitutions were not sufficient to convert the subgroup A virus to the subgroup C phenotype or vice versa. Thus, certain distinct structural changes within the N-terminal region of FeLV SU can result in convergent viral phenotypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Holly R. D., Grant C. K. Retrovirus-induced feline pure red cell aplasia. Hematopoietic progenitors are infected with feline leukemia virus and erythroid burst-forming cells are uniquely sensitive to heterologous complement. J Clin Invest. 1987 Oct;80(4):1056–1063. doi: 10.1172/JCI113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altstein A. D., Zhdanov V. M., Omelchenko T. N., Dzagurov S. G., Miller G. G., Zavada J. Phenotypic mixing of vesicular stomatitis virus and D-type oncornavirus. Int J Cancer. 1976 Jun 15;17(6):780–784. doi: 10.1002/ijc.2910170614. [DOI] [PubMed] [Google Scholar]
- Bova-Hill C., Olsen J. C., Swanstrom R. Genetic analysis of the Rous sarcoma virus subgroup D env gene: mammal tropism correlates with temperature sensitivity of gp85. J Virol. 1991 Apr;65(4):2073–2080. doi: 10.1128/jvi.65.4.2073-2080.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Quackenbush S. L., Gallo M. V., deNoronha C. M., Overbaugh J., Hoover E. A., Mullins J. I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1991 Aug;65(8):4461–4469. doi: 10.1128/jvi.65.8.4461-4469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Stoye J. P., Coffin J. M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985 Jan;53(1):32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornsife R. E., Gasper P. W., Mullins J. I., Hoover E. A. Induction of aplastic anemia by intra-bone marrow inoculation of a molecularly cloned feline retrovirus. Leuk Res. 1989;13(9):745–755. doi: 10.1016/0145-2126(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Bachmann M. H., Hoover E. A., Mullins J. I. Identification of a putative receptor for subgroup A feline leukemia virus on feline T cells. J Virol. 1992 Jun;66(6):3707–3714. doi: 10.1128/jvi.66.6.3707-3714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Kociba G. J., Hardy W. D., Jr, Yohn D. S. Erythroid hypoplasia in cats inoculated with feline leukemia virus. J Natl Cancer Inst. 1974 Nov;53(5):1271–1276. doi: 10.1093/jnci/53.5.1271. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Olsen R. G., Hardy W. D., Jr, Schaller J. P., Mathes L. E. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Natl Cancer Inst. 1976 Aug;57(2):365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Russell P. H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int J Cancer. 1978 Apr 15;21(4):466–472. doi: 10.1002/ijc.2910210411. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Mackey L., Jarrett W., Jarrett O., Laird H. Anemia associated with feline leukemia virus infection in cats. J Natl Cancer Inst. 1975 Jan;54(1):209–217. doi: 10.1093/jnci/54.1.209. [DOI] [PubMed] [Google Scholar]
- Onions D., Jarrett O., Testa N., Frassoni F., Toth S. Selective effect of feline leukaemia virus on early erythroid precursors. Nature. 1982 Mar 11;296(5853):156–158. doi: 10.1038/296156a0. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak V. K., Temin H. M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: substitutions, frameshifts, and hypermutations. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6019–6023. doi: 10.1073/pnas.87.16.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush S. L., Donahue P. R., Dean G. A., Myles M. H., Ackley C. D., Cooper M. D., Mullins J. I., Hoover E. A. Lymphocyte subset alterations and viral determinants of immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1990 Nov;64(11):5465–5474. doi: 10.1128/jvi.64.11.5465-5474.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Dornsife R. E., Mullins J. I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Jain D., Hill P. R., Huebner R. J. Differential host range of viruses of feline leukemia-sarcoma complex. Virology. 1975 Apr;64(2):438–446. doi: 10.1016/0042-6822(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Weiss R. A., Zavada J. Pseudotypes of vesicular stomatitis virus with the envelope properties of mammalian and primate retroviruses. J Virol. 1977 Sep;23(3):449–454. doi: 10.1128/jvi.23.3.449-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Neckameyer W. S., Hayward W. S., Smith R. E. Genetic determinants of neoplastic diseases induced by a subgroup F avian leukosis virus. J Virol. 1987 Apr;61(4):1203–1212. doi: 10.1128/jvi.61.4.1203-1212.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S. HIV genome variability in vivo. AIDS. 1989;3 (Suppl 1):S13–S18. doi: 10.1097/00002030-198901001-00003. [DOI] [PubMed] [Google Scholar]



