Abstract
Respiratory failure is a serious complication of scrub typhus. In this prospective study, all patients with a diagnosis of scrub typhus were included from a single center Intensive Care Unit (ICU). Demographic, clinical characteristics, laboratory, and imaging parameters of these patients at the time of ICU admission were compared. Of the 55 scrub typhus patients, 27 (49%) had an acute respiratory failure. Seventeen patients had acute respiratory distress syndrome, and ten had cardiogenic pulmonary edema. Respiratory supported patients were older had significant chronic lungs disease and high severity illness scores (Acute Physiology and Chronic Health Evaluation-II and Sequential Organ Failure Assessment score). At ICU admission, these patients presented with more deranged laboratory markers, including high bilirubin, high creatine kinase, high lactate, metabolic acidosis, low serum albumin, and presence of ascites. The average ICU and hospital stay were 4.27 ± 2.74 and 6.53 ± 3.52 days, respectively, in the respiratory supported group. Three patients died in respiratory failure group, while only one patient died in nonrespiratory failure group.
Key words: Acute respiratory distress syndrome, respiratory failure, scrub typhus
Introduction
Scrub typhus is a life-threatening zoonotic infectious disease and represents one of the most underdiagnosed and underreported acute undifferentiated febrile illnesses. The organism causing scrub typhus, Orientia tsutsugamushi, is an obligate intracytosolic Gram-negative bacterium that is transmitted by feeding larval trombiculid mites, which is both the vector and reservoir of the disease.[1]
In the rural area of Asia, where scrub typhus is endemic, it is among major cause (up to 20%) of acute febrile illness requiring hospital admissions.[2] Scrub typhus clinical manifestation is diverse ranging from a nonspecific febrile illness to severe multiorgan dysfunction, with an overall mortality of 6-24%.[3,4] Acute respiratory failure (ARF) is a serious manifestation of untreated scrub typhus.[5] The purpose of the present study was to investigate the risk factor, clinical course, and outcome of scrub typhus patients complicated with ARF.
Materials and Methods
This was a prospective observational study conducted in a medical Intensive Care Unit (ICU) of a Tertiary Care Hospital in Southern India between June 2013 and December 2014. All consecutive adult patients with a diagnosis of scrub typhus, which was based on the detection of antibodies by a solid phase immunochromatographic assay, were studied. Demographic and clinical characteristics of these patients, including comorbidities, sign and symptoms, biochemical and microbiological investigations, severity scores Sequential Organ Failure Assessment (SOFA) scores, and Acute Physiology and Chronic Health Evaluation (APACHE-II) scores at the time of ICU admission, were documented. In laboratory workup, complete blood picture and serum chemistry including blood sugar, serum creatinine, bilirubin, albumin, transaminases, lactic dehydrogenase (LDH), creatine kinase (CK), and electrolytes and blood lactate and arterial blood gas were measured. The diagnostic workup for other acute febrile illness such as dengue, leptospira, Q-fever, malaria, and typhoid were also performed. Chest radiography, echocardiography, and electrocardiogram were also obtained. Organ dysfunction was said to be present if the organ specific SOFA score was ≥1 and organ failure if SOFA score was ≥3.
Criteria for ARF are requirement of any form of mechanical ventilation (MV), i.e., either noninvasive ventilation (NIV) or invasive MV (IMV) for signs of exhaustion with high respiratory rate (>35 breath/min), use of accessory muscle of respiration, severe hypoxemia, hypercapnia, and hypotension (shock). The decision for type of MV was determined by intensivist, and if MV changed from NIV to IMV within 24 h of admission, then considered as IMV. During IMV, lung protective ventilator strategy (tidal volume ≤6 ml/kg of ideal body weight, plateau pressure ≤30 cm of H2 O) was followed. Patient on a respiratory support having abnormal echocardiographic finding was diagnosed as cardiogenic pulmonary edema. Other remaining (noncardiogenic cause of respiratory failure) patients were diagnosed as per the Berlin definition of acute respiratory distress syndrome (ARDS). The severity of hypoxemia is defined as per Berlin definition.
All patients were treated with oral doxycycline 100 mg twice daily for 7 days. Other supportive management was given as per the clinician decision. Patients were followed up until discharge from hospital or death. Duration of ICU stay, hospital stay, and duration of MV, vasoactive use, and ultimate hospital outcome were also documented.
Statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp.). Independent samples t-test was performed for continuous variables and was expressed using the mean ± standard deviation. Statistical significance was defined as P < 0.05.
Results
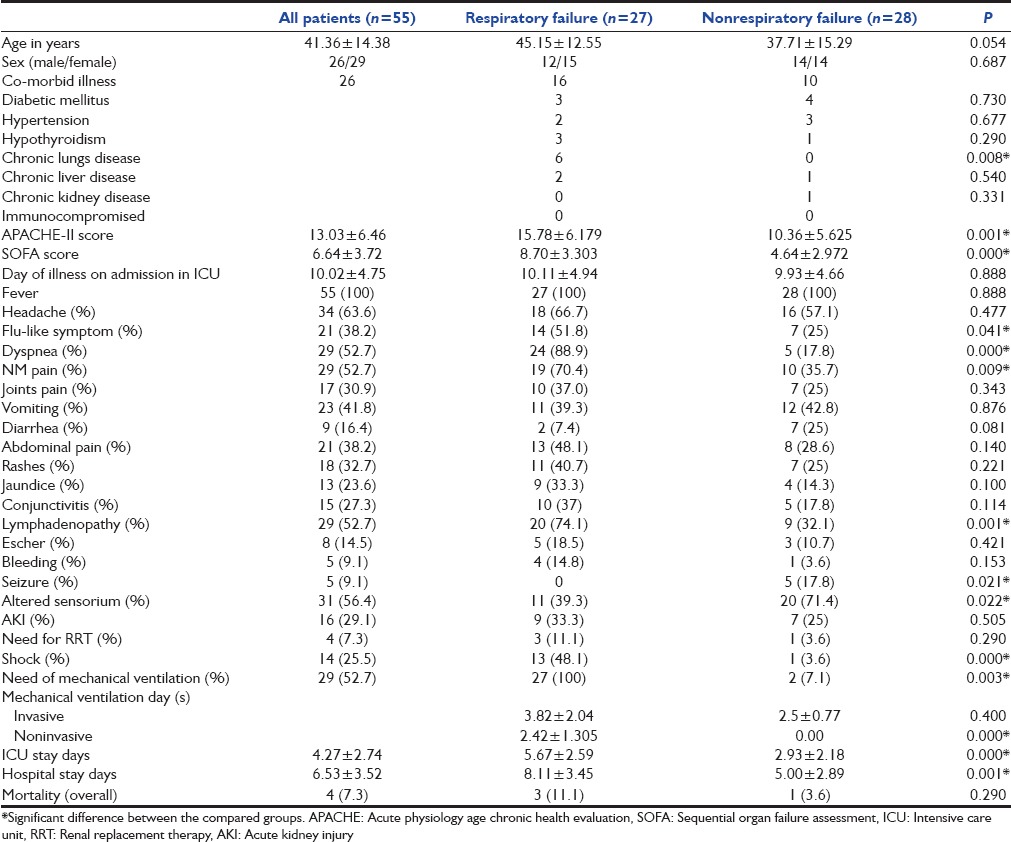
During the study period, 1587 patients admitted to our ICU; 55 patients (3.4%) were diagnosed to have scrub typhus. The mean age of all 55 patients were 41.36 years, and 26 were male [Table 1]. Diabetes mellitus was common comorbid present in seven patients, followed by chronic lungs disease (six patients) and hypertension (five patients). The mean severity of illness at ICU admission, SOFA score and APACHE-II score were 6.64 ± 3.72 and 13.03 ± 6.46, respectively. The most common symptoms were fever (100%), headache (63.3%), myalgia (52.5%), vomiting (41.8%), diarrhea (16.4%), abdominal pain (38.2%), and altered sensorium (56.4%). Respiratory symptom such as dyspnea (52.2%) and flu-like illness (38.2%) was present in more than half of the patients. Five patients (9%) had seizure (three generalized and two focal) and with 2 having Glasgow Coma Scale (GCS) <8. Other common presentation were skin rashes 18 (32.7%), 13 (23.6%) jaundice, 8 (14.5%) eschar, 29 (52.7%) lymphadenopathy, and 5 (9.1%) presented with minor bleeding.
Table 1.
Demographic, clinical characteristics of scrub typhus patients at intensive care unit admission and their outcome
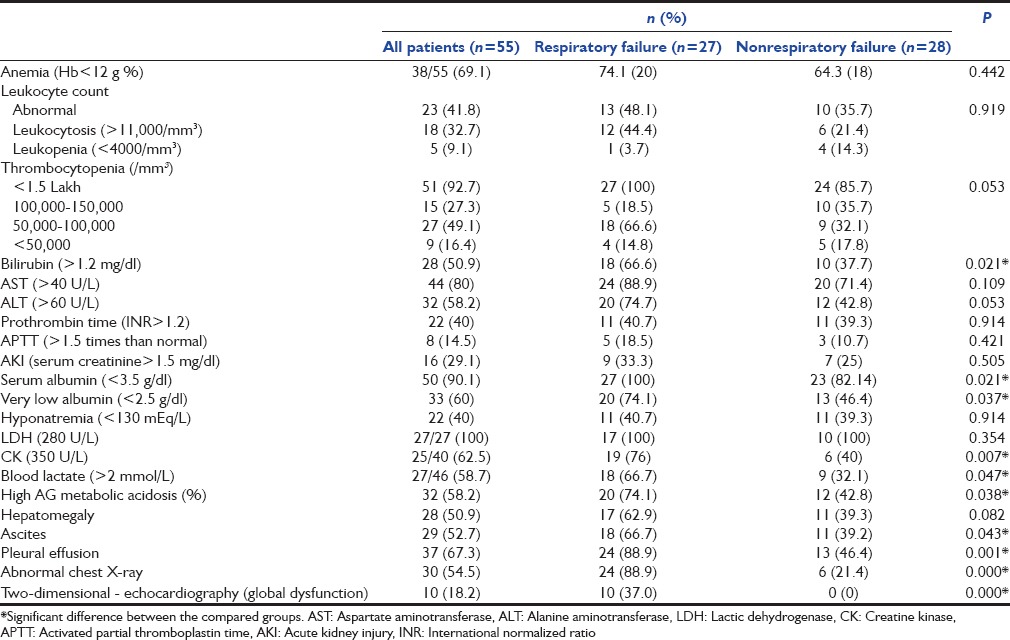
Common laboratory abnormality [Table 2] seen in these patients were thrombocytopenia (92.7%), anemia (69.1%), leukocytosis (32.7%), leukopenia (9.1), hyperbilirubinemia (50.9%), hypoalbuminemia (90.1%), hyponatremia (40%), high anion gap metabolic acidosis (58.7%), and high blood lactate, i.e., >2 mmol/L (58.7%). High serum LDH and CK were present in 100% and 62.5% of patients, respectively. Ten patients had co-infection (seven dengue, one leptospirosis, and two malaria). Ultrasonography revealed splenomegaly in 38 (69.1%), hepatomegaly 28 (50.9%), ascites 29 (52.7%), pleural effusion 37 (67.3%), and global ventricular dysfunction in 10 (18.2%) patients.
Table 2.
Laboratory and imaging parameters of scrub typhus patients at intensive care unit admission
The most common organ failures were respiratory (49.1%), followed by coagulation (16.4%), cardiovascular (12.7%), central nervous system (9.1%), liver (9.1%), and kidney (7.4%). Twenty-nine (52.7%) patients required respiratory support for the neurological cause (GCS <8), while 27 (49.1%) needed ventilation for ARF. Fourteen (25.5%) patients presented with shock (ten cardiogenic and four septic shock) required vasoactive drugs, and 7.3% patients required renal replacement therapy for acute kidney injury (AKI). The average ICU and hospital stay is 4.27 ± 2.74 and 6.53 ± 3.52, respectively, which was significantly higher in respiratory failure patients (P = 0.000). In follow-up, overall four (7.3%) patients died (three in respiratory failure group).
ARDS was cause of MV support in 17 patients and rest 10 patients required respiratory support for cardiogenic pulmonary edema [Table 3]. Respiratory supported patients were older (45.15 ± 12.55, P = 0.054), had significant chronic lungs disease (P = 0.008), high severity illness score APACHE-II (P = 0.001), and SOFA (P = 0.000) score [Table 1]. Signs and symptoms such as flu-like illness (P = 0.041), dyspnea (P = 0.000), myalgia (P = 0.009), lymphadenopathy (P = 0.001), and hypotension (P = 0.000) were more commonly seen in respiratory supported group. Laboratory and imaging parameter that were significantly associated with respiratory support cohort are hyperbilirubinemia (P = 0.021), low serum albumin (P = 0.021), elevated serum CK (P = 0.007), blood lactate (P = 0.047), high anion gap metabolic acidosis (P = 0.038), pleural effusion (P = 0.001), ascites (P = 0.043), and global hypokinesia of left ventricle (P = 0.000) [Table 2]. Clinical feature that were negatively associated with respiratory failure group were seizure (P = 0.021) and altered sensorium (P = 0.022). Although anemia (69.1%), thrombocytopenia (92.7%), leukocytosis (32.7%), leukopenia (9.1%), liver injury (58.1%), AKI (29.1%), and hyponatremia (40%) are commonly found in scrub typhus patients, none were significantly associated with respiratory support group.
Table 3.
Severity of hypoxemia and ventilator support in patients with respiratory failure
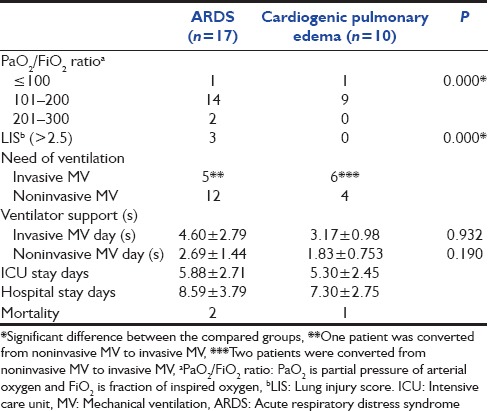
Among the respiratory failure patients, ARDS group were found to have significant low PaO2 /FiO2 (P = 0.000) and high Murray lung injury score (P = 0.000), with no difference in mortality [Table 3]. The mean MV and NIV support days were nonsignificant (4.60 ± 2.79 vs. 3.17 ± 0.98 P = 0.932, 2.69 ± 1.44 vs. 1.83 ± 0.753 P = 0.190, respectively) between ARDS and cardiogenic pulmonary edema groups.
Discussion
In the present cohort of patients with scrub typhus, half (49.1%) of the patients require MV support for respiratory failure. Eleven (40.7%) patients required IMV, 16 (59.26%) required NIV. Respiratory manifestations of scrub typhus are usually seen during the 2nd week of untreated illness,[4,5] and the presentation ranged from flu-like symptoms, bronchitis, interstitial pneumonitis to ARDS.[6,7] Respiratory failure can also be seen secondary to pulmonary edema due to cardiogenic shock. The previous report shows that ARDS can complicate scrub typhus in 44% of patients (in preantibiotic era) to 11.1-29% in recent studies[5,8] and in one recent study, the requirement of ventilator support was 87.9%.[5]
In the current study, scrub typhus patients with respiratory failure were older (45.15 ± 12.55 years) and had high SOFA and APACHE-II score compare to the nonsupported patient. The incidence of respiratory failure was higher in patients with chronic lung disease (22.2%, P = 0.008), which is contrary to previous studies.[5]
Clinical manifestation of scrub typhus ranges from a mild to a fatal illness. The typical scrub typhus begins insidiously as an acute febrile illness with fever, headache, malaise, rashes, myalgia, sore throat, cough, vomiting, diarrhea, lymphadenopathy, eschar, and abdominal pain.[6] In the respiratory failure group, dyspnea, cough, myalgia, and lymphadenopathy were significantly present, and this finding is consistent with the findings of the previous study.[5] If treated with appropriate antibiotic, symptom resolve rapidly.[6] Untreated patients entering the 2nd week of illness develop a serious complication such as severe respiratory distress syndrome,[5] encephalitis,[9] interstitial pneumonia, myocarditis,[7] AKI,[10] acute hepatic failure,[11] pancreatitis, and septic shock.[12] These severe clinical manifestations are due to generalized vasculitis due to the invasion of the endothelial cell by the organism and perivascular infiltrate of leukocyte. The common organ failures seen in our cohort were respiratory (49.1%), coagulation (16.4%), and cardiovascular system (12.7%), which were significantly lower compared to other studies.[3,4]
Although the previous study showed a significant high incidence of leukocytosis and low hematocrit in ARDS group, our study did not found.[5] Abnormal liver biochemical test (bilirubin, alanine aminotransferase, and aspartate aminotransferase) was increased in both group,[13] but only bilirubin level was significantly higher in the respiratory supported group in accordance with other studies.[5,11] Other laboratory abnormalities significantly seen in our patients with respiratory support were hypoalbuminemia, hyperlactemia, high serum creatinine kinase, and high anion gap metabolic acidosis, which was not observed in other studies except hypoalbuminemia.[5,7,14] The mechanism of hypoalbuminemia in scrub typhus is due to poor oral intake, decrease synthesis of protein from the injured liver, increase catabolism of protein, and vascular leakage of protein due to vasculitis or perivasculitis.[14] This widespread vascular permeability leads to anasarca and polyserositis such as pleural effusion and ascites. The reported incidence of abnormal chest radiographic in scrub typhus infection varies from 59% to 72%, which was slightly higher from our cohort (54.5%). The common findings are the bilateral diffuse area of reticulonodular opacity predominantly in middle and lower lobe of lungs.[15] Pleural effusion is a common finding with a reported incident varies from 12% to 43%, but our cohort had a higher incidence of pleural effusion (overall, 54.4%) and 88.9% in respiratory supported patients.[15]
Mortality rates of severe complicated scrub typhus are estimated to be approximately 35% to 40% and scrub typhus complicating ARDS mortality is about 22-45%.[5] In our cohort of patients, the overall mortality was 7.3% (4/55), while 11.1% in respiratory failure patients, which was insignificant compared to nonrespiratory failure group. This low mortality in our cohort could be due to appropriate early antibiotic (doxycycline) therapy and better ICU care down the years compared to older studies.
Conclusion
ARF is a serious and common complication of patients with scrub typhus who require ICU admission. These patients presented with more deranged laboratory markers, including high bilirubin, high CK, high lactate, metabolic acidosis, low serum albumin, and presence of ascites, at the time of ICU admission. Moreover, these patients require more days of ICU and hospital stay in comparison to patients without respiratory failure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–7. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, et al. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–62. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith M, Peter JV, Karthik G, Ramakrishna K, Prakash JA, Kalki RC, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18:497–502. doi: 10.4103/0972-5229.138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CS, Hwang JH, Lee HB, Kwon KS. Risk factors leading to fatal outcome in scrub typhus patients. Am J Trop Med Hyg. 2009;81:484–8. [PubMed] [Google Scholar]
- 5.Wang CC, Liu SF, Liu JW, Chung YH, Su MC, Lin MC. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–52. [PubMed] [Google Scholar]
- 6.Lee JH, Lee JH, Chung KM, Kim ES, Kwak YG, Moon C, et al. Dynamics of clinical symptoms in patients with scrub typhus. Jpn J Infect Dis. 2013;66:155–7. doi: 10.7883/yoken.66.155. [DOI] [PubMed] [Google Scholar]
- 7.Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsay RW, Chang FY. Acute respiratory distress syndrome in scrub typhus. QJM. 2002;95:126–8. doi: 10.1093/qjmed/95.2.126. [DOI] [PubMed] [Google Scholar]
- 9.Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry. 2015;86:761–6. doi: 10.1136/jnnp-2014-308722. [DOI] [PubMed] [Google Scholar]
- 10.Attur RP, Kuppasamy S, Bairy M, Nagaraju SP, Pammidi NR, Kamath V, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17:725–9. doi: 10.1007/s10157-012-0753-9. [DOI] [PubMed] [Google Scholar]
- 11.Goswami D, Hing A, Das A, Lyngdoh M. Scrub typhus complicated by acute respiratory distress syndrome and acute liver failure: A case report from Northeast India. Int J Infect Dis. 2013;17:e644–5. doi: 10.1016/j.ijid.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Thap LC, Supanaranond W, Treeprasertsuk S, Kitvatanachai S, Chinprasatsak S, Phonrat B. Septic shock secondary to scrub typhus: Characteristics and complications. Southeast Asian J Trop Med Public Health. 2002;33:780–6. [PubMed] [Google Scholar]
- 13.Hu ML, Liu JW, Wu KL, Lu SN, Chiou SS, Kuo CH, et al. Short report: Abnormal liver function in scrub typhus. Am J Trop Med Hyg. 2005;73:667–8. [PubMed] [Google Scholar]
- 14.Lee CS, Min IS, Hwang JH, Kwon KS, Lee HB. Clinical significance of hypoalbuminemia in outcome of patients with scrub typhus. BMC Infect Dis. 2010;10:216. doi: 10.1186/1471-2334-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: Clinical, pathologic, and imaging findings. Radiographics. 2007;27:161–72. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]


