Abstract
Background: Remote-access techniques have been described over the recent years as a method of removing the thyroid gland without an incision in the neck. However, there is confusion related to the number of techniques available and the ideal patient selection criteria for a given technique. The aims of this review were to develop a simple classification of these approaches, describe the optimal patient selection criteria, evaluate the outcomes objectively, and define the barriers to adoption.
Methods: A review of the literature was performed to identify the described techniques. A simple classification was developed. Technical details, outcomes, and the learning curve were described. Expert opinion consensus was formulated regarding recommendations for patient selection and performance of remote-access thyroid surgery.
Results: Remote-access thyroid procedures can be categorized into endoscopic or robotic breast, bilateral axillo-breast, axillary, and facelift approaches. The experience in the United States involves the latter two techniques. The limited data in the literature suggest long operative times, a steep learning curve, and higher costs with remote-access thyroid surgery compared with conventional thyroidectomy. Nevertheless, a consensus was reached that, in appropriate hands, it can be a viable option for patients with unilateral small nodules who wish to avoid a neck incision.
Conclusions: Remote-access thyroidectomy has a role in a small group of patients who fit strict selection criteria. These approaches require an additional level of expertise, and therefore should be done by surgeons performing a high volume of thyroid and robotic surgery.
Introduction
Over the last two decades, techniques developed for remote-access thyroid surgery have been cosmetically appealing to some patients. However, they have been approached with caution by the medical community because of technical challenges, new complications introduced, concerns about oncologic equivalency, and medicolegal and cost issues. This report is a review and appraisal of these evolving techniques.
Remote-Access Techniques
A number of different access options have been developed, many of which are variations on a central anatomic approach. Based on expert opinion, this review focuses on the four techniques most commonly utilized in 2015 while acknowledging that this is not an exhaustive list.
Endoscopic Breast Approach
This approach, developed by Ohgami et al. (1) in 2000, involves two circumareolar incisions measuring 12–15 mm. After an injection of a diluted adrenalin solution into the subcutaneous space, subcutaneous and subplatysmal dissections are performed. Another 5-mm port is inserted about 3 cm below the clavicle on the side of the pathology. Carbon dioxide (CO2) insufflation is established at 5–6 mm Hg. The central neck is subsequently entered, and, using an energy device, the thyroidectomy is performed. The significance of this approach is that it was the first remote-access technique developed. However, the disadvantages are the amount of dissection necessary and the CO2 insufflation, which can diffuse into remote tissue planes.
Endoscopic and Robotic Bilateral Axillo Breast Approach
The Bilateral Axillo Breast Approach (BABA), developed by Choe et al. (2) in 2007 as a modification of the axillo-bilateral breast technique described by Shimazu et al. (3), involves 8–12-mm ports placed around both areolae and axillae (Fig. 1). The subcutaneous dissection is performed as in the endoscopic breast approach. CO2 insufflation is used at 5–6 mm Hg. Thyroidectomy is performed by using various endoscopic instruments and a vessel sealer, with steps similar to the conventional neck approach. As this procedure required significant endoscopic surgical skills and the learning curve was steep, the use of a robot was subsequently adapted to this approach by Lee et al. in 2009 (4). The endoscopic camera is inserted from the left breast port and the vessel sealer from the right breast port. The axillary ports are used for a robotic grasper and dissector. This approach most closely resembles conventional thyroid surgery, as it provides a midline access to the gland. However, the extent of dissection required for exposure and the use of CO2 insufflation are disadvantages.
FIG. 1.
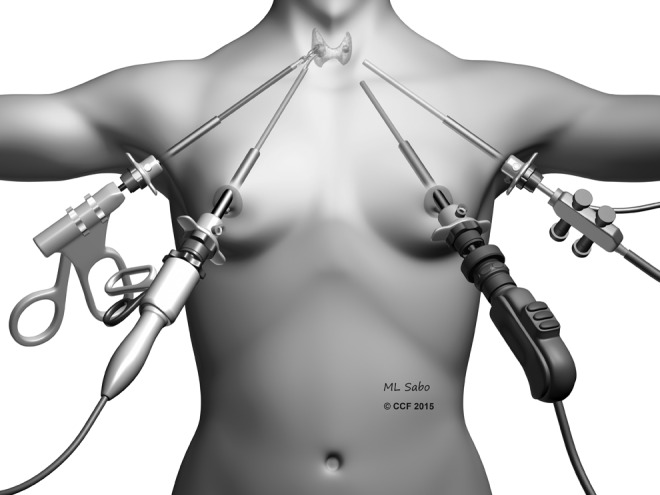
Illustration showing the bilateral axillary breast approach.
Endoscopic and Robotic Axillary Approach
The original axillary approach described by Ikeda et al. involves a 30-mm incision made in the axilla, with subsequent insertion of 12- and 5-mm trocars through this opening (5,6). The space is then insufflated to 4 mm Hg, and an additional 5-mm trocar is inserted adjacently. The central neck is entered by dissecting the sternocleidomastoid muscle (SCM) off the sternohyoid muscle. After the thyroid is exposed, thyroidectomy is performed using an endoscopic vessel sealer and a grasper. This approach was then modified by Chung et al. in 2006 by utilizing a gasless approach, and it became popular worldwide (7,8). This technique involves a 5–6-cm incision in the axilla, followed by creation of a subcutaneous flap extending to the clavicle. By identifying the sternal and clavicular heads of the SCM, the central neck is entered, and a static elevating retractor is placed. The thyroid is exposed by separating the strap muscles, and a robotic platform is introduced (Fig. 2). A robotic 30-degree down camera, vessel sealer, and a grasper are introduced through this incision. Although the original description involved another parasternal incision for a fourth trocar, this was subsequently abandoned, with the use of three or four robotic arms through this single incision. A drawback of this technique is that the removal of the contralateral lobe requires significant expertise and can be limited.
FIG. 2.
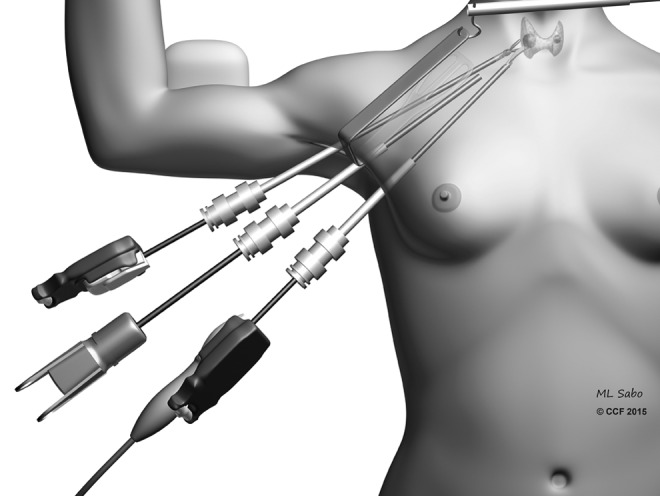
Illustration showing the setup and instrumentation for a transaxillary thyroidectomy.
There have been advocates for both unilateral (9), and bilateral axillary (10) approaches for total thyroidectomy. The advocates of a single incision propose that adequate exposure of the contralateral side is possible with the use of a 30-degree scope and rotation of the operative bed toward the endoscope, whereas proponents of the latter approach contend the complexity of the procedure necessitates bilateral axillary incisions. As there could be brachial plexus injuries with the extension of the arm, another modification in the United States has been a more conservative positioning of the ipsilateral extremity with limited extension across elbow and shoulder joints. The advantages of this approach are that it provides a direct access to the thyroid gland on the ipsilateral side, CO2 insufflation is not necessary, and exposure of the contralateral lobe is also possible. However, the creation of the axillary flap involves a learning curve, and removal of the contralateral thyroid lobe from a single incision is technically more challenging compared with ipsilateral lobectomy.
Endoscopic and Robotic Facelift Approach
The facelift approach was described by Terris et al. (11) with the goal of combining the principles of gasless surgery with a facelift incision approach to perform remote-access thyroidectomy (Fig. 3). The technique entails a facelift incision that is marked out in or adjacent to the post-auricular crease, and crossing over to the occipital hairline at a position that will be obscured by the ear. A flap is elevated over the SCM, which is retracted to reveal the omohyoid muscle. This muscle is retracted ventrally and serves as the landmark for visualizing the strap muscles that guide entry to the central neck. A modified static retractor is used to retract the flap, and a second fixed retractor is used to retract the SCM laterally. The procedure is done using a robotic 30-degree down camera, vessel sealer, and a Maryland grasper. The upper pole of the thyroid is divided first, followed by identification of the recurrent laryngeal nerve (RLN) and parathyroid glands. This procedure enables the performance of a thyroid lobectomy, with limited access to the contralateral lobe. The benefits of this approach are that the flap distance is the shortest of all the remote-access techniques, and CO2 is not used. However, the disadvantages are that the thyroid lobe is viewed from the top down, and the access to the contralateral lobe is limited.
FIG. 3.
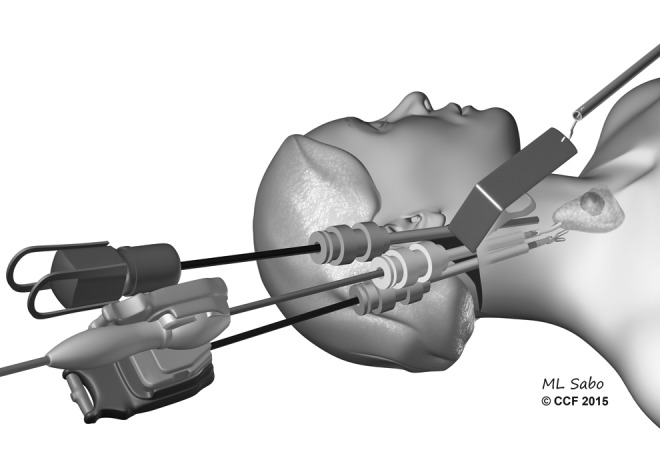
Illustration demonstrating the technique for the facelift approach for thyroidectomy.
Patient Selection
There are certain selection criteria for remote-access thyroid surgery. First, patients should be worked up based on American Thyroid Association (ATA) guidelines for the management of thyroid nodules (12). The indications for thyroidectomy should be the same as for conventional surgery. The following factors should be reviewed for patient selection. Factors relating to the patient include: (i) thin body habitus (except for the facelift approach), and (ii) the absence of excessive body fat along the flap trajectory (except for the facelift approach). Factors relating to the thyroid pathology include: (i) well-circumscribed nodule ≤3 cm, and thyroid lobe <5–6 cm in the largest dimension; and (ii) underlying thyroid pathology with no evidence of thyroiditis on ultrasound. Factors relating to specific approaches include the fact that the distance between the axilla and the sternal notch should ideally be <15–17 cm for an axillary approach. Absolute contraindications include: (i) evidence of thyroid cancer with extrathyroidal extension or lymph node involvement; (ii) Graves' disease; (iii) substernal extension; and (iv) previous neck surgery. Overall, the ideal patient is a patient with <3 cm unilateral nodule who wishes to avoid a neck scar.
Outcomes
Due to the differences in patient populations, most of the experience with remote-access thyroidectomy has been described from South Korea. In the largest study to date, Ban et al. (13) reported on 3000 patients who underwent a robotic transaxillary thyroidectomy by Dr. Chung's team. This series was comprised of thin patients (average body mass index [BMI]: 22 kg/m2) with small thyroid nodules (average 0.66 cm) across all age groups (Mage = 39 years; range 13–70 years). The majority of patients (80%) had microscopic papillary thyroid carcinomas on final pathology, while 19% had papillary thyroid cancer. The operative time for a total thyroidectomy was 141 minutes, compared with 115 minutes for less than a total thyroidectomy. Complications were similar to those seen with conventional thyroidectomy, including transient symptomatic hypocalcemia (37%), permanent hypocalcemia (1%), transient recurrent laryngeal injury (1.2%), permanent recurrent laryngeal injury (0.3%), seroma (1.7%), and hematoma (0.4%), as well as additional complications rarely if ever seen with conventional thyroidectomy, including chyle leakage (0.4%), tracheal injury (0.2%), Horner's syndrome (0.03%), carotid artery injury (0.03%), brachiocephalic vein injury (0.03%), traction injury (0.1%), and axillary flap perforation (0.1%). The largest size of the tumor removed robotically in Dr. Chung's series is reported to be 6 cm (8).
In the largest experience with the BABA approach, Lee et al. (14) reported on 1026 patients operated on robotically. The patients had similar characteristics compared to the series by Kang et al. (8), with a mean age of 40 years (range 13–70 years), small nodules (0.8 cm), and mostly papillary microcarcinomas on final pathology (81%). Central neck dissection was performed along with total thyroidectomy in 872 patients, and in this study, bleeding was observed in four patients, pneumothorax in one patient, transient hypoparathyroidism in 39%, permanent hypoparathyroidism in 1.5%, transient vocal cord palsy in 14.2%, and permanent vocal cord palsy in 0.2%. The variety of complications seen even in very experienced hands and in operations for early disease suggest that these procedures should be done in selected centers under strict protocols by very experienced surgical teams.
Despite the popularity of robotic thyroidectomy in South Korea, the acceptance in the United States has been slower because of differences in patient population, practice patterns, and patient interest. An initial report on 31 patients who underwent robotic transaxillary thyroid surgery by Kuppersmith and Holsinger (15) underscores these differences, with an average BMI of 25 kg/m2 (range 18–34 kg/m2) and nodule size of 2.7 cm (range 1.0–4.6 cm). With two-thirds of the patients undergoing lobectomy and the rest total thyroidectomy, the intraoperative data highlight the learning curve involved, with a mean total surgical time for total thyroidectomies of 278 minutes in the first part of the series versus 168 minutes in the later part. One patient had neuropraxia, another temporary vocal cord paresis, and two patients developed intraoperative hemorrhage (>500 cc) related to anterior jugular injuries.
The largest series of robotic transaxillary thyroidectomy in the United States reported to date was by Kandil et al. (16). In this study, the patient characteristics reflected the nature of the disease in the United States, with a BMI of 28.5 kg/m2 (range 16–55 kg/m2), and an average nodule size of 2.4 cm. Seventy percent of the procedures were hemithyroidectomies, with a 24% complication rate, including seroma (4%), wound infection (1%), tracheal injury (1%), transient hoarseness (7%), transient hypocalcemia (10%), and neuropraxia (1%). The operative time for a thyroid lobectomy was 108 minutes versus 118 minutes for a total thyroidectomy.
In another report from the United States, a single-incision three-arm technique for transaxillary total thyroidectomy was described in 16 patients (17). With a similar patient demographic and clinical profile (Table 1), there were no nerve injuries while asymptomatic hypocalcemia was observed in two patients and hematoma in one.
Table 1.
A Summary of Results from Major U.S. Remote-Access Thyroidectomy Studies
| Author (year) | Number of procedures | Approach | Pathology (n) | Tumor size (cm), M (range) | Operative time (minutes), M ± SD or range | Complications, n (%) | Conversion to open, n (%) | Hospital stay (days), median |
|---|---|---|---|---|---|---|---|---|
| Kandil et al. (2012) (16) | 100 | TAA | — | — | 108.1 ± 60.5 | 1 (1) | 2 (2) | 1 |
| Kuppersmith and Holsinger (2011) (15) | 31 | TAA | PTC (3), benign (28) | 2.7 (1.0–4.6) | 132–328 | 4 (13) | 0 (0) | 1 |
| Aliyev et al. (2012) (17) | 16 | TAA | PTC (10), FTC (1), benign (5) | 1.8 | 183 ± 11 | 3 (19) | 0 (0) | 1 |
| Landry et al. (2011) (10) | 12 | TAA | PTC (1), FTC (1), benign (9) | 2.1 (0.8–2.8) | 142a | 4 (30) | 0 (0) | 1 |
| Terris et al. (2011) (18) | 18 | Facelift | PTC (2), FTC (1), benign (15) | — | 154.9 ± 23.8 | 2 (11) | 0 (0) | 0 |
| Kandil et al. (2014) (19) | 12 | Facelift | PTC (1), FTC (2), benign (9) | 1.2 | 156 ± 15.8 | 0 (0) | 0 (0) | 1 |
All of these series have utilized a robotic approach.
Further details of the operative time were not given in this study.
TAA, transaxillary approach; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; SD, standard deviation.
The experience with robotic facelift thyroidectomy has been more limited in the United States. In the largest experience, Terris et al. (18) described 18 procedures in 14 patients. The mean BMI was 27 kg/m2, and operative time was 155 minutes; all but the first case were completed on a drainless, outpatient basis. There were two seromas and one instance of transient vocal fold dysfunction. Kandil et al. (19) subsequently reported on 12 patients who underwent a retroauricular robotic hemithyroidectomy. The operative time was 156 minutes, with nine (75%) patients developing minor complications, including seroma (n = 2), hypoesthesia (n = 3), transient hypocalcemia (n = 3), and transient hoarseness (n = 1).
A recent national review of 68,393 patients with thyroid cancer who had a thyroidectomy between 2010 and 2011 summarizes the status of remote-access thyroid surgery in the United States. In this study, 225 patients were found to have undergone robotic and the rest conventional thyroid surgery. Robotic cases were reported from 93 centers, with 89 centers reporting fewer than 10 cases. Compared to the open group, the robotic group was younger (51 vs. 47 years) and included more Asian and privately insured patients. Patients were more likely to undergo robotic surgery if they were female or underwent lobectomy. On multivariate analysis, there were no differences in the number of lymph nodes removed between the groups in operations for cancer (20).
The paucity of experience from the United States underscores the most challenging nature of the disease and patient characteristics compared with South Korea. The expertise and data are very scant, but still draw attention to the unusual complications that may arise with these remote-access procedures, which are also associated with much longer operative times, especially taking into consideration that these patients have less complicated disease compared with most patients undergoing conventional thyroidectomy.
Comparison with Conventional Thyroidectomy
In their recent meta-analysis of 2375 patients from 11 studies, Lang et al. reported longer operative times (mean difference = 56 minutes), hospital stay, and increased transient RLN injury rates in patients undergoing robotic thyroidectomy compared with those who underwent conventional transcervical thyroidectomy (21). There was no difference between the two procedures regarding blood loss, hypocalcemia, and overall morbidity.
In a study by Lee et al. (22), cosmetic satisfaction at three months postoperatively was assessed using a five-point scale (extremely satisfied, satisfied, acceptable, dissatisfied, or extremely dissatisfied). The patients in the robotic group reported significantly greater satisfaction than those in the open group (p < 0.0001). In fact, 24 (58.5%) patients in the robotic group were extremely satisfied compared with five (11.6%) patients in the open group. There were no patients in the robotic group expressing dissatisfaction. On the contrary, eight (18.6%) patients in the open group were dissatisfied, and one (2.3%) extremely dissatisfied. In a similar study, Tae et al. (23) measured cosmetic satisfaction at one week, one month, and three months postoperatively. A significantly better mean satisfaction was achieved in the robotic group (p < 0.001 for each time point). A criticism concerning this study is that in reality, cosmetic outcome might be better assessed at one year after surgery when hyperpigmentation has had a chance to resolve.
The quality of life after robotic or open thyroidectomy has been investigated by a number of authors. In a study by Lee et al. (22), the Voice Handicap Index (24) was used preoperatively and at one week and three months postoperatively for patient self-assessment of voice impairment. Findings from this study indicated no statistically significant difference in voice symptom scores at any time point between the two groups. In a separate study by the same group (25), the authors adopted both subjective and objective methods to evaluate patients preoperatively and at one week and three months postoperatively. In this study, subjective evaluation, performed by an experienced voice therapist using the GRBAS (Grade, Roughness, Breathiness, Asthenia, Strain) scale (26), revealed no difference at any of the three time points. With sophisticated objective evaluation, very few differences were identified between both groups, and all were temporary. The other studies have described similar findings (27).
When the incidence of difficulty swallowing after thyroidectomy was assessed using a validated six-item Swallowing Impairment Index (SIS-6) (22), patients in both robotic and open cohorts were found to have similar scores preoperatively. Postoperatively, the open cohort had significantly worse mean SIS-6 scores at both one week (p = 0.001) and three months (p = 0.007). In another study, using a three-item questionnaire to evaluate pain or difficulty swallowing, foreign-body sensation, and choking or coughing when swallowing up to six months postoperatively (27), no differences at any time points were identified between robotic and conventional groups.
There are no randomized clinical trials or comparative studies with long-term follow-up data to comment on the oncologic equivalency of remote-access thyroidectomy to conventional surgery. A recent meta-analysis of the surgical safety and oncologic effectiveness reported that the robotic approach was associated with a less estimated blood loss, better cosmetic satisfaction, and a low level of swallowing impairment compared with conventional thyroidectomy, which was related to a shorter operative time and more retrieved lymph nodes in patients with thyroid cancer (28).
The Learning Curve
Compared with conventional endoscopic techniques, a faster learning curve for robotic techniques has been reported. In a comparative study by Lee et al. (29), the superiority of robotic thyroidectomy to endoscopic thyroidectomy in terms of operative time, lymph node dissection, and learning curve was described. For both procedures, the operative time was found to decrease gradually with increasing experience and reach a steady state after 35–40 cases for robotic thyroidectomy, and 55–60 cases for endoscopic thyroidectomy (29). In another prospective multicenter study, outcomes of robotic total or subtotal thyroidectomy were compared between one experienced and three inexperienced robotic thyroid surgeons. Overall, mean operative time and complication rates were higher for the inexperienced surgeons. However, once the inexperienced surgeons had performed 50 total or 40 subtotal thyroidectomy procedures, both the operative time and complication rates were similar to those of the experienced robotic thyroid surgeon (30).
The experience from the United States also supports at least 40 cases to overcome the learning curve of remote-access surgery. In the largest study, Kandil et al. (16) demonstrated a decrease in overall total operative time from 122 minutes to 104 minutes after 45 cases (p = 0.02). In the same study, a significant increase (37 minutes) in total operative time was observed in obese patients (BMI >30 kg/m2). Although the complications of normal-weight and overweight patients were similar, their data highlight the technical challenges to be expected in obese patients.
Cost of Robotic Thyroidectomy
In a study done by Cabot et al. (31), conventional transaxillary endoscopic and transaxillary robotic thyroidectomy approaches were compared based on medical costs in the United States. Although a higher total cost for the transaxillary approaches was reported, compared with the conventional technique ($13,087 vs. $9028), equivalence in cost for all three procedures was noted, once the total operative time was decreased in the robotic and endoscopic approaches to 68 and 111 minutes, respectively. To date, most studies on robotic thyroidectomy have been conducted in South Korea. Reimbursement for robotic thyroidectomy in South Korea is four times more than that of open thyroidectomy. The implications for U.S.-based reimbursement strategies therefore remain uncertain. Higher costs are also generated from related equipment usage, prolonged operative time, and associated facility and staffing fees. In the context of growing health expenses in the United States, this represents a major concern at the national policy level. Overall, it is agreed that remote-access surgery is not cost-effective, as the procedure is longer when compared with conventional thyroidectomy. Furthermore, in the case of a robotic procedure, additional instrumentation and draping may contribute to the higher costs.
Other Considerations
In addition to the concerns about surgical outcomes, there have also been additional controversies around robotic surgery related to the safety and efficacy of a number of procedures over the past years, with remote-access thyroid surgery being at the center of these debates. A notice was sent to the surgeons in October 2011 by Intuitive Surgical (Sunnyvale, CA) indicating that the company was “working with the FDA to modify the indication for use with respect to thyroidectomy procedures.” This notification, and a series of poor outcomes among low-volume centers, has resulted in a reduced enthusiasm for remote-access thyroid surgery in the United States. The approaches are now predominantly limited to a small number of academic centers, and the procedures are being scrutinized carefully by hospital administrations.
The experience so far suggests that the transaxillary and retroauricular approaches are more appropriate in the United States. The experience has also shown that despite the technical difficulty and more extensive surgical dissection field associated, there is a niche group of patients for whom avoidance of a neck incision is of utmost value, because of either cosmetic reasons or history of bad wound healing. The availability and feasibility of a remote-access option allow the wishes of these patients to be acknowledged and fulfilled.
There are no uniform guidelines about credentialing in remote-access robotic thyroid surgery, with each institution having its own regulations. However, as suggested for training in any other robotic procedure, recommendations include starting a program initially with a series of simple lobectomies performed under the supervision of a proctor and only after a certain experience to target more challenging procedures.
A recent report has looked at the trends in robotic surgery in the United States from 2009 through 2013. This study found out that the increase in the initial annual case volume dropped after 2011, with lower volume centers (fewer than five cases annually) contributing to the recent increases in utilization. Overall, 22% of the procedures were total thyroidectomies and 72% lobectomies. Complication rates at lower-volume centers (fewer than five annual cases) were higher than those at higher-volume (five or more annual cases) institutions. The average cost of a robotic thyroidectomy was $13,287 (32).
Conclusions and Recommendations
There are significant barriers to the performance of remote-access thyroid surgery in the United States, related to patient selection, technical challenges, outcomes, cost, and medicolegal considerations. Although equivalency to the conventional thyroidectomy has not been proven, the data show that remote-access thyroidectomy may be done safely in high-volume centers. There are insufficient data to draw conclusions about oncologic equivalency. The following recommendations are offered. First, remote-access thyroid surgery has a role in selected circumstances. Second, adherence to strict selection criteria can help to ensure safe outcomes. Third, because these approaches require an additional level of expertise, they should be done by surgeons performing a high volume of thyroid surgery. Finally, ongoing remote-access thyroid surgery quality outcomes should continue to be acquired so that the proper indications may be further refined.
Contributor Information
Collaborators: for the American Thyroid Association Surgical Affairs Committee
Acknowledgments
The authors would like to thank Dr. Alexis Kofi Okoh for his help with the editing of this manuscript and Mark Sabo of Cleveland Clinic for creating the medical illustrations describing the techniques.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ohgami M, Ishii S, Arisawa Y, Ohmori T, Noga K, Furukawa T, Kitajima M. 2000. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech 10:1–4 [PubMed] [Google Scholar]
- 2.Choe JH, Kim SW, Chung KW, Park KS, Han W, Noh DY, Oh SK, Youn YK. 2007. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 31:601–606 [DOI] [PubMed] [Google Scholar]
- 3.Shimazu K, Shiba E, Tamaki Y, Takiguchi S, Taniguchi E, Ohashi S, Noguchi S. 2003. Endoscopic thyroid surgery through the axillo-bilateral-breast approach. Surg Laparosc Endosc Percutan Tech 13:196–201 [DOI] [PubMed] [Google Scholar]
- 4.Lee KE, Rao J, Youn YK. 2009. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech 19:e71–e75 [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Takami H, Sasaki Y, Kan S, Niimi M. 2000. Endoscopic resection of thyroid tumors by the axillary approach. J Cardiovasc Surg (Torino) 41:791–792 [PubMed] [Google Scholar]
- 6.Takami H, Ikeda Y. 2002. Minimally invasive thyroidectomy. ANZ J Surg 72:841–842 [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Park CH, Chung WY. 2006. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech 16:226–231 [DOI] [PubMed] [Google Scholar]
- 8.Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, Nam KH, Chang HS, Chung WY, Park CS. 2009. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 146:1048–1055 [DOI] [PubMed] [Google Scholar]
- 9.Berber E, Siperstein A. 2011. Robotic transaxillary total thyroidectomy using a unilateral approach. Surg Laparosc Endosc Percutan Tech 21:207–210 [DOI] [PubMed] [Google Scholar]
- 10.Landry CS, Grubbs EG, Morris GS, Turner NS, Holsinger FC, Lee JE, Perrier ND. 2011. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 149:549–555 [DOI] [PubMed] [Google Scholar]
- 11.Terris DJ, Singer MC, Seybt MW. 2011. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 21:237–242 [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 13.Ban EJ, Yoo JY, Kim WW, Son HY, Park S, Lee SH, Lee CR, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS. 2014. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single-center experience with 3,000 patients. Surg Endosc 28:2555–2563 [DOI] [PubMed] [Google Scholar]
- 14.Lee KE, Kim E, Koo do H, Choi JY, Kim KH, Youn YK. 2013. Robotic thyroidectomy by bilateral axillo-breast approach; review of 1,026 cases and surgical completeness. Surg Endosc 27:2955–2962 [DOI] [PubMed] [Google Scholar]
- 15.Kuppersmith RB, Holsinger FC. 2011. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 121:521–526 [DOI] [PubMed] [Google Scholar]
- 16.Kandil EH, Noureldine SI, Yao L, Slakey DP. 2012. Robotic trans axillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 214:558–564 [DOI] [PubMed] [Google Scholar]
- 17.Aliyev S, Taskin HE, Agcaoglu O, Aksoy E, Milas M, Siperstein A, Berber E. 2013. Robotic trans axillary total thyroidectomy through a single axillary incision. Surgery 153:705–710 [DOI] [PubMed] [Google Scholar]
- 18.Terris DJ, Singer MC, Seybt MW. 2011. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 121:1636–1641 [DOI] [PubMed] [Google Scholar]
- 19.Kandil E, Saeed A, Mohamed SE, Alsaleh N, Aslam R, Moulthrop T. 2015. Modified robotic-assisted thyroidectomy: an initial experience with the retro auricular approach. Laryngoscope 125:767–771 [DOI] [PubMed] [Google Scholar]
- 20.Adam MA, Speicher P, Pura J, Dinan MA, Reed SD, Roman SA, Sosa JA. 2014. Robotic thyroidectomy for cancer in the US: patterns of use and short-term outcomes. Ann Surg Oncol 21:3859–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang BH, Wong CK, Tsang JS, Wong KP, Wan KY. 2014. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol 21:850–861 [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Nah KY, Kim RM, Ahn YH, Soh EY, Chung WY. 2010. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 24:3186–3194 [DOI] [PubMed] [Google Scholar]
- 23.Tae K, Ji YB, Jeong JH, Lee SH, Jeong MA, Park CW. 2011. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: our early experiences. Surg Endosc 25:221–228 [DOI] [PubMed] [Google Scholar]
- 24.Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. 2004. Development and validation of the voice handicap index-10. Laryngoscope 114:1549–1556 [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Na KY, Kim RM, Oh Y, Lee JH, Lee J, Lee JS, Kim CH, Soh EY, Chung WY. 2012. Postoperative functional voice changes after conventional open or robotic thyroidectomy: a prospective trial. Ann Surg Oncol 19:2963–2970 [DOI] [PubMed] [Google Scholar]
- 26.Hirano M. 1981. Clinical examination of voice. In: Amold GE, Winckel F, Wyke BD. (eds) Disorders of Human Communication. Springer-Verlag, New York, NY, pp 81–84 [Google Scholar]
- 27.Tae K, Kim KY, Yun BR, Ji YB, Park CW, Kim DS, Kim TW. 2012. Functional voice and swallowing outcomes after robotic thyroidectomy by a gasless unilateral axillo-breast approach: comparison with open thyroidectomy. Surg Endosc 26:1871–1877 [DOI] [PubMed] [Google Scholar]
- 28.Son SK, Kim JH, Bae JS, Lee SH. 2015. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol 22:3022–3032 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Yun JH, Choi UJ, Kang SW, Jeong JJ, Chung WY. 2012. Robotic versus endoscopic thyroidectomy for thyroid cancers: a multi-institutional analysis of early postoperative outcomes and surgical learning curves. J Oncol 2012:734541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. 2011. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol 18:226–232 [DOI] [PubMed] [Google Scholar]
- 31.Cabot JC, Lee CR, Brunaud L, Kleiman DA, Chung WY, Fahey TJ, 3rd, Zarnegar R. 2012. Robotic and endoscopic trans axillary thyroidectomies may be cost prohibitive when compared to standard cervical thyroidectomy: a cost analysis. Surgery 152:1016–1024 [DOI] [PubMed] [Google Scholar]
- 32.Hinson AM, Kandil E, O'Brien S, Spencer HJ, Bodenner DL, Hohmann SF, Stack BC., Jr 2015. Trends in robotic thyroid surgery in the United States from 2009 through 2013. Thyroid 25:919–926 [DOI] [PubMed] [Google Scholar]


